Two-Step PEF Processing for Enhancing the Polyphenol Concentration and Decontaminating a Red Grape Juice
Abstract
:1. Introduction
2. Material and Methods
2.1. Grape Samples and Grape Juice Processing
2.2. PEF Treatments
2.2.1. PEF1-Treatment: Grapes
2.2.2. PEF2-Treatment: Juice
2.3. Microbiological Analysis
2.4. Physico-Chemical Analysis
2.4.1. pH, °Brix, Total Acidity
2.4.2. Color Intensity (CI), CIELab Coordinates, Total Polyphenol Index (TPI) and Total Anthocyanin Content (TAC)
2.4.3. Folin-Ciocalteu Index
2.4.4. DPPH Assay: Antioxidant Activity
2.5. Determination of Phenolic Compounds by High-Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD)
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Grape Electroporation by PEF on the Physico-Chemical Properties and the Individual Polyphenol Content of Juices
3.2. Decontamination of Red Grape Juices by Pulsed Electric Fields
3.3. Changes in the Microbial Population and Quality Attributes of Grape Juices during Storing at 4 and 10 °C
3.3.1. Change of Microbial Population of Grape Juices during Storage
3.3.2. Evolution of Quality Attributes of Grape Juices during Storage
3.4. Sensory Analyses of Grape Juices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, K.E. Health beneficial consumer products—status and trends. In Developing Food Products for Consumers with Specific Dietary Needs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 15–42. ISBN 9780081003404. [Google Scholar]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal Technologies for Fruit and Vegetable Juices and Beverages: Overview and Advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef] [Green Version]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Grapes. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–163. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Rahmani, A.H. Grapes and their Bioactive Compounds: Role in Health Management Through Modulating Various Biological Activities. Pharmacogn. J. 2020, 12, 1455–1462. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.-Y. Grape Phytochemicals and Associated Health Benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, P.; Darwish, A.G.; Tsolova, V.; El-Sharkawy, I.; Soliman, K.F.A. The anticancer and antioxidant effects of muscadine grape extracts on racially different triple-negative breast cancer cells. Anticancer Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Reddivari, L.; Charepalli, V.; Radhakrishnan, S.; Vadde, R.; Elias, R.J.; Lambert, J.D.; Vanamala, J.K.P. Grape compounds suppress colon cancer stem cells in vitro and in a rodent model of colon carcinogenesis. BMC Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [Green Version]
- Restani, P.; Fradera, U.; Ruf, J.C.; Stockley, C.; Teissedre, P.L.; Biella, S.; Colombo, F.; Di Lorenzo, C. Grapes and their derivatives in modulation of cognitive decline: A critical review of epidemiological and randomized-controlled trials in humans. Crit. Rev. Food Sci. Nutr. 2020, 61, 566–576. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; de Magalhães Carrapeiro, M.; Fogliano, V.; van Ruth, S.M. Effects of geographical origin, varietal and farming system on the chemical composition and functional properties of purple grape juices: A review. Trends Food Sci. Technol. 2016, 52, 31–48. [Google Scholar] [CrossRef]
- Morris, J.R. Factors Influencing Grape Juice Quality. Horttechnology 1998, 8, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.M.; Delso, C.; Maza, M.; Álvarez, I.; Raso, J. Utilising Pulsed Electric Field Processing to Enhance Extraction Processes. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–7. [Google Scholar]
- Timmermans, R.A.H.; Mastwijk, H.C.; Berendsen, L.B.J.M.; Nederhoff, A.L.; Matser, A.M.; Van Boekel, M.A.J.S.; Nierop Groot, M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D.; Martín-Belloso, O. Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends Food Sci. Technol. 2009, 20, 544–556. [Google Scholar] [CrossRef]
- Luengo, E.; Franco, E.; Ballesteros, F.; Álvarez, I.; Raso, J. Winery Trial on Application of Pulsed Electric Fields for Improving Vinification of Garnacha Grapes. Food Bioprocess Technol. 2014, 7, 1457–1464. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Cebrián, G.; Sánchez-Gimeno, A.C.; Camargo, A.; Álvarez, I.; Raso, J. Evolution of Polyphenolic Compounds and Sensory Properties of Wines Obtained from Grenache Grapes Treated by Pulsed Electric Fields during Aging in Bottles and in Oak Barrels. Foods 2020, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.Y.; Treadwell, M.; Liu, T.; Hochberg, M.; Sack, M.; Mueller, G.; Sigler, J.; Silcock, P.; Oey, I. Influence of Pulsed Electric Fields processing at high-intensity electric field strength on the relationship between anthocyanins composition and colour intensity of Merlot (Vitis vinifera L.) musts during cold maceration. Innov. Food Sci. Emerg. Technol. 2020, 59, 102243. [Google Scholar] [CrossRef]
- López-Giral, N.; González-Arenzana, L.; González-Ferrero, C.; López, R.; Santamaría, P.; López-Alfaro, I.; Garde-Cerdán, T. Pulsed electric field treatment to improve the phenolic compound extraction from Graciano, Tempranillo and Grenache grape varieties during two vintages. Innov. Food Sci. Emerg. Technol. 2015, 28, 31–39. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.; Versari, A. Recent Advances and Applications of Pulsed Electric Fields (PEF) to Improve Polyphenol Extraction and Color Release during Red Winemaking. Beverages 2018, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Maza, M.A.; Pereira, C.; Martínez, J.M.; Camargo, A.; Álvarez, I.; Raso, J. PEF treatments of high specific energy permit the reduction of maceration time during vinification of Caladoc and Grenache grapes. Innov. Food Sci. Emerg. Technol. 2020, 63, 102375. [Google Scholar] [CrossRef]
- Huang, K.; Yu, L.; Wang, W.; Gai, L.; Wang, J. Comparing the pulsed electric field resistance of the microorganisms in grape juice: Application of the Weibull model. Food Control 2014, 35, 241–251. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Bursać Kovačević, D.; Putnik, P.; Santos, M.D.; Queirós, R.P.; Moreira, S.A.; Inácio, R.S.; Fidalgo, L.G.; Saraiva, J.A. Pulsed Electric Field Processing of Fruit Juices. Fruit Juices Extr. Compos. Qual. Anal. 2018, 437–449. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Álvarez, I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, O.N.; Khorokhorina, V.A.; Bowman, A.M.; Rodaite-Riševičiene, R.; Saulis, G.; Xiao, S.; Pakhomov, A.G. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 2012, 527, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abca, E.E.; Akdemir Evrendilek, G. Processing of Red Wine by Pulsed Electric Fields with Respect to Quality Parameters. J. Food Process. Preserv. 2015, 39, 758–767. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Portu, J.; López, N.; Santamaría, P.; Gutiérrez, A.R.; López, R.; López-Alfaro, I. Pulsed Electric Field treatment after malolactic fermentation of Tempranillo Rioja wines: Influence on microbial, physicochemical and sensorial quality. Innov. Food Sci. Emerg. Technol. 2019, 51, 57–63. [Google Scholar] [CrossRef]
- Marsellés-Fontanet, Á.R.; Puig-Pujol, A.; Olmos, P.; Mínguez-Sanz, S.; Martín-Belloso, O. A Comparison of the Effects of Pulsed Electric Field and Thermal Treatments on Grape Juice. Food Bioprocess Technol. 2013, 6, 978–987. [Google Scholar] [CrossRef]
- Min, S.; Jin, Z.T.; Min, S.K.; Yeom, H.; Zhang, Q.H. Commercial-Scale Pulsed Electric Field Processing of Orange Juice. J. Food Sci. 2003, 68, 1265–1271. [Google Scholar] [CrossRef]
- Salehi, F. Physico-chemical properties of fruit and vegetable juices as affected by pulsed electric field: A review. Int. J. Food Prop. 2020, 23, 1036–1050. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G.; Qin, Z.; Munk, D.; Otte, J.; Ahrné, L. Effects of Pulsed Electric Fields on Food Constituents, Microstructure and Sensorial Attributes of Food Products. In Effect of Emerging Processing Methods on the Food Quality; Roohinejad, S., Koubaa, M., Greiner, R., Mallikarjunan, K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 27–67. ISBN 978-3-030-18191-8. [Google Scholar]
- OIV (Organization Internationale de la Vigne et du Vin). Compendium of Internation Methods of Analysis of Wines and Musts; International Organisation of Vine and Wine: Paris, France, 2018. [Google Scholar]
- Maza, M.A.; Martínez, J.M.; Hernández-Orte, P.; Cebrián, G.; Sánchez-Gimeno, A.C.; Álvarez, I.; Raso, J. Influence of pulsed electric fields on aroma and polyphenolic compounds of Garnacha wine. Food Bioprod. Process. 2019, 116, 249–257. [Google Scholar] [CrossRef]
- Ayala, J.F.F.; Echávarri, A.N. MSCV®. Available online: https://www.unirioja.es/color/descargas.shtml (accessed on 1 November 2021).
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl Jasmonate Foliar Application to Tempranillo Vineyard Improved Grape and Wine Phenolic Content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef]
- Agcam, E.; Akyildiz, A.; Akdemir Evrendilek, G. A comparative assessment of long-term storage stability and quality attributes of orange juice in response to pulsed electric fields and heat treatments. Food Bioprod. Process. 2016, 99, 90–98. [Google Scholar] [CrossRef]
- Arcena, M.R.; Leong, S.Y.; Then, S.; Hochberg, M.; Sack, M.; Mueller, G.; Sigler, J.; Kebede, B.; Silcock, P.; Oey, I. The effect of pulsed electric fields pre-treatment on the volatile and phenolic profiles of Merlot grape musts at different winemaking stages and the sensory characteristics of the finished wines. Innov. Food Sci. Emerg. Technol. 2021, 70, 102698. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Arias-Gil, M.; Marsellés-Fontanet, A.R.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effects of thermal and non-thermal processing treatments on fatty acids and free amino acids of grape juice. Food Control 2007, 18, 473–479. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of Ethanol Concentration on the Extraction of Color and Phenolic Compounds from the Skin and Seeds of Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors affecting extraction and evolution of phenolic compounds during red wine maceration and the role of process modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. Oeno One 2017, 51. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J.A. Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J. Food Compos. Anal. 2015, 38, 19–26. [Google Scholar] [CrossRef]
- Granato, D.; Koot, A.; Schnitzler, E.; van Ruth, S.M. Authentication of Geographical Origin and Crop System of Grape Juices by Phenolic Compounds and Antioxidant Activity Using Chemometrics. J. Food Sci. 2015, 80, C584–C593. [Google Scholar] [CrossRef]
- Tenore, G.C.; Manfra, M.; Stiuso, P.; Coppola, L.; Russo, M.; Gomez Monterrey, I.M.; Campiglia, P. Antioxidant Profile and in Vitro Cardiac Radical-Scavenging versus Pro-oxidant Effects of Commercial Red Grape Juices (Vitis vinifera L. cv. Aglianico N.). J. Agric. Food Chem. 2012, 60, 9680–9687. [Google Scholar] [CrossRef] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of Pulsed Electric Field Treatments for the Enhancement of Mass Transfer from Vegetable Tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Control of Pathogenic and Spoilage Microorganisms in Fresh-cut Fruits and Fruit Juices by Traditional and Alternative Natural Antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009, 8, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Heinz, V.; Alvarez, I.; Angersbach, A.; Knorr, D. Preservation of liquid foods by high intensity pulsed electric fields—basic concepts for process design. Trends Food Sci. Technol. 2001, 12, 103–111. [Google Scholar] [CrossRef]
- Pol, I.E.; van Arendonk, W.G.C.; Mastwijk, H.C.; Krommer, J.; Smid, E.J.; Moezelaar, R. Sensitivities of Germinating Spores and Carvacrol-Adapted Vegetative Cells and Spores of Bacillus cereus to Nisin and Pulsed-Electric-Field Treatment. Appl. Environ. Microbiol. 2001, 67, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Siemer, C.; Toepfl, S.; Heinz, V. Inactivation of Bacillus subtilis spores by pulsed electric fields (PEF) in combination with thermal energy—I. Influence of process- and product parameters. Food Control 2014, 39, 163–171. [Google Scholar] [CrossRef]
- Ertugay, M.F.; Baslar, M.; Ortaki, F. Effect of pulsed electric field treatment on polyphenol oxidase, total phenolic compounds, and microbial growth of apple juice. Turkish J. Agric. For. 2013, 37, 772–780. [Google Scholar] [CrossRef]
- Noci, F.; Riener, J.; Walkling-Ribeiro, M.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple Juice. J. Food Eng. 2008, 85, 141–146. [Google Scholar] [CrossRef]
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.V.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT Food Sci. Technol. 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- Wu, Y.; Mittal, G.S.; Griffiths, M.W. Effect of Pulsed Electric Field on the Inactivation of Microorganisms in Grape Juices with and without Antimicrobials. Biosyst. Eng. 2005, 90, 1–7. [Google Scholar] [CrossRef]
- Saldaña, G.; Puértolas, E.; Álvarez, I.; Meneses, N.; Knorr, D.; Raso, J. Evaluation of a static treatment chamber to investigate kinetics of microbial inactivation by pulsed electric fields at different temperatures at quasi-isothermal conditions. J. Food Eng. 2010, 100, 349–356. [Google Scholar] [CrossRef]
