Determination of Cultivation Regions and Quality Parameters of Poria cocos by Near-Infrared Spectroscopy and Chemometrics
Abstract
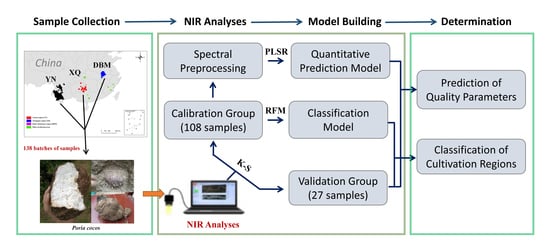
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemicals and Reagents
2.3. Extraction of Water-Soluble Content and Ethanol-Soluble Content of PC
2.4. Polysaccharides Content and Absorbance Curve
2.5. Quantitative Analysis of Five Triterpene Acids Using High-Performance Liquid Chromatography (HPLC)
2.6. NIR Spectroscopy Determination
2.7. Chemometric Methods
2.7.1. Geographical Discriminant Analysis of Random Forest Method (RFM)
2.7.2. Partial Least Squares Regression (PLSR) and Determination of Chemical Composition
2.8. Software Requirements and Data Analysis
3. Results and Discussion
3.1. Chemical Composition of PC Samples
3.1.1. Polysaccharides, Water-Soluble Extract and Ethanol-Soluble Extract in PC Samples
3.1.2. HPLC Analysis of Five Triterpene Acids
3.2. NIR Spectra Characteristics
3.3. Elimination of Outliers
3.4. Determination of PC Cultivation Regions
3.5. Development of Quantitative Prediction Model
3.5.1. Model Development and Optimization
3.5.2. Validation of Quantitative Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, L.; Xu, J.; Zhang, S.; Wang, R.; Huang, Q.; Chen, H.; Dong, X.; Zhao, Z. Qualitatively and quantitatively comparing secondary metabolites in three medicinal parts derived from Poria cocos (Schw.) Wolf using UHPLC-QTOF-MS/MS-based chemical profiling. J. Pharm. Biomed. Anal. 2018, 150, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Hou, C.C.; Chang, C.L.; Yang, W.C. Anti-Hyperglycemic Properties of Crude Extract and Triterpenes from Poria cocos. Evid. Based Complement. Altern. Med. 2011, 2011, 128402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Fan, L. The effect of Poria cocos ethanol extract on the intestinal barrier function and intestinal microbiota in mice with breast cancer. J. Ethnopharmacol. 2021, 266, 113456. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Li, P.; Zhao, J.; Duan, J. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int. J. Biol. Macromol. 2018, 120 Pt B, 1696–1704. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Q.; Chen, Y.; Wang, W.; Li, S. Simultaneous extraction of polysaccharides from Poria cocos by ultrasonic technique and its inhibitory activities against oxidative injury in rats with cervical cancer. Carbohydr. Polym. 2010, 79, 409–413. [Google Scholar] [CrossRef]
- Sun, S.S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- Rigling, M.; Liu, Z.; Hofele, M.; Prozmann, J.; Zhang, C.; Ni, L.; Fan, R.; Zhang, Y. Aroma and catechin profile and in vitro antioxidant activity of green tea infusion as affected by submerged fermentation with Wolfiporia cocos (Fu Ling). Food Chem. 2021, 361, 130065. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef]
- Dong, T.T.X.; Cui, X.M.; Song, Z.H.; Zhao, K.J.; Ji, Z.N.; Lo, A.C.K.; Tsim, K.W.K. Chemical assessment of roots of Panax notoginseng in China: Regional and seasonal variations in its active constituents. J. Agric. Food Chem. 2003, 51, 4617–4623. [Google Scholar] [CrossRef]
- Yu, L.; Sun, S.Q.; Fan, K.F.; Zhou, Q.; Noda, I. Research on processing medicinal herbs with multi-steps infrared macro-fingerprint method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 22–29. [Google Scholar] [CrossRef]
- Tian, S.-S.; Liu, X.-Q.; Feng, W.-H.; Zhang, Q.-W.; Yan, L.-H.; Wang, Z.-M.; Gao, L. Quality evaluation of Poria based on specific chromatogram and quantitative analysis of multicomponents. Zhongguo Zhong Yao Za Zhi 2019, 44, 1371–1380. [Google Scholar]
- Nie, A.; Chao, Y.; Zhang, X.; Jia, W.; Zhou, Z.; Zhu, C. Phytochemistry and Pharmacological Activities of Wolfiporia cocos (F.A. Wolf) Ryvarden & Gilb. Front. Pharmacol. 2020, 11, 505249. [Google Scholar]
- Zhu, L.-X.; Xu, J.; Wang, R.-J.; Li, H.-X.; Tan, Y.-Z.; Chen, H.-B.; Dong, X.-P.; Zhao, Z.-Z. Correlation between Quality and Geographical Origins of Poria cocos Revealed by Qualitative Fingerprint Profiling and Quantitative Determination of Triterpenoid Acids. Molecules 2018, 23, 2200. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Zhou, R.; Xie, J.; Ye, H.; Liang, X.; Zhong, C.; Shen, B.; Qin, Y.; Zhang, S.; Huang, L. Insights into Triterpene Acids in Fermented Mycelia of Edible Fungus Poria cocos by a Comparative Study. Molecules 2019, 24, 1331. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.L.; Zhang, L.; Bian, X.K.; Qian, D.W.; Guo, S.; Yan, H. Analysis of 8 triterpene acids in Poria from different habitats based on UPLC-QTRAP-MS. Chin. J. Pharm. Anal. 2020, 40, 1169–1177. [Google Scholar]
- Lee, S.R.; Lee, S.; Moon, E.; Park, H.J.; Park, H.B.; Kim, K.H. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorg. Chem. 2017, 70, 94–99. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Yang, S.-M.; Ryoo, R.; Moon, E.; Kim, S.-H.; Kim, K.H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorg. Chem. 2018, 81, 27–34. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Jurutka, P.W.; Wu, S.; Chen, Y.; Cao, C.; Chen, G.; Tian, B.; Wang, S.; Cheng, S. 16α-Hydroxytrametenolic Acid from Poria cocos Improves Intestinal Barrier Function Through the Glucocorticoid Receptor-Mediated PI3K/Akt/NF-κB Pathway. J. Agric. Food Chem. 2019, 67, 10871–10879. [Google Scholar] [CrossRef]
- Zou, Y.-T.; Zhou, J.; Wu, C.-Y.; Zhang, W.; Shen, H.; Xu, J.-D.; Zhang, Y.-Q.; Long, F.; Li, S.-L. Protective effects of Poria cocos and its components against cisplatin-induced intestinal injury. J. Ethnopharmacol. 2021, 269, 113722. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Tang, C.; Xiao, J.; Xie, B.; Sun, Z. Synergistic effect of B-type oligomeric procyanidins from lotus seedpod in combination with water-soluble Poria cocos polysaccharides against E. coli and mechanism. J. Funct. Foods 2018, 48, 134–143. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.; Li, X.; Leong, W.; Xia, W.; Hsiao, W.L.W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 2018, 41, 191–201. [Google Scholar] [CrossRef]
- Hua, H.; Yi, Y.; Li, H.; Chen, C. Monitoring the extraction process of acidic polysaccharides in Poria cocos by near infrared spectroscopy combined with chemometrics. Infrared Phys. Technol. 2021, 113, 103613. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Hsu, N.-Y.; Lu, K.-H.; Lin, Y.-E.; Lin, S.-H.; Lu, Y.-S.; Liu, W.-T.; Chen, M.-H.; Sheen, L.-Y. Poria cocos water extract ameliorates the behavioral deficits induced by unpredictable chronic mild stress in rats by down-regulating inflammation. J. Ethnopharmacol. 2020, 258, 112566. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Lu, Y.; Chaurasiya, B.; Mi, G.; Shi, D.; Chen, D.; Webster, T.J.; Tu, J.; Shen, Y. Co-delivery of Poria cocos extract and doxorubicin as an ‘all-in-one’ nanocarrier to combat breast cancer multidrug resistance during chemotherapy. Nanomedicine 2020, 23, 102095. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, H.; Yan, F.; Yuan, S.; Li, S.; Zhu, P.; Cheng, W.; Peng, C.; Peng, D. An UPLC-Q-Orbitrap method for pharmacokinetics and tissue distribution of four triterpenoids in rats after oral administration of Poria cocos ethanol extracts. J. Pharm. Biomed. Anal. 2021, 203, 114237. [Google Scholar] [CrossRef]
