Green Extraction-Assisted Pseudo-Targeted Profile of Alkaloids in Lotus Seed Epicarp Based on UPLC-QTOF MS with IDA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Reagents and Chemicals
2.2. Sample Preparation
2.3. Ultrasonic-Assisted Extraction
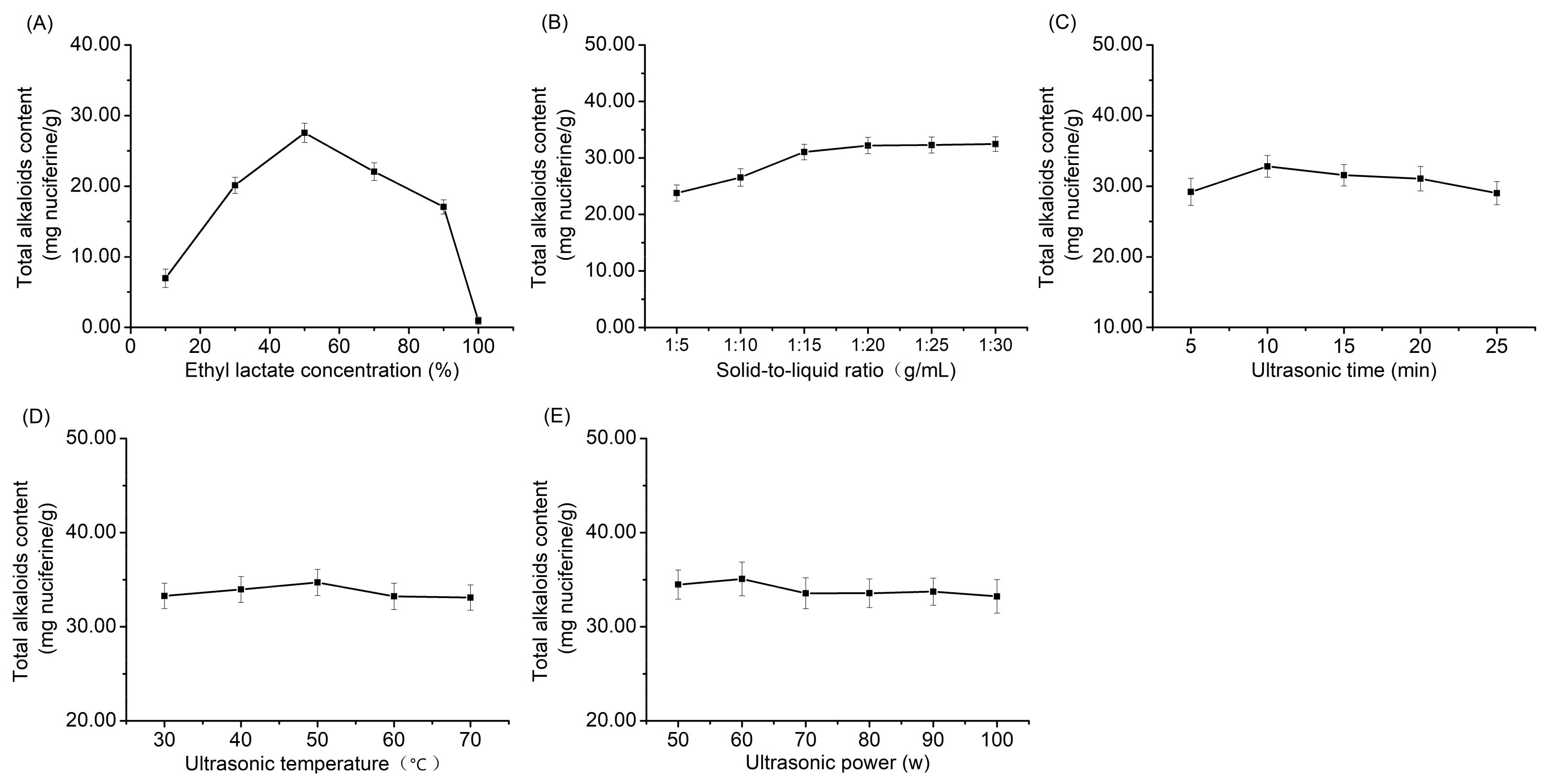
2.4. Optimization of Ultrasonic Extraction Method
2.4.1. Single-Factor Experiments
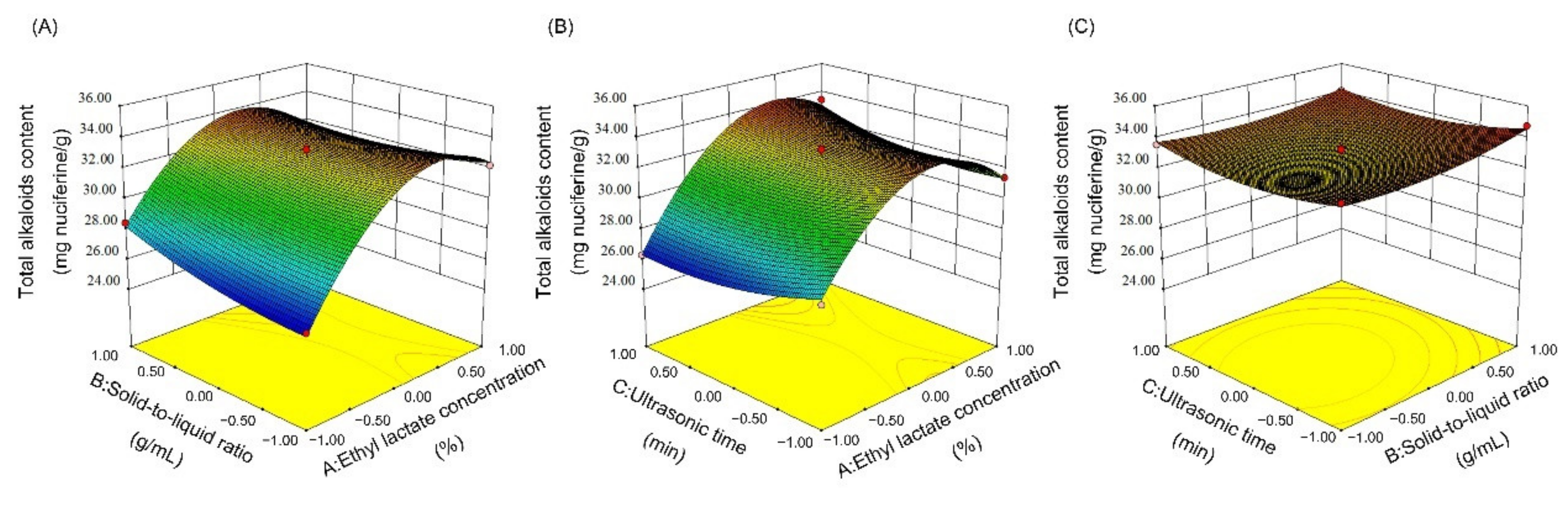
2.4.2. Response Surface Methodology (RSM) Experiments
2.5. Conventional Reference Extraction Methods
2.6. Antioxidant Activities
2.7. Conditions of UPLC-QTOF-MS/MS
2.8. Quantification of Ten Representative Alkaloids in Lotus Seed Epicarp at Different Growth Stages
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extraction Conditions
3.1.1. Single-Factor Experiments
3.1.2. Response Surface Optimization
3.1.3. Comparison with Previously Reported Methods
3.1.4. Antioxidant Activity of Lotus Seed Epicarp Extracts
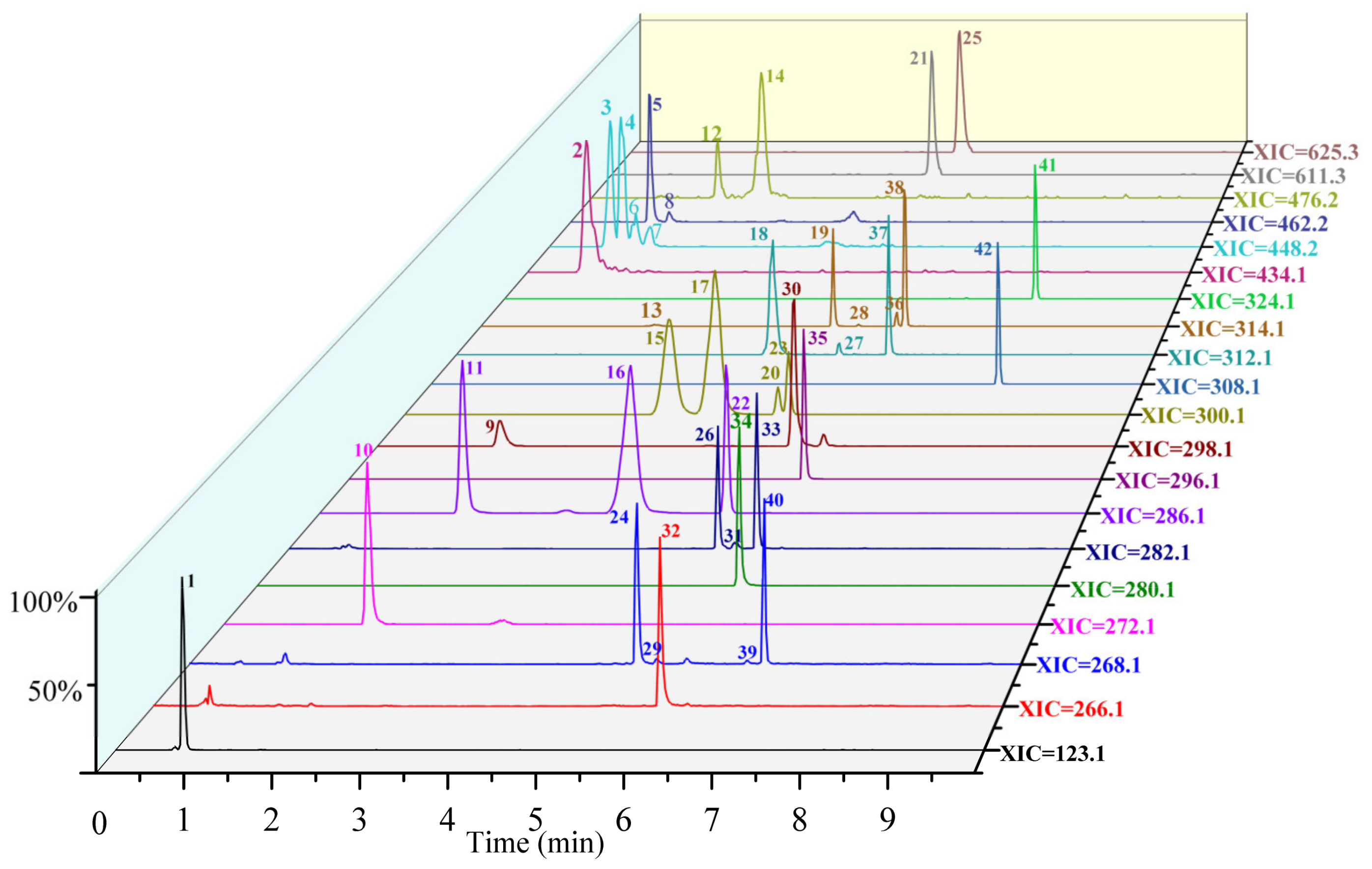
3.2. Pseudo-Targeted Profile of Alkaloids in Lotus Seed Epicarp
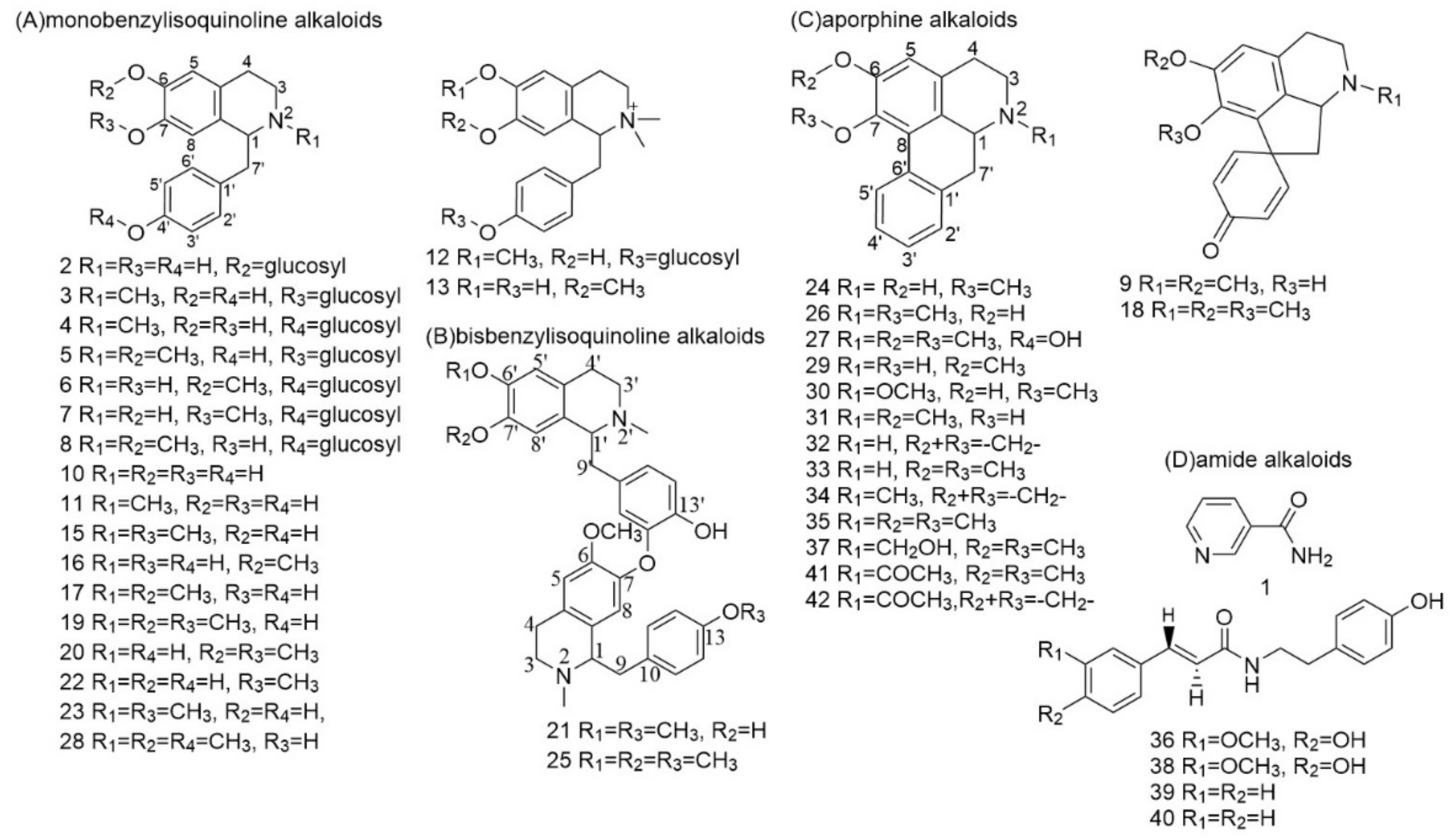
3.2.1. Identification of Monobenzylisoquinoline Alkaloids
3.2.2. Identification of Bisbenzylisoquinoline Alkaloids
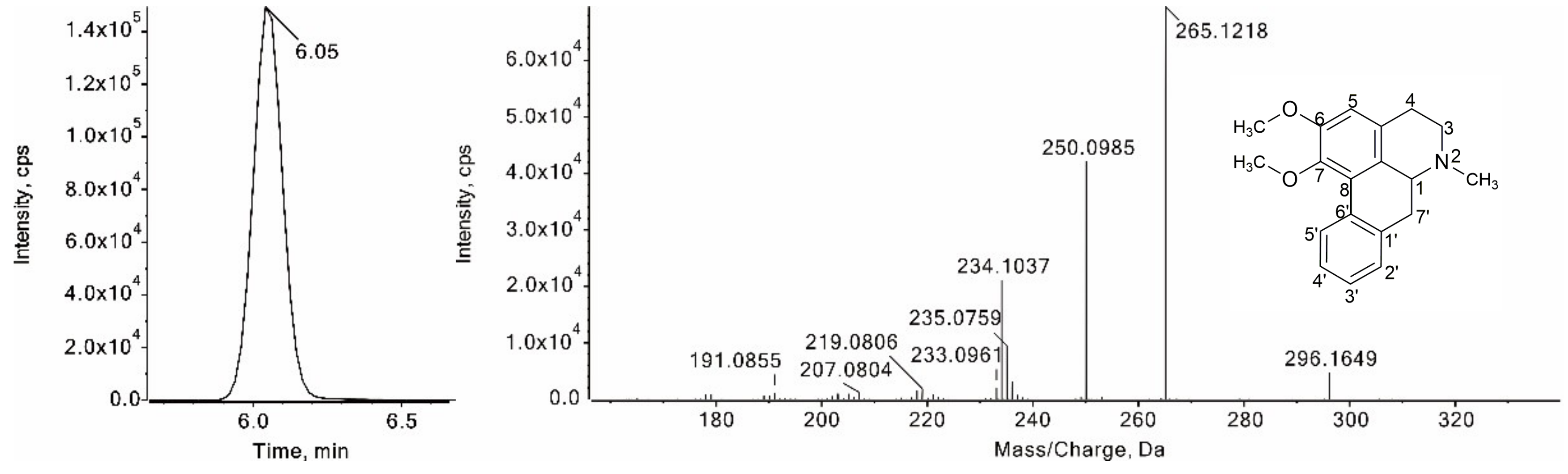
3.2.3. Identification of Aporphine Alkaloids
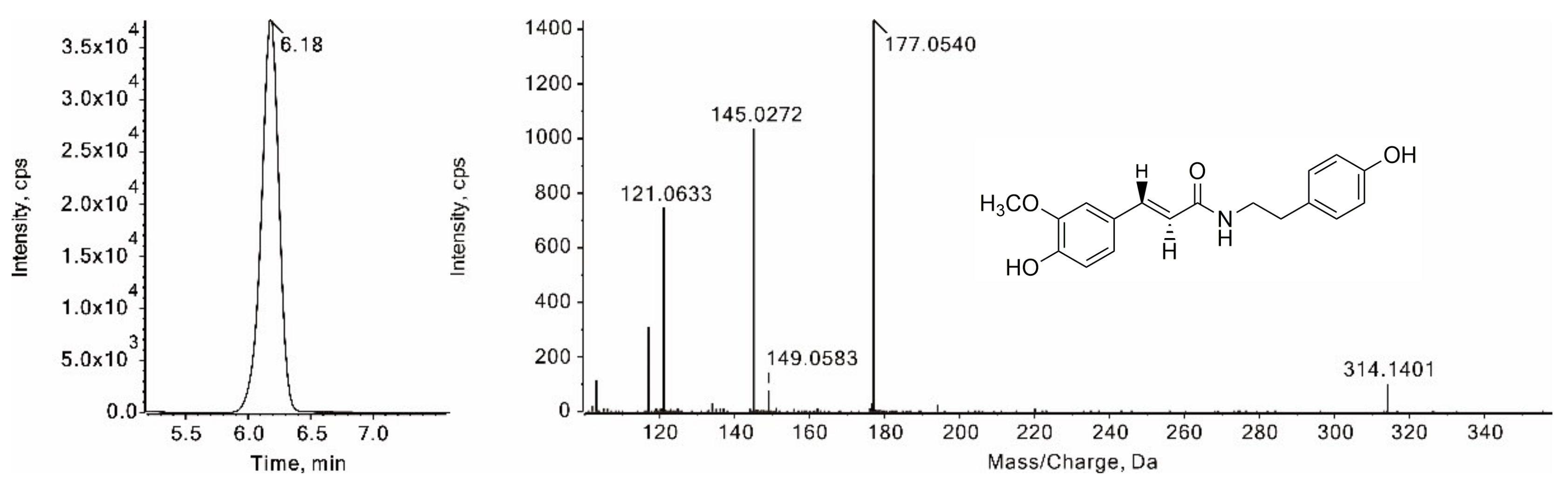
3.2.4. Identification of Amide Alkaloids
3.3. Analysis of Ten Representative Alkaloids in Lotus Seed Epicarp at Different Growth Stages
3.3.1. Validation of Quantitative Analysis
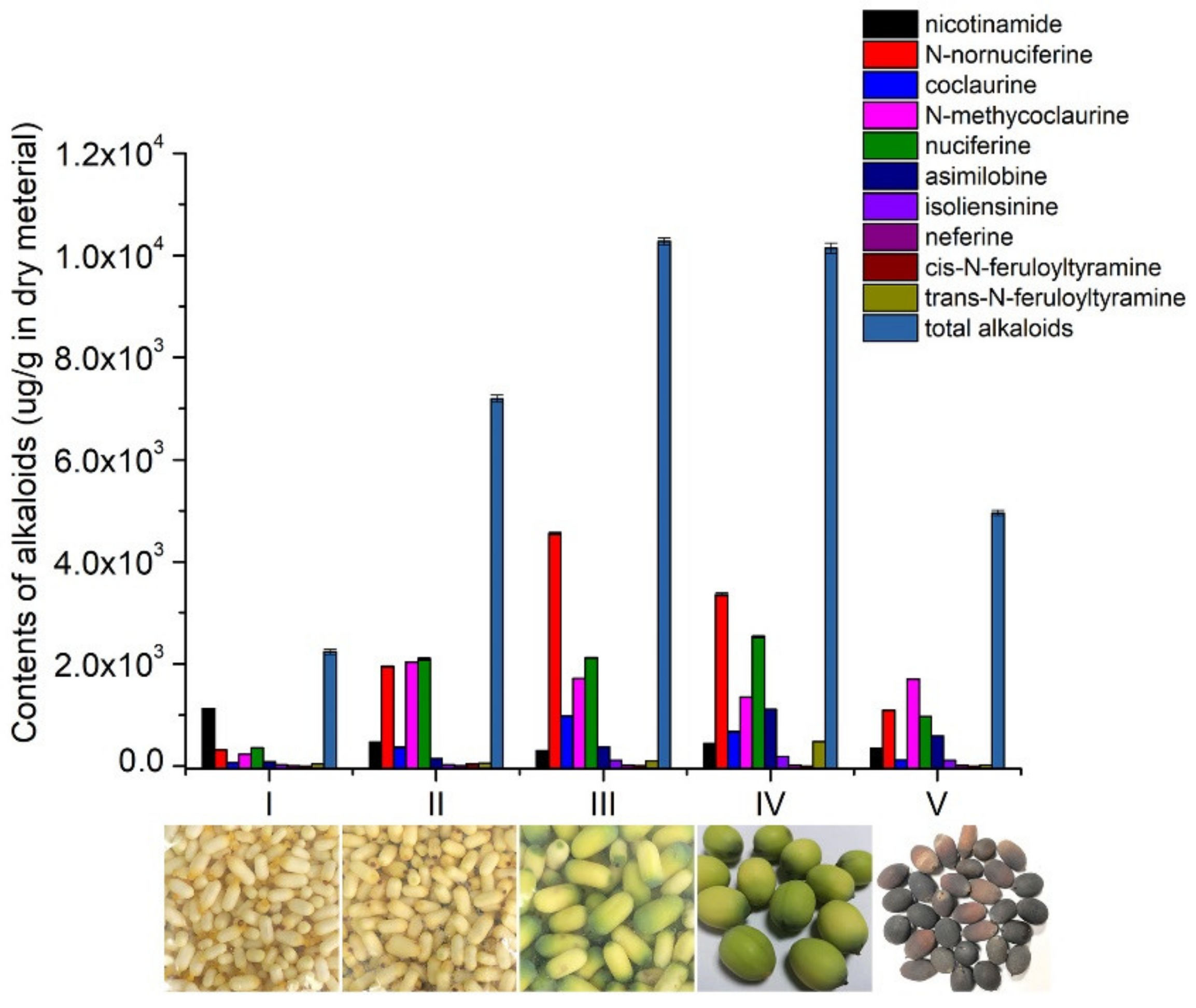
3.3.2. Determination of Ten Alkaloids in Lotus Seed Epicarp at Different Growth Stages
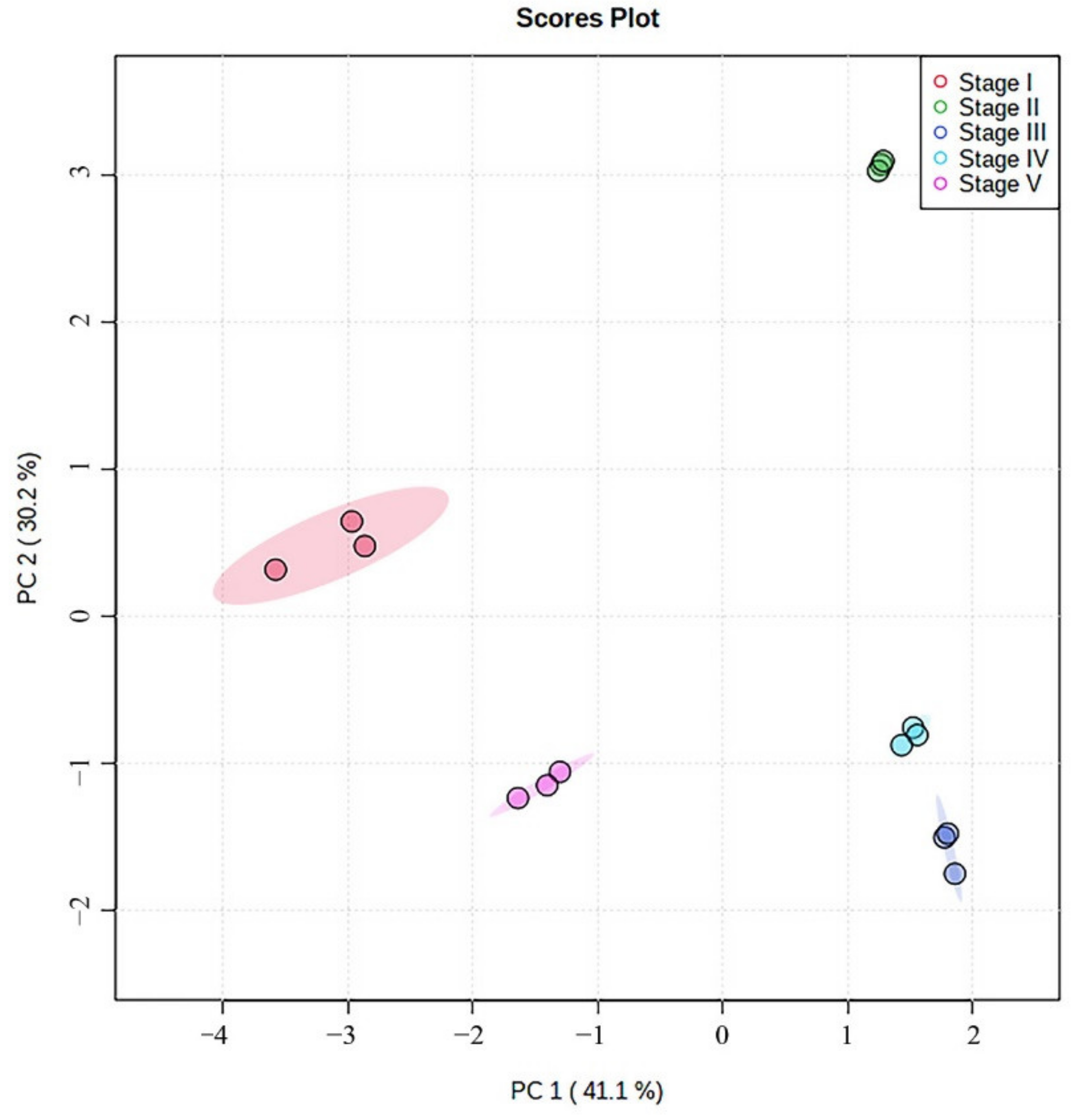
3.3.3. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.B. Cultivation of lotus (Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China. Genet. Resour. Crop Evol. 2009, 56, 323–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Zeng, S.; Huang, X.; Guo, Z.; Zheng, Y.; Tian, Y.; Zheng, B. Nutritional composition, physiological functions and processing of lotus (Nelumbo nucifera Gaertn.) seeds: A review. Phytochem. Rev. 2015, 14, 321–334. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, E.S.; Lee, C.; Kim, S.; Cho, S.-H.; Hwang, B.Y.; Lee, M.K. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg. Med. Chem. Lett. 2013, 23, 3604–3608. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Yi, R.; Mu, J.; Zhou, X.; Zhao, X. Preventive Effect of Alkaloids from Lotus plumule on Acute Liver Injury in Mice. Foods 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhou, J. A study on the chemical composition oflotus seed epicarp. J. Agric. Food Chem. 2011, 66, 139–141. [Google Scholar]
- Chen, H.; Sun, K.; Yang, Z.; Guo, X.; Wei, S. Identification of Antioxidant and Anti-α-amylase Components in Lotus (Nelumbo nucifera, Gaertn.) Seed Epicarp. Appl. Biochem. Biotechnol. 2018, 187, 677–690. [Google Scholar] [CrossRef]
- Kredy, H.M.; Huang, D.; Xie, B.; He, H.; Yang, E.; Tian, B.; Xiao, D. Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential. Eur. Food Res. Technol. 2010, 231, 387–394. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Y.; Huang, W.; Feng, X.; Yang, F.; Li, D. Separation, Identification, and Antioxidant Activity of Polyphenols from Lotus Seed Epicarp. Molecules 2019, 24, 4007. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ma, S.-S.; Ibrahim, S.; Li, E.-H.; Yang, H.; Huang, W. Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chem. 2015, 185, 159–164. [Google Scholar] [CrossRef]
- Cao, J.; Yu, X.; Deng, Z.; Pan, Y.; Zhang, B.; Tsao, R.; Li, H. Chemical Compositions, Antiobesity, and Antioxidant Effects of Proanthocyanidins from Lotus Seed Epicarp and Lotus Seed Pot. J. Agric. Food Chem. 2018, 66, 13492–13502. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, R.; Liu, M.; Yi, L.; Liu, S. An integrated strategy to rapidly characterize non-targeted benzylisoquinoline alkaloids from Plumula nelumbinis ethanol extract using UHPLC/Q-orbitrap HRMS. Int. J. Mass Spectrom. 2018, 432, 26–35. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, Z.D.; Xie, B.J.; Yang, E.N. Optimization of Alkaloid Extraction from Lotus Leaves by Response Surface Method. Food Sci. 2009, 30, 157–161. [Google Scholar]
- Hu, J.-N.; Shan, B.; Deng, Z.-Y.; Li, J.; Fan, Y.-W.; Ruan, Z.; Liu, R. Application of high-speed counter-current chromatography for the isolation of 5 alkaloids from lotus (Nelumbo nucifera Gaertn.) leaves. Food Sci. Biotechnol. 2010, 19, 1661–1665. [Google Scholar] [CrossRef]
- Wu, S.; Sun, C.; Cao, X.; Zhou, H.; Zhang, H.; Pan, Y. Preparative counter-current chromatography isolation of liensinine and its analogues from embryo of the seed of Nelumbo nucifera GAERTN. Using upright coil planet centrifuge with four multilayer coils connected in series. J. Chromatogr. A 2004, 1041, 153–162. [Google Scholar] [CrossRef]
- Bao, N.; Wang, D.; Fu, X.; Xie, H.; Gao, G.; Luo, Z. Green Extraction of Phenolic Compounds from Lotus Seedpod (Receptaculum Nelumbinis) Assisted by Ultrasound Coupled with Glycerol. Foods 2021, 10, 239. [Google Scholar] [CrossRef]
- Rao, M.V.; Sengar, A.S.; K, S.C.; Rawson, A. Ultrasonication—A green technology extraction technique for spices: A review. Trends Food Sci. Technol. 2021, 116, 975–991. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.-N.; Li, H.-B. Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha integerrima by Response Surface Methodology. Molecules 2015, 21, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zheng, J.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules 2017, 22, 638. [Google Scholar] [CrossRef] [Green Version]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and kinetic modeling of ultrasonic-assisted extraction of fucoxanthin from edible brown algae Sargassum fusiforme using green solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef] [PubMed]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Ethyl lactate as a potential green solvent to extract hydrophilic (polar) and lipophilic (non-polar) phytonutrients simultaneously from fruit and vegetable by-products. Sustain. Chem. Pharm. 2016, 4, 21–31. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Remonatto, D.; Paula, A.V.; Herculano, R.D.; Santos-Ebinuma, V.C.; Coutinho Joao, A.P.; Pereira Jorge, F.B. Selective recovery and purification of carotenoids and fatty acids from Rhodotorula glutinis using mixtures of biosolvents. Sep. Purif. Technol. 2021, 266, 118548. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Extraction of curcuminoids by using ethyl lactate and its optimisation by response surface methodology. J. Pharm. Biomed. Anal. 2018, 149, 89–95. [Google Scholar] [CrossRef]
- Sepulveda, B.; Benites, D.; Albornoz, L.; Simirgiotis, M.; Castro, O.; Garcia-Beltran, O.; Areche, C. Green ultrasound-assisted extraction of lichen substances from Hypotrachyna cirrhata. Ethyl lactate, a better extracting agent than methanol toxic organic solvent? Nat. Prod. Res. 2021, 35, 1–5. [Google Scholar] [CrossRef]
- Ma, J.; Li, Q.; Fan, S.; He, L.; Sun, L.; Wen, D.; Zhai, H.; Zhang, Y. Determination of 8 Endogenous Alkaloid Components inBoletusUsing Ultrahigh-Performance Liquid Chromatography Combined with Quadrupole-Time of Flight Mass Spectrometry. J. Food Qual. 2020, 2020, 8865725. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, Z.; Chen, A.; Lu, X.; Zhao, C.; Hu, C.; Zhou, L.; Liu, X.; Wang, X.; Hou, X.; et al. Comprehensive Strategy to Construct In-House Database for Accurate and Batch Identification of Small Molecular Metabolites. Anal. Chem. 2018, 90, 7635–7643. [Google Scholar] [CrossRef]
- Wu, X.-L.; Wu, M.-J.; Chen, X.-Z.; Ma, H.-L.; Ding, L.-Q.; Qiu, F.; Pan, Q.; Zhang, D.-Q. Metabolic profiling of nuciferine in rat urine, plasma, bile and feces after oral administration using ultra-high performance liquid chromatography-diode array detection-quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2017, 140, 71–80. [Google Scholar] [CrossRef]
- Cao, X.; Ye, X.; Lu, Y.; Yu, Y.; Mo, W. Ionic liquid-based ultrasonic-assisted extraction of piperine from white pepper. Anal. Chim. Acta 2009, 640, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, M.; Shao, J.; Lin, B.; Shen, Q. Rapid determination of alkaloids in Macleaya cordata using ionic liquid extraction followed by multiple reaction monitoring UPLC–MS/MS analysis. J. Pharm. Biomed. Anal. 2017, 135, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Chen, X.; Lv, G.; Hu, D.; Zhao, J.; Li, S. Optimization of microwave-assisted extraction of bioactive alkaloids from lotus plumule using response surface methodology. J. Pharm. Anal. 2016, 6, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Yang, R.; Guan, Z.; Chen, A.; Li, W. Ultra-performance