- Sharma, P.; Bremer, P.; Oey, I.; Everett, D.W. Bacterial inactivation in whole milk using pulsed electric field processing. Int. Dairy J. 2014, 35, 49–56. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Nederhoff, A.L.; Nierop Groot, M.N.; van Boekel, M.A.J.S.; Mastwijk, H.C. Effect of electrical field strength applied by PEF processing and storage temperature on the outgrowth of yeasts and moulds naturally present in a fresh fruit smoothie. Int. J. Food Microbiol. 2016, 230, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Evelyn; Chairul; Komalasari; Pebrianti, E.; Vazirani, W. Use of Pulsed Electric Field for the Inactivation of Eupenicillium Javanicum Ascospores in Pineapple Juice. J. Phys. Conf. Ser. 2021, 2049, 012020. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Ayestarán, B. Changes in the color components and phenolic content of red wines from Vitis vinifera L. Cv. “Tempranillo” during vinification and aging. Eur. Food Res. Technol. 2008, 228, 29–38. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, L.; Yang, Y.; Yang, W.; Wang, H.; Lan, T.; Zhang, Q.; Sun, X. Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality. Foods 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Margean, A.; Lupu, M.I.; Alexa, E.; Padureanu, V.; Canja, C.M.; Cocan, I.; Negrea, M.; Calefariu, G.; Poiana, M.-A. An Overview of Effects Induced by Pasteurization and High-Power Ultrasound Treatment on the Quality of Red Grape Juice. Molecules 2020, 25, 1669. [Google Scholar] [CrossRef] [Green Version]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sahar, A.; Khalil, A.A.; Rahman, U.U.; Khan, M.; Mehmood, T. Combined effects of pulsed electric field and ultrasound on bioactive compounds and microbial quality of grapefruit juice. J. Food Process. Preserv. 2018, 42, e13507. [Google Scholar] [CrossRef]
- Agcam, E.; Akyildiz, A.; Evrendilek, G.A. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurisation. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Buckow, R.; Ng, S.; Toepfl, S. Pulsed Electric Field Processing of Orange Juice: A Review on Microbial, Enzymatic, Nutritional, and Sensory Quality and Stability. Compr. Rev. Food Sci. Food Saf. 2013, 12, 455–467. [Google Scholar] [CrossRef] [PubMed]

| Juice from Untreated Grapes | Juice from PEF-Treated Grapes | |
|---|---|---|
| pH | 3.52 ± 0.03 a | 3.56 ± 0.04 a |
| °Brix (g/100 g)a | 25.95 ± 0.14 a | 26.35 ± 0.21 a |
