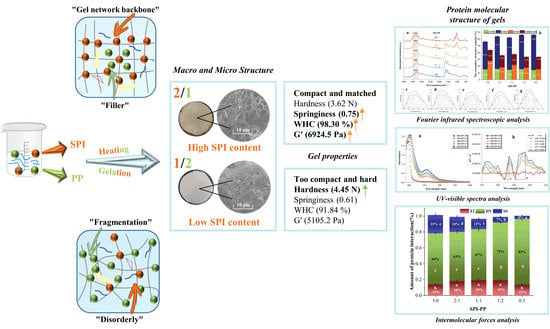
Structural Analysis and Study of Gel Properties of Thermally-Induced Soybean Isolate–Potato Protein Gel System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Protein and Gel
2.2.1. Preparation of SPI
2.2.2. Preparation of PP
2.2.3. Gel Preparation
2.3. Non-Network Protein Ratio (Rnon)
2.4. Electrophoresis
2.5. Turbidity Measurement
2.6. Texture Analysis
2.7. Water Holding Capacity (WHC)
2.8. Dynamic Rheology
2.9. Fourier Transform Infrared Spectrum (FT-IR)
2.10. Measurement of UV Spectrum
2.11. Intermolecular Forces
2.12. Scanning Electron Microscopy (SEM)
2.13. Statistical Analysis of Data
3. Results and Discussion
3.1. Gel Structure Analyses
3.1.1. Rnon Analysis
3.1.2. Protein Electrophoresis and Turbidity Analysis
3.2. Characterization of Gel Properties
3.2.1. Texture Analysis
3.2.2. WHC Analysis
3.2.3. Rheological Properties Analysis
3.3. Protein Molecular Structure of Gels
3.3.1. Fourier Infrared Spectroscopic Analysis
3.3.2. UV-Visible Spectra Analysis
3.3.3. Intermolecular Forces Analysis
3.3.4. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Zheng, T.; Li, X.; Taha, A.; Wei, Y.; Hu, T.; Fatamorgana, P.B.; Zhang, Z.; Liu, F.; Xu, X.; Pan, S.; et al. Effect of high intensity ultrasound on the structure and physicochemical properties of soy protein isolates produced by different denaturation methods. Food Hydrocoll. 2019, 97, 105216. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Damgaard, H.; Greve-Poulsen, M.; Sunds, A.V.; Larsen, L.B.; Hammershøj, M. Gel properties of potato protein and the isolated fractions of patatins and protease inhibitors—Impact of drying method, protein concentration, pH and ionic strength. Food Hydrocoll. 2019, 96, 246–258. [Google Scholar] [CrossRef]
- Fitzsimons, S.M.; Mulvihill, D.M.; Morris, E.R. Denaturation and aggregation processes in thermal gelation of whey proteins resolved by differential scanning calorimetry. Food Hydrocoll. 2007, 21, 638–644. [Google Scholar] [CrossRef]
- Andrews, J.M.; Roberts, C.J. A Lumry-Eyring nucleated polymerization model of protein aggregation kinetics: 1. Aggregation with pre-equilibrated unfolding. J. Phys. Chem. 2007, 111, 7897–7913. [Google Scholar] [CrossRef]
- Niu, H.; Xia, X.; Wang, C.; Kong, B.; Liu, Q. Thermal stability and gel quality of myofibrillar protein as affected by soy protein isolates subjected to an acidic pH and mild heating. Food Chem. 2018, 242, 188–195. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the rheological and textural properties of calcium sulfate-induced soy protein isolate gels by the incorporation of different polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef]
- Hussain, M.; Qayum, A.; Xiuxiu, Z.; Liu, L.; Hussain, K.; Yue, P.; Yue, S.; Koko, M.Y.; Hussain, A.; Li, X. Potato protein: An emerging source of high quality and allergy free protein, and its possible future based products. Food Res. Int. 2021, 148, 110583. [Google Scholar] [CrossRef]
- Creusot, N.; Wierenga, P.A.; Laus, M.C.; Giuseppin, M.L.; Gruppen, H. Rheological properties of patatin gels compared with beta-lactoglobulin, ovalbumin, and glycinin. J. Sci. Food Agric. 2011, 91, 253–261. [Google Scholar] [CrossRef] [PubMed]
- González, A.D.; Frostell, B.; Carlsson-Kanyama, A. Protein efficiency per unit energy and per unit greenhouse gas emissions: Potential contribution of diet choices to climate change mitigation. Food Policy 2011, 36, 562–570. [Google Scholar] [CrossRef]
- Pots, A.M.; ten Grotenhuis, E.; Gruppen, H.; Voragen, A.G.; de Kruif, K.G. Thermal aggregation of patatin studied in situ. J. Agric. Food Chem. 1999, 47, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, X.Q.; He, X.T.; Wu, N.N.; Wang, J.M.; Gu, W.; Zhang, Y.Y. Limited aggregation behavior of beta-conglycinin and its terminating effect on glycinin aggregation during heating at pH 7.0. J. Agric. Food Chem. 2012, 60, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Katzav, H.; Chirug, L.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. Comparison of Thermal and High-Pressure Gelation of Potato Protein Isolates. Foods 2020, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, J.; Yan, X.; Ma, W.; Wu, D.; Du, M. Effect of partial replacement of water-soluble cod proteins by soy proteins on the heat-induced aggregation and gelation properties of mixed protein systems. Food Hydrocoll. 2020, 100, 105417. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Wang, T.; Xu, P.; Chen, Z.; Wang, R. Mechanism of structural interplay between rice proteins and soy protein isolates to design novel protein hydrocolloids. Food Hydrocoll. 2018, 84, 361–367. [Google Scholar] [CrossRef]
- Kim, J.H.; Varankovich, N.V.; Stone, A.K.; Nickerson, M.T. Nature of protein-protein interactions during the gelation of canola protein isolate networks. Food Res. Int. 2016, 89, 408–414. [Google Scholar] [CrossRef]
- Lakemond, C. Gelation of soy glycinin; influence of pH and ionic strength on network structure in relation to protein conformation. Food Hydrocoll. 2003, 17, 365–377. [Google Scholar] [CrossRef]
- Wu, C.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effect of temperature, ionic strength and 11S ratio on the rheological properties of heat-induced soy protein gels in relation to network proteins content and aggregates size. Food Hydrocoll. 2017, 66, 389–395. [Google Scholar] [CrossRef]
- Wu, C.; Navicha, W.B.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effects of removal of non-network protein on the rheological properties of heat-induced soy protein gels. LWT-Food Sci. Technol. 2018, 95, 193–199. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Guo, S. Characteristics of soy protein isolate gel induced by glucono-δ-lactone: Effects of the protein concentration during preheating. Food Hydrocoll. 2021, 113, 106525. [Google Scholar] [CrossRef]
- Zhao, Z.-K.; Mu, T.-H.; Zhang, M.; Richel, A. Effects of Sulfur-Containing Amino Acids and High Hydrostatic Pressure on Structure and Gelation Properties of Sweet Potato Protein. Food Bioprocess Technol. 2019, 12, 1863–1873. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ishiguro, T.; Ozeki, A.; Kawai, K.; Suzuki, T. Property changes of frozen soybean curd during frozen storage in “Kori-tofu” manufacturing process. Food Hydrocoll. 2020, 104, 105714. [Google Scholar] [CrossRef]
- Feng, L.; Jia, X.; Zhu, Q.; Liu, Y.; Li, J.; Yin, L. Investigation of the mechanical, rheological and microstructural properties of sugar beet pectin /soy protein isolate-based emulsion-filled gels. Food Hydