3.1. Stable Isotopic Composition in the Milk and Its Constituents
Stable isotope ratios of 142 milk samples collected from four different agroclimatic zones of Sri Lanka varied from −15.5 to −28.0‰ for δ
13C, 11.6 to 3.3‰ for δ
15N, −48 to −155‰ for δ
2H, and 26.0 to 16.7‰ for δ
18O. The mean δ
18O of whole milk samples showed a significant difference among the four agroclimatic zones (
Table 2). Milk from the dry zone region showed relatively higher δ
18O values than the other zones, while up country milk showed the lowest values (
Figure 2).
The present study showed a significant correlation of δ
18O values with latitude, a moderate correlation with altitude (
Figure 3), and a lower correlation with distance to the coast. It has been revealed that the isotopic enrichment of exchangeable oxygen and hydrogen in plants and animals is usually shifted in relation to the local precipitation, as the enrichment of all sources of absorbed water is altered by plant or animal metabolism. Temperature is an important factor governing the enrichment of δ
18O and δ
2H in precipitation [
27]. The milk samples collected from Sri Lanka showed higher δ
18O values at lower elevation and lower values at high elevation. This observation could have resulted from the influence of climatic factors, such as temperature and rainfall, as relative humidity affects the fractionation of oxygen isotopes. Each agroclimatic zone receives rainfall from different rainfall regimes by the two main monsoon rains and two convectional (inter-monsoon) rains known to have characteristic isotopic signatures [
28]. The isotopic composition of monsoon rains in Sri Lanka is affected by terrestrial and oceanic moisture sources, depression, and cyclonic conditions of the Bay of Bengal, Arabian Sea, and the country’s topography [
29].
Higher evapotranspiration rates in the dry zone may also lead to enriched δ
18O values in plants and animals. In Sri Lanka, the temperature decreases at a steady rate of about 6.5 °C for each 1000-m rise. Higher temperatures are experienced generally in the dry zone areas of northern, northcentral, and eastern regions of the island that range between 33.3–34.7 °C [
30]. The dry zone receives a mean annual rainfall of less than 1000 mm with a distinct dry season, while the intermediate zone receives a mean annual rainfall of about 1750 mm. The wet zone receives over 2500 mm of annual rainfall [
30].
In the present study, the δ
15N values of whole milk, milk casein, and whey revealed a moderate potential for discriminating cattle farming zones. However, this study found that δ
15N as well as δ
13C values of dry zone samples were significantly different from other zones (
Table 2). The highest δ
15N values were reported in the dry zone samples, and the lowest δ
15N values were reported in the up country. The whole milk δ
15N values were between their casein and whey δ
15N values for all the agroclimatic zones except the upcountry. In up country, the whole milk δ
15N values were higher than their respective casein and whey δ
15N values. Further, up country recorded the lowest temperature range (18–23 °C) and the other zones showed a higher temperature range (27–34 °C) during sampling. According to literature, ambient temperature could influence casein δ
15N values [
17]. The isotopic fractionation of nitrogen in dairy cows was estimated to be high in metabolic origin and affected by temperature as the metabolic rates of animals are indirectly affected by environmental temperatures. In up country, animals are subjected to minimal heat stress compared to the other zones.
Complete milk protein is composed of 20% whey protein and 80% casein. δ
15N values of milk whey were more depleted than that of milk casein and whole milk in all four agroclimatic regions. A similar observation has been reported by Kornexl et al. [
31]. This finding may be attributed to the different amino acid compositions and relative abundance of amino acids originating from body tissues or diet [
31,
32]. Further, the δ
15N value in agricultural products is influenced by the differences in soil conditions, amount of rainfall, soil age, nitrogen turnover rate in soils, and other complex parameters [
17,
31]. Regional differences in the δ
15N value of milk may reflect the differences in feeding regimes depending on the regional dairy farm [
11]. There is a trend for increasing foliar δ
15N with decreasing rainfall [
33].
The difference in δ
13C reflects the relative amounts of C3 and C4 plants used by the regional feeding regimes. According to the Calvin cycles in photosynthetic CO
2 fixation, C3 plants show δ
13C values between −24 and −32‰. Plants following the C4-dicarboxylic acid pathway show δ
13C values between −10 and −19‰ [
34]. Except in the dry zone, all other cattle management zones used particularly the grasses of the genera
Brachiaria and
Panicum to feed cattle, which fall into the C4 plant category.
Gliricidia sepium, which is in the C3 category, was fed to cattle of all the zones except the up country. In up country, Ryegrass (
Lolium multiflorum) was given to the animals which is also a C3 plant. However, a considerable amount of concentrate feed, such as rice bran, rice polish, coconut poonac were given as cattle feed in most of the farms. Cattle reared in dry zone eat various types of plants, while freely grazing on communal grazing lands. Therefore, the δ
13C values showed a value in between C3 and C4 plants in cattle feed for most of the samples. However, in this study, the δ
2H value displayed weak potential to differentiate milk samples of different agroclimatic zones. The variations in δ
18O, δ
13C, and δ
15N values for each of the agroclimatic zone investigated are presented in
Figure 4. This representation helps visualize the potential of stable isotope values as a ‘screening’ parameter to differentiate milk produced in different agroclimatic zones. Samples from the dry zone could be visualized separately from the mid country and up country.
3.2. Influence of Sampling Period on Stable Isotope Values of Milk
Although δ
13C, δ
15N, δ
18O, and δ
2H in whole milk and milk components were significantly influenced by agro-climatic zone, these variables were also moderately influenced by the sampling period (
Table 3) and less influenced by interactions of the sampling period and the region. Mean δ
18O values in whole milk displayed a significant difference in wet and dry sampling periods for each agroclimatic zone except for the dry zone (
p < 0.001). The only variable that displayed a significant difference in wet and dry periods for the dry zone was δ
15N of milk casein (
p < 0.001). Significant variation between sampling periods in the δ
15N values of milk casein and whole milk samples collected from coconut triangle and up country was observed. Mean δ
15N of whole milk and milk fractions and δ
18O showed relatively enriched values for the wet period compared with the dry period.
Forage production and availability in Sri Lanka mainly depend on the rainfall. The availability of lush pastures is high during rainy season and forage is scanty during dry season. Due to the scarcity of forage during the dry season, cattle are often fed with different feeding materials, such as silage and concentrate feeds. The difference of δ
15N during wet and dry seasons may have resulted from variations in ambient temperature and feed material [
17,
31].
The topographical features and regional scale monsoons wind regimes dominate the climate dynamics in each agroclimatic zone. Monsoonal, convectional, and depressional rain contribute to the seasonal rainfall in Sri Lanka. The topographical features of the various agroclimatic zones mean they receive rain in different months of the year. The two main monsoonal rains and two inter-monsoon rains have characteristic isotopic signatures [
28]. The up country agroclimatic zone falls in the most affected area of the southwest monsoon. North-east monsoon and second inter-monsoon rains are the significant rainy seasons in mid country. The dry zone and coconut triangle receive abundant rainfall from the northeast monsoon [
30]. The source of precipitation, temperature, isotopic depletion of moisture in clouds moving inland, elevation effect, and cooling of the vapor masses as they rise over the landscape may change the isotopic signatures of δ
18O and δ
2H in different months of the year in agroclimatic zones.
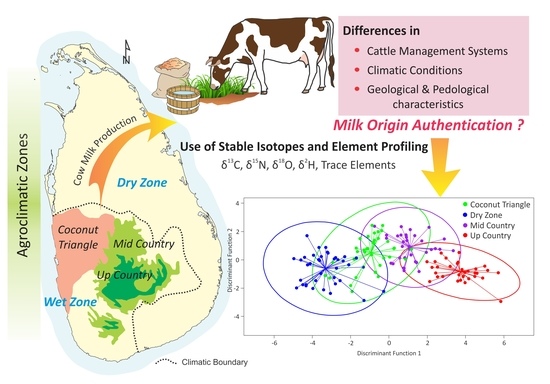
3.3. Determination of Geographical Origin by Statistical Analysis
The combined isotope parameters obtained from milk samples were evaluated to distinguish agroclimatic zones using multivariate statistics. The first two principal components explained 69% of the total variance (
Table 4).
However, clear clustering was not observed in the score plot of PC1 and PC2 (
Figure 5). As the PCA could not clearly group the samples based on the origin, LDA was employed with backward stepwise refinement of stable isotope ratios to provide a mathematical model to classify samples simultaneously according to their agroclimatic origin. The selected variables in the model were whole milk δ
15N, milk casein δ
15N, milk whey δ
15N, whole milk δ
13C, milk fat δ
13C, milk casein δ
13C, whole milk δ
2H, and whole milk δ
18O. The efficacy of the model in discriminating milk samples originated in coconut triangle, dry zone, mid country, and up country from the rest agroclimatic regions were 83%, 92%, 89%, and 97%, respectively. After cross-validation, the above percentages were 75%, 92%, 81%, and 91%, respectively.
Table 5 summarized original and cross-validated classification by discriminant analysis for the samples collected from four different agroclimatic zones. The discriminant analysis also showed a degree of overlap. As illustrated in
Figure 1, farms located closer to the border areas of these agroclimatic zones share similar climatic conditions.
Samples collected during the dry period were evaluated for elemental composition and stable isotope ratios. Previous studies have shown that there is a correlation between the elemental profile of animal products and geographic origin. Further, the elemental profiles of milk have shown to be a useful tool for verification of geographic origin [
35,
36,
37]. The elements present in the natural environment go through a complex metabolic process inside the feed (eg., grass) and animal, and the variability of trace elements in milk may be affected by both intrinsic and extrinsic factors related to feed and the animal. A significant variation in climate, soil, and cattle management practices can be observed between the agroclimatic zones of Sri Lanka, though geographic distances between these zones are small (
Figure 1). Al, Cr, Mn, Fe, Co, Ni, Cd, and Pb may be related to intrinsic factors of the animal, such as breed, stage of lactation, parity, and health status together with feeding and dietary adaptations based on the availability of feeding material. Li, Cu, Zn, As, Se, Sr, Ba, and Pb could be attributed to dietary supplements and feed additives used to feed animals [
38,
39,
40,
41,
42]. Se, Cu, and Zn are present in mineral mixtures fed to intensively manage high yielding animals [
40]. However, there are very limited data available on correlations between elemental content of milk and geographic origin for the South Asian region. The value of such information is vital to study the applicability of elemental content analysis for geographic origin verification for the region, as cattle management practices vary widely in the region even within the same agro-climatic zone.
Geologically, underlying over 90% of Sri Lanka is Precambrian high-grade metamorphic rocks which can be subdivided into three main litho-tectonic units, namely Highland Complex, Vijayan Complex, and Wanni Complex. The remaining 10% is underlain by Miocene sedimentary rocks. Rocks underlain in the Highland Complex are mainly granulite facies suites, including charnockites, garnet-biotite gneisses, quartzite, marbles, and calc-silicate gneisses. The Vijayan region consists of gneisses and granitoid varieties, while the Wanni Complex is dominated by supracrustal rocks and meta-igneous suits, such as granodioritic gneisses. All the sampling locations of up country and mid country, and D1 and D4 locations of the dry zone belong to the Highland Complex of the metamorphic terrain while other sampling locations belong to Wanni Complex. The major and trace element content in the natural environment is mainly determined by their content in rocks, which significantly varies within a very short distance in a metamorphic terrain like Sri Lanka [
43]. However, it is difficult to directly correlate the local geology with the resulted elemental contents in milk, as there are other factors influencing the elemental content in milk such as management practices. Li, Al, Cr, Mn, and Sr contents in samples collected in the dry period indicated significant differences in their mean contents among four agroclimatic zones (
Table 6). Fe, Ni, Cu, Zn, As, Se, Ba, Pb, and Bi displayed moderate potential in differentiating agroclimatic zones, and Co and Cd composition were similar among the different farming zones. Sr and Mn concentrations together with isotope ratios have been used as markers for geographical traceability of raw milk in previous studies [
36,
37]. The first two principal components explained 58% of the total variance and clear clustering was observed in the score plot of PC1 and PC2 (
Figure 6). The LDA carried out using both trace element and stable isotope data with backward stepwise variable selection (i.e., milk casein δ
15N, whole milk δ
15N, milk casein δ
13C, whole milk δ
18O, whole milk δ
2H, Li, Al, Cr, Mn, Fe, Co, Ba, and Sr) showed excellent discrimination in samples from the dry zone, coconut triangle, mid country, and up country (100%) even after cross-validation (
Figure 7).
One limitation of the study is that the authors could not select farms in each of the agro-climatic zones with a good geographic distribution, due to unavailability of suitable farms throughout the zones. This uneven distribution of farms resulted from suitable agricultural land, water sources and other infrastructure availability, and environmental considerations. In this study, only large to medium scale farms were selected. Even though some of these farms were very close to the agroclimatic boundaries, they could be clearly categorized into respective agroclimatic zones in the LDA carried out with trace elements and stable isotopes data. This could have been possible due to the differences in elemental contents in the environment. However, we could not confirm whether the LDA model can differentiate milk coming from locations that were not sampled, especially in the eastern and southern regions of the dry zone where suitable farms are not available.