| Acidity (g/L)b | 3.95 ± 0.09 a | 4.17 ± 0.08 a |
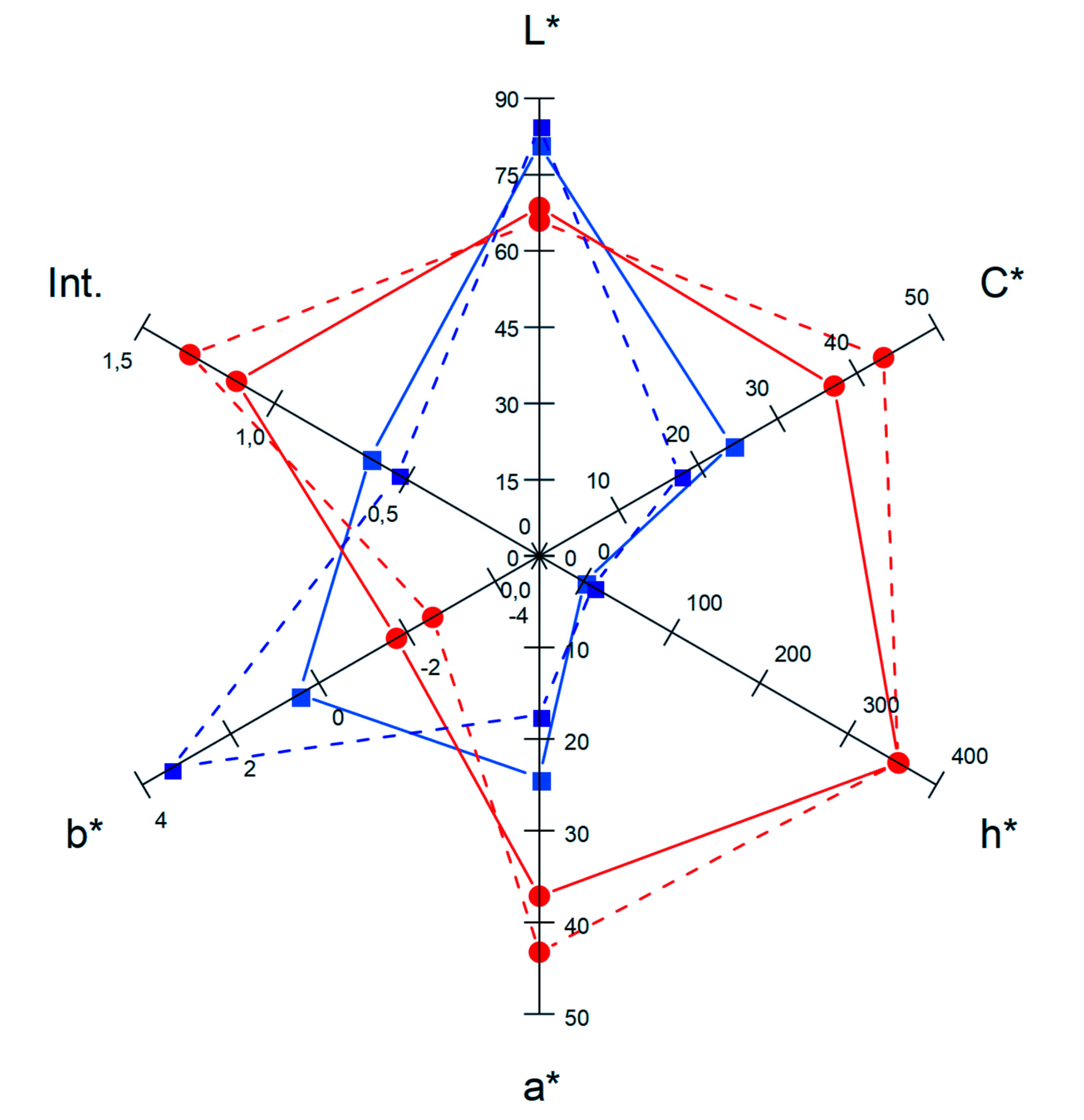
| CI * (A.U.) | 8.46 ± 0.035 a | 16.41 ± 0.09 b |
| TPI ** (A.U.) | 28.65 ± 0.07 a | 45.30 ± 0.28 b |
| TAC *** (mg/L) c | 242.39 ± 1.60 a | 522.34 ± 24.14 b |
| Total polyphenols (mgGAE/L) d | 916.10 ± 13.10 a | 1434.30 ± 154.60 b |
| DPPH (ETμg/mL) e | 419.03 ± 19.05 a | 604.28 ± 33.4 b |
| Anthocyanin Content (mg/L) | ||
| Juice from Untreated Grapes | Juice from PEF Treated Grapes | |
| delphinidin 3-glc | 1.35 ± 0.06 a | 11.04 ± 0.72 b |
| cyanidin 3-glc | 3.06 ± 0.04 a | 12.27 ± 0.77 b |
| petunidin 3-glc | 3.24 ± 0.11 a | 17.26 ± 0.76 b |
| peonidin 3-glc | 28.11 ± 0.69 a | 54.68 ± 1.89 b |
| malvidin 3-glc | 85.25 ± 2.07 a | 198.53 ± 6.33 b |
| delphinidin 3-acglc | 0.79 ± 0.06 a | 1.24 ± 0.04 b |
| cyanidin 3-acglc | n.d. | 0.51 ± 0.04 |
| petunidin 3-acglc | 0.84 ± 0.02 a | 1.39 ± 0.01 b |
| peonidin 3-acglc | 0.51 ± 0.00 a | 0.85 ± 0.02 b |
| malvidin 3-acglc | 0.66 ± 0.01 a | 1.61 ± 0.03 b |
| delphinidin 3-cmglc | 2.38 ± 0.07 a | 5.19 ± 0.14 b |
| cyanidin 3-cmglc | n.d. | 0.63 ± 0.01 |
| petunidin 3-cmglc | n.d. | 0.60 ± 0.00 |
| peonidin 3-cmglc | n.d. | 0.77 ± 0.04 |
| malvidin 3-cis-cmglc | 0.50 ± 0.01 a | 0.77 ± 0.01 b |
| malvidin 3-trans-cmglc | 0.70 ± 0.03 a | 2.19 ± 0.16 b |
| malvidin 3-cfglc | 2.16 ± 0.14 a | 10.07 ± 0.88 b |
| total anthocyanins | 125.91 ± 2.86 a | 309.45 ± 11.32 b |
| vitisin A | 0.50 ± 0.01 a | 0.70 ± 0.06 b |
| visitin B | 0.69 ± 0.01 a | 1.26 ± 0.01 b |
| Flavonol Content (mg/L) | ||
| Juice from Untreated Grapes | Juice from PEF Treated Grapes | |
| myricetin 3-gal + glcu | 4.69 ± 0.15 a | 11.00 ± 0.04 b |
| myricetin 3-glc | 3.06 ± 0.04 a | 12.27 ± 0.77 b |
| quercetin 3-glcu | 9.41 ± 0.08 a | 19.53 ± 0.64 b |
| quercetin 3-glc | 12.04 ± 0.54 a | 27.45 ± 1.38 b |
| laricitrin 3-glc | 3.45 ± 0.11 a | 9.40 ± 0.49 b |
| kaempferol 3-gal | 0.13 ± 0.04 a | 0.56 ± 0.03 b |
| kaempferol 3-glc | 0.45 ± 0.00 a | 3.27 ± 0.06 b |
| isorhamnetin 3-gal | 3.14 ± 0.18 a | 7.40 ± 0.54 b |
| syringetin 3-glc | 5.46 ± 0.15 a | 11.12 ± 0.18 |
| quercetin | 0.40 ± 0.01 a | 1.23 ± 0.04 b |
| laricitrin | n.d. | 0.06 ± 0.02 b |
| total flavonols | 46.88 ± 0.45 a | 120.37 ± 5.43 b |
| Flavanol Content (mg/L) | ||
| Juice from Untreated Grapes | Juice from PEF Treated Grapes | |
| epigallocatechin | 6.17 ± 0.33 a | 9.69 ± 0.35 b |