- Ríos, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Qian, Q.; Zhou, N.; Qi, P.; Zhang, Y.; Mu, X.; Shi, X.; Wang, Q. A UHPLC-QTOF-MS/MS method for the simultaneous determination of eight triterpene compounds from Poria cocos (Schw.) Wolf extract in rat plasma: Application to a comparative pharmacokinetic study. J. Chromatogr. B 2018, 1102–1103, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Gapter, L.A.; Ling, H.; Agarwal, R.; Ng, K.Y. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem. Pharm. Bull. 2008, 56, 1459–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musingarabwi, D.M.; Nieuwoudt, H.H.; Young, P.R.; Eyéghè-Bickong, H.A.; Vivier, M.A. A rapid qualitative and quantitative evaluation of grape berries at various stages of development using Fourier-transform infrared spectroscopy and multivariate data analysis. Food Chem. 2016, 190, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef] [Green Version]
- Benes, E.; Gere, A.; Fodor, M. Predicting macronutrients and energy content of snack products using FT-NIR analysis and chemometric techniques. J. Food Eng. 2020, 280, 109954. [Google Scholar] [CrossRef]
- Fodor, M.; Mikola, E.E.; Geösel, A.; Stefanovits-Bányai, É.; Mednyánszky, Z. Application of Near-Infrared Spectroscopy to Investigate Some Endogenic Properties of Pleurotus ostreatus Cultivars. Sensors 2020, 20, 6632. [Google Scholar] [CrossRef]
- Yi, C.; Xie, M.; Hui, Z.; Wang, Y.; Nie, S.; Chang, L. Quantification of total polysaccharides and triterpenoids in Ganoderma lucidum and Ganoderma atrum by near infrared spectroscopy and chemometrics. Food Chem. 2012, 135, 268–275. [Google Scholar]
- Mikola, E.; Gesel, A.; Stefanovits-Bányai, V.; Fodor, M. Quantitative determination of macro components and classification of some cultivated mushrooms using near-infrared spectroscopy. J. Food Process. Preserv. 2020, 44, e14540. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. Application of vibrational spectroscopy for classification, authentication and quality analysis of mushroom: A concise review. Food Chem. 2019, 289, 545–557. [Google Scholar] [CrossRef]
- Benes, E.; Bajusz, D.; Gere, A.; Fodor, M.; Rácz, A. Comprehensive chemometric classification of snack products based on their near infrared spectra. LWT 2020, 133, 110130. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Yang, G. Development of a Non-Destructive Method for Detection of the Juiciness of Pear via VIS/NIR Spectroscopy Combined with Chemometric Methods. Foods 2020, 9, 1778. [Google Scholar] [CrossRef]
- Pereira, A.C.; Reis, M.S.; Saraiva, P.M.; Marques, J.C. Madeira wine ageing prediction based on different analytical techniques: UV–vis, GC-MS, HPLC-DAD. Chemom. Intell. Lab. Syst. 2011, 105, 43–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, J.; Zhu, C.; Wang, J. Combination of efficient signal pre-processing and optimal band combination algorithm to predict soil organic matter through visible and near-infrared spectra. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118553. [Google Scholar] [CrossRef]
- Ren, G.; Ning, J.; Zhang, Z. Multi-variable selection strategy based on near-infrared spectra for the rapid description of dianhong black tea quality. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 245, 118918. [Google Scholar] [CrossRef]
- Zhou, R.-R.; Yu, Y.; Zeng, W.; Hu, M.-H.; Fan, L.-D.; Chen, L.; Qiu, Z.-D.; Song, C.; Zhang, S.-H.; Guo, L.-P.; et al. Rapid identification of geographical origins and determination of polysaccharides contents in Ganoderma lucidum based on near infrared spectroscopy and chemometrics. Zhongguo Zhong Yao Za Zhi 2018, 43, 3243–3248. [Google Scholar]
- Huang, J.-H.; Zhou, R.-R.; He, D.; Chen, L.; Yang, Y.-Y.; Xie, H.-L.; Zhang, S.-H.; Zhao, C.-X.; Huang, L.-Q. Rapid identification of Lilium species and polysaccharide contents based on near infrared spectroscopy and weighted partial least square method. Int. J. Biol. Macromol. 2020, 154, 182–187. [Google Scholar] [CrossRef]
- Masithoh, R.E.; Lohumi, S.; Yoon, W.; Amanah, H.Z.; Cho, B. Development of multi-product calibration models of various root and tuber powders by fourier transform near infra-red (FT-NIR) spectroscopy for the quantification of polysaccharide contents. Heliyon 2020, 6, e05099. [Google Scholar] [CrossRef]





| Compounds | Range (μg mL−1) | Regression Equation a | R2 | LOD b (μg mL−1) | LOQ c (μg mL−1) |
|---|---|---|---|---|---|
| DTUA | 0.81~40.60 | y = 16,985,351.48000 x + 4404.85745 | 0.996 | 0.209 | 0.696 |
| PAA | 0.40~20.00 | y = 14,178,282.95000 x − 1464.46374 | 0.998 | 0.186 | 0.619 |
| PAC | 0.41~20.40 | y = 16,709,116.59000 x − 399.56329 | 0.999 | 0.166 | 0.554 |
| DPA | 0.79~39.60 | y = 15,817,965.09000 x + 518.67483 | 0.998 | 0.236 | 0.788 |
| DTRA | 1.21~60.40 | y = 18,347,795.59000 x + 9040.36403 | 0.994 | 0.157 | 0.525 |
| Regions | Yunan Region | Xiangqian Region | Dabie Mountains Region | |
|---|---|---|---|---|
| Number of Samples | 58 | 28 | 52 | |
| PSC (mg g−1) | Min | 1.24 | 1.20 | 1.40 |
| Max | 31.16 | 22.66 | 8.96 | |
| Mean | 6.81 | 5.67 | 4.59 | |
| SD | 5.62 | 4.59 | 1.89 | |
| WSE (mg g−1) | Min | 8.80 | 15.52 | 5.68 |
| Max | 73.95 | 73.76 | 57.58 | |
| Mean | 25.22 | 23.95 | 19.57 | |
| SD | 12.81 | 12.18 | 7.40 | |
| ASE (mg g−1) | Min | 16.29 | 24.20 | 17.01 |
| Max | 90.00 | 53.88 | 48.70 | |
| Mean | 37.85 | 33.60 | 31.24 | |
| SD | 14.31 | 7.53 | 5.83 | |
| DTUA (mg g−1) | Min | 0.08 | 0.08 | 0.08 |
| Max | 0.39 | 0.33 | 0.50 | |
| Mean | 0.15 | 0.16 | 0.24 | |
| SD | 0.05 | 0.05 | 0.10 | |
| PAA (mg g−1) | Min | 0.01 | 0.01 | 0.01 |
| Max | 0.30 | 0.20 | 0.34 | |
| Mean | 0.06 | 0.07 | 0.05 | |
| SD | 0.06 | 0.05 | 0.05 | |
| PAC (mg g−1) | Min | 0.02 | 0.07 | 0.09 |
| Max | 0.21 | 0.20 | 0.36 | |
| Mean | 0.13 | 0.13 | 0.14 | |
| SD | 0.03 | 0.03 | 0.04 | |
| DPA (mg g−1) | Min | 0.11 | 0.13 | 0.15 |
| Max | 0.27 | 0.35 | 0.33 | |
| Mean | 0.18 | 0.21 | 0.21 | |
| SD | 0.04 | 0.05 | 0.04 | |
| DTRA (mg g−1) | Min | 0.13 | 0.15 | 0.07 |
| Max | 4.65 | 4.04 | 2.12 | |
| Mean | 0.73 | 0.80 | 0.50 | |
| SD | 0.89 | 0.73 | 0.41 | |
| SFT (mg g−1) | Min | 0.51 | 0.64 | 0.52 |
| Max | 5.68 | 4.82 | 2.77 | |
| Mean | 1.26 | 1.36 | 1.13 | |
| SD | 1.00 | 0.80 | 0.47 | |
| Sample | Reference Values | NIR Values | Results |
|---|---|---|---|