LC Separation and Quadrupole Time-of-flight MS Identification of Major Alkaloids in Plumula Nelumbinis. Phytochem. Anal. 2014, 25, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Chen, X.; Qi, J.; Yu, B. Simultaneous qualitative and quantitative analysis of flavonoids and alkaloids from the leaves ofNelumbo nuciferaGaertn. Using high-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 2499–2507. [Google Scholar] [CrossRef]
- Morikawa, T.; Kitagawa, N.; Tanabe, G.; Ninomiya, K.; Okugawa, S.; Motai, C.; Kamei, I.; Yoshikawa, M.; Lee, I.-J.; Muraoka, O. Quantitative Determination of Alkaloids in Lotus Flower (Flower Buds of Nelumbo nucifera) and Their Melanogenesis Inhibitory Activity. Molecules 2016, 21, 930. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Q.; Qing, Z.X.; Zeng, J.G. Research advances on chemical constituents and pharmacological effects of various parts of Nelumbo nucifera. Chin. Tradit. Herbal. Drugs 2019, 50, 6162–6189. [Google Scholar]
- Tian, W.; Zhi, H.; Yang, C.; Wang, L.; Long, J.; Xiao, L.; Liang, J.; Huang, Y.; Zheng, X.; Zhao, S.; et al. Chemical composition of alkaloids of Plumula nelumbinis and their antioxidant activity from different habitats in China. Ind. Crop. Prod. 2018, 125, 537–548. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Liu, C.; Mao, F.; LI, X. Analysis of chemical constituents of Lotus leaf by HPLC-TOF /MS. J. Pharm. Prac. 2011, 29, 342–346. [Google Scholar]
- Shan, F.; Yuan, Y.; Kang , L.P.; Huang, L.Q. Study on chemical constituents in stems of Nelumbo nucifera by UPLC-ESI /Q-TOF-MS /MS. Chin. J. Chin. Mater. Med. 2015, 40, 3233–3238. [Google Scholar]