| Catechin | 3.23 ± 0.36 a | 10.12 ± 0.46 b |
| Epicatechin | 4.96 ± 0.01 a | 8.32 ± 1.45 b |
| Epicatechin gallate | 0.53 ± 0.08 a | 0.68 ± 0.06 b |
| Procianidin B1 | 6.95 ± 0.04 a | 9.19 ± 0.32 b |
| Procianidin B2 | 1.10 ± 0.06 a | 2.87 ± 0.29 b |
| total flavanols | 25.18 ± 0.65 a | 43.11 ± 1.47 b |
| Non-Flavonoids Content (mg/L) | ||
| Juice from Untreated Grapes | Juice from PEF Treated Grapes | |
| Hydroxybenzoic acids | ||
| gallic acid | n.d. | n.d. |
| Hydroxycinnamic acids | ||
| caftaric acid | 44.26 ± 0.69 a | 77.45 ± 1.39 b |
| coutaric acid | 8.88 ± 0.21 a | 16.35 ± 0.13 b |
| fertaric acid | 5.51 ± 0.16 a | 6.83 ± 0.02 b |
| caffeic acid | 1.29 ± 0.14 a | 1.41 ± 0.04 a |
| coumaric acid | 0.25 ± 0.00 a | 0.23 ± 0.02 a |
| ferulic acid | 1.75 ± 0.01 a | 3.58 ± 0.15 b |
| total hydroxycinnamic acids | 59.86 ± 1.18 a | 103.75 ± 1.63 b |
| Stilbenes | ||
| trans-piceid | 0.78 ± 0.01 a | 1.73 ± 0.13 b |
| cis-piceid | 0.56 ± 0.01 a | 0.83 ± 0.06 b |
| trans-resveratrol | 0.12 ± 0.06 a | 0.18 ± 0.02 a |
| cis-resveratrol | 0.46 ± 0.01 a | 0.99 ± 0.09 b |
| total stilbenes | 1.95 ± 0.09 a | 3.74 ± 0.09 b |
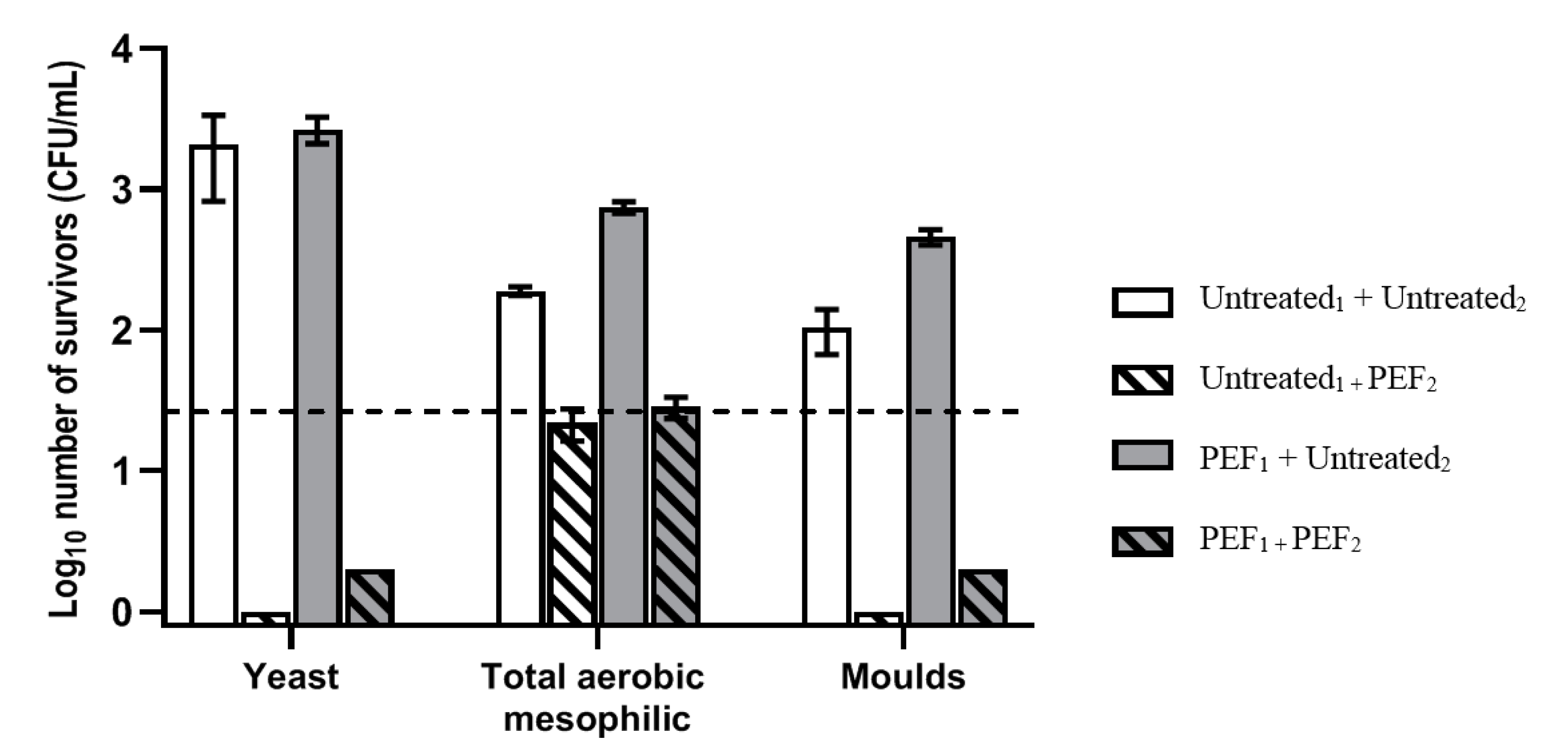
| Total Aerobic Mesophilic Bacteria | ||||||
| 0 days | 10 days | 15 days | 30 days | 45 days | ||
| Untreated1 + Untreated2 | 4 °C | 2.28 ± 0.03 a | 2.12 ± 0.16 a | 5.61 ± 0.29 b | 6.10 ± 0.05 b | 6.29 ± 0.05 b |
| 10 °C | 5.17 ± 0.25 b | 5.96 ± 0.31 bc | 6.80 ± 0.43 cd | 7.26 ± 0.03 d | ||
| Untreated1 + PEF2 | 4 °C | <1.5 | <1.5 | <1.5 | <1.5 | <1.5 |
| 10 °C | <1.5 | <1.5 | <1.5 | <1.5 | ||
| PEF1 + Untreated2 | 4 °C | 2.87 ± 0.04 a | 4.17 ± 0.09 ab | 5.35 ± 0.01 bc | 5.92 ± 0.02 c | 5.86 ± 0.11 c |
| 10 °C | 6.42 ± 0.08 b | 6.78 ± 0.19 bc | 7.44 ± 0.28 c | 7.26 ± 0.17 bc | ||
| PEF1 + PEF2 | 4 °C | <1.5 | <1.5 | <1.5 | <1.5 | <1.5 |
| 10 °C | <1.5 | <1.5 | <1.5 | <1.5 | ||
| Yeasts | ||||||
| 0 days | 10 days | 15 days | 30 days | 45 days | ||
| Untreated1 + Untreated2 | 4 °C | 3.28 ± 0.28 a | 2.70 ± 0.26 a | 6.54 ± 0.09 a | 6.82 ± 0.02 b | 6.55 ± 0.11 b |
| 10 °C | 6.65 ± 0.07 b | 7.39 ± 0.02 b | 7.44 ± 0.06 b | 7.26 ± 0.09 b | ||
| Untreated1 + PEF2 | 4 °C | n.d. | n.d. | <1.5 | <1.5 | <1.5 |
| 10 °C | n.d. | <1.5 | <1.5 | <1.5 | ||
| PEF1 + Untreated2 | 4 °C | 3.42 ± 0.08 a | 3.58 ± 0.14 a | 6.39 ± 0.12 b | 6.60 ± 0.02 b | 6.62 ± 0.14 b |