| S16 | YN a | YN | Correct |
| S19 | YN | YN | Correct |
| S22 | YN | YN | Correct |
| S25 | YN | YN | Correct |
| S35 | YN | YN | Correct |
| S50 | YN | YN | Correct |
| S57 | DBM b | DBM | Correct |
| S64 | DBM | DBM | Correct |
| S65 | DBM | DBM | Correct |
| S67 | DBM | DBM | Correct |
| S68 | DBM | DBM | Correct |
| S69 | DBM | DBM | Correct |
| S73 | DBM | DBM | Correct |
| S76 | DBM | DBM | Correct |
| S80 | DBM | DBM | Correct |
| S83 | XQ c | XQ | Correct |
| S88 | XQ | XQ | Correct |
| S92 | XQ | DBM | False |
| S93 | DBM | DBM | Correct |
| S101 | DBM | DBM | Correct |
| S102 | DBM | DBM | Correct |
| S109 | DBM | DBM | Correct |
| S112 | DBM | DBM | Correct |
| S121 | YN | YN | Correct |
| S128 | DBM | DBM | Correct |
| S133 | YN | YN | Correct |
| S136 | XQ | DBM | False |
| Indexes | Calibration | Validation | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Range | Mean | SD | N | Range | Mean | SD | |
| PSC | 108 | 1.20–31.16 | 5.89 | 4.84 | 27 | 1.95–8.90 | 4.97 | 2.02 |
| WSE | 108 | 5.68–73.95 | 23.31 | 11.08 | 27 | 10.40–25.69 | 18.28 | 3.10 |
| ASE | 108 | 16.29–90.00 | 35.27 | 11.73 | 27 | 25.29–42.38 | 30.77 | 4.51 |
| SFT | 108 | 0.51–5.68 | 1.25 | 0.87 | 27 | 0.58–2.53 | 1.12 | 0.40 |
| Indexes | Evaluations a | Raw | MSC | SNV | Smooth | Smooth + SNV | Smooth + MSC | SG-1D |
|---|---|---|---|---|---|---|---|---|
| PSC | RMSEC | 0.122 | 0.107 | 0.114 | 0.112 | 0.101 | 0.093 | 0.056 |
| R2cal | 0.855 | 0.888 | 0.872 | 0.879 | 0.901 | 0.915 | 0.987 | |
| RMSEP | 0.179 | 0.165 | 0.188 | 0.108 | 0.138 | 0.124 | 0.079 | |
| R2pre | 0.739 | 0.790 | 0.701 | 0.863 | 0.805 | 0.859 | 0.965 | |
| WSE | RMSEC | 2.802 | 2.924 | 2.455 | 2.343 | 3.116 | 2.489 | 1.083 |
| R2cal | 0.824 | 0.822 | 0.864 | 0.877 | 0.782 | 0.861 | 0.978 | |
| RMSEP | 3.315 | 3.090 | 3.174 | 3.467 | 3.610 | 3.516 | 1.934 | |
| R2pre | 0.758 | 0.808 | 0.768 | 0.725 | 0.696 | 0.710 | 0.962 | |
| ASE | RMSEC | 2.996 | 2.701 | 1.502 | 2.066 | 2.489 | 3.749 | 3.512 |
| R2cal | 0.910 | 0.920 | 0.972 | 0.946 | 0.911 | 0.897 | 0.909 | |
| RMSEP | 2.772 | 3.763 | 1.926 | 2.968 | 4.285 | 3.894 | 3.763 | |
| R2pre | 0.910 | 0.862 | 0.940 | 0.918 | 0.836 | 0.873 | 0.862 | |
| SFT | RMSEC | 0.298 | 0.228 | 0.165 | 0.225 | 0.225 | 0.229 | 0.197 |
| R2cal | 0.862 | 0.919 | 0.975 | 0.921 | 0.921 | 0.919 | 0.940 | |
| RMSEP | 0.315 | 0.279 | 0.191 | 0.298 | 0.283 | 0.270 | 0.259 | |
| R2pre | 0.846 | 0.880 | 0.961 | 0.862 | 0.876 | 0.887 | 0.896 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Huang, J.; Ren, G.; Jin, J.; Chen, L.; Zhong, C.; Cai, Y.; Liu, H.; Zhou, R.; Qin, Y.; et al. Determination of Cultivation Regions and Quality Parameters of Poria cocos by Near-Infrared Spectroscopy and Chemometrics. Foods 2022, 11, 892. https://doi.org/10.3390/foods11060892
Xie J, Huang J, Ren G, Jin J, Chen L, Zhong C, Cai Y, Liu H, Zhou R, Qin Y, et al. Determination of Cultivation Regions and Quality Parameters of Poria cocos by Near-Infrared Spectroscopy and Chemometrics. Foods. 2022; 11(6):892. https://doi.org/10.3390/foods11060892
Chicago/Turabian StyleXie, Jing, Jianhua Huang, Guangxi Ren, Jian Jin, Lin Chen, Can Zhong, Yuan Cai, Hao Liu, Rongrong Zhou, Yuhui Qin, and et al. 2022. "Determination of Cultivation Regions and Quality Parameters of Poria cocos by Near-Infrared Spectroscopy and Chemometrics" Foods 11, no. 6: 892. https://doi.org/10.3390/foods11060892