- Sharma, B.R.; Gautam, L.N.S.; Adhikari, D.; Karki, R. A Comprehensive Review on Chemical Profiling of Nelumbo Nucifera: Potential for Drug Development. Phytother. Res. 2016, 31, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Do, T.C.M.V.; Nguyen, T.D.; Tran, H.; Stuppner, H.; Ganzera, M. Analysis of alkaloids in Lotus (Nelumbo nucifera Gaertn.) leaves by non-aqueous capillary electrophoresis using ultraviolet and mass spectrometric detection. J. Chromatogr. A 2013, 1302, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.T.; Li, Q.; Shao, Q.; Wang, B.H.; Wei, Y. A general ionic liquid pH-zone-refining countercurrent chromatography method for separation of alkaloids from Nelumbo nucifera Gaertn. J. Chromatogr. A 2017, 1507, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-T.; Huang, J.-C.; Kuo, P.-L.; Chen, C.-Y. Bioactive Constituents from Michelia champaca. Nat. Prod. Commun. 2011, 6, 1251–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.-L.; He, Y.-Q.; Wang, M.; Zhang, L.; Yaang, L.; Wang, Z.-T.; Ji, G. Characterization of nuciferine metabolism by P450 enzymes and uridine diphosphate glucuronosyltransferases in liver microsomes from humans and animals. Acta Pharmacol. Sin. 2010, 31, 1635–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S. Molecular Mechanism Study on Efficacy Differernce of Lotus Leaf and Lotus Plumule; Chengdu University of Traditional Chinese Medicine: Chengdu, China, 2015. [Google Scholar]
- Liu, J.; Shi, K.; Shi, J.; Feng, Y.; Hao, C.; Peng, J.; Chen, S. A simple strategy to monitor the temporal and spatial distribution of alkaloids in sacred lotus leaves. Biosci. Biotechnol. Biochem. 2021, 85, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, B.; Liu, J.; Yao, S. Simultaneous analysis of N-nornuciferine, O-nornuciferine, nuciferine, and roemerine in leaves of Nelumbo nucifera Gaertn by high-performance liquid chromatography–photodiode array detection–electrospray mass spectrometry. Anal. Chim. Acta 2005, 538, 129–133. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Li, L.; Li, J.; Xu, L.; Feng, J.; Liu, Y. Digital Gene Expression Analysis Provides Insight into the Transcript Profile of the Genes Involved in Aporphine Alkaloid Biosynthesis in Lotus (Nelumbo nucifera). Front. Plant Sci. 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]



| Method | Solid-to-Liquid (g/mL) | Extraction Time (min) | Temperature (°C) | Total Alkaloid Content (mg Nuciferine/g) | Ref. |
|---|---|---|---|---|---|
| Ethyl lactate with ultrasonic | 1:10 | 15 | 50 | 34.75 ± 1.55 | This work |
| Ethanol with ultrasonic | 1:25 | 30 | 27.484 ± 1.56 | [34] | |
| Methanol with ultrasonic | 1:50 | 30 | 35.55 ± 1.42 | [35] | |
| Ethanol with reflux | 1:10 | 60 | 90 | 35.69 ± 1.51 | [11] |
| Methanol with reflux | 1:10 | 60 | 25 | 21.29 ± 1.53 | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Lin, X.; Wu, C.; Zhang, M.; Wang, M. Green Extraction-Assisted Pseudo-Targeted Profile of Alkaloids in Lotus Seed Epicarp Based on UPLC-QTOF MS with IDA. Foods 2022, 11, 1056. https://doi.org/10.3390/foods11071056
Cao X, Lin X, Wu C, Zhang M, Wang M. Green Extraction-Assisted Pseudo-Targeted Profile of Alkaloids in Lotus Seed Epicarp Based on UPLC-QTOF MS with IDA. Foods. 2022; 11(7):1056. https://doi.org/10.3390/foods11071056
Chicago/Turabian StyleCao, Xiaoji, Xupin Lin, Congcong Wu, Minghua Zhang, and Mingwei Wang. 2022. "Green Extraction-Assisted Pseudo-Targeted Profile of Alkaloids in Lotus Seed Epicarp Based on UPLC-QTOF MS with IDA" Foods 11, no. 7: 1056. https://doi.org/10.3390/foods11071056