| 10 °C | 6.65 ± 0.08 b | 7.35 ± 0.01 b | 7.52 ± 0.22 b | 7.23 ± 0.19 b | ||
| PEF1 + PEF2 | 4 °C | n.d. | n.d. | <1.5 | <1.5 | n.d. |
| 10 °C | n.d. | <1.5 | <1.5 | n.d. | ||
| Moulds | ||||||
| 0 days | 10 days | 30 days | 45 days | |||
| Untreated1 + Untreated2 | 4 °C | 2.00 ± 0.16 a | 2.31 ± 0.06 a | 2.15 ± 0.21 a | 2.27 ± 0.16 a | |
| 10 °C | 2.21 ± 0.36 a | 2.07 ± 0.16 a | 2.26 ± 0.20 a | |||
| Untreated1 + PEF2 | 4 °C | n.d. | n.d. | n.d. | n.d. | |
| 10 °C | n.d. | n.d. | n.d. | |||
| PEF1 + Untreated2 | 4 °C | 2.66 ± 0.05 a | 2.52 ± 0.21 a | 2.44 ± 0.14 a | 2.54 ± 0.21 a | |
| 10 °C | 2.21 ± 0.29 a | 2.57 ± 0.12 a | 2.62 ± 0.11 a | |||
| PEF1 + PEF2 | 4 °C | n.d. | n.d. | n.d. | n.d. | |
| 10 °C | n.d. | n.d. | n.d. | |||
| Untreated1 + PEF2 | PEF1 + PEF2 | ||
|---|---|---|---|
| Anthocyanins | 0 days | 146.36 ± 3.34 a | 340.30 ± 10.09 b |
| 45 days 4 °C | 94.16 ± 14.06 a | 267.86 ± 28.02 b | |
| 45 days 10 °C | 82.96 ± 8.92 a | 204.88 ± 33.34 b | |
| Flavonols | 0 days | 64.96 ± 1.92 a | 139.59 ± 0.52 b |
| 45 days 4 °C | 45.90 ± 0.40 a | 125.08 ± 2.18 b | |
| 45 days 10 °C | 45.63 ± 6.39 a | 105.03 ± 12.60 b | |
| Flavanols | 0 days | 32.15 ± 4.06 a | 48.65 ± 0.65 b |
| 45 days 4 °C | 30.79 ± 1.84 a | 51.77 ± 0.61 b | |
| 45 days 10 °C | 26.65 ± 3.32 a | 44.93 ± 4.93 b | |
| Stilbenes | 0 days | 2.76 ± 0.08 a | 3.78 ± 0.06 b |
| 45 days 4 °C | 2.51 ± 0.04 a | 4.31 ± 0.03 b | |
| 45 days 10 °C | 2.40 ± 0.68 a | 3.98 ± 0.03 b | |
| Hydroxycinnamic acids | 0 days | 69.47 ± 0.48 a | 110.64 ± 1.10 b |
| 45 days 4 °C | 64.64 ± 3.84 a | 108.69 ± 5.83 b | |
| 45 days 10 °C | 59.81 ± 9.66 a | 105.28 ± 7.74 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Two-Step PEF Processing for Enhancing the Polyphenol Concentration and Decontaminating a Red Grape Juice. Foods 2022, 11, 621. https://doi.org/10.3390/foods11040621
Delso C, Berzosa A, Sanz J, Álvarez I, Raso J. Two-Step PEF Processing for Enhancing the Polyphenol Concentration and Decontaminating a Red Grape Juice. Foods. 2022; 11(4):621. https://doi.org/10.3390/foods11040621
Chicago/Turabian StyleDelso, Carlota, Alejandro Berzosa, Jorge Sanz, Ignacio Álvarez, and Javier Raso. 2022. "Two-Step PEF Processing for Enhancing the Polyphenol Concentration and Decontaminating a Red Grape Juice" Foods 11, no. 4: 621. https://doi.org/10.3390/foods11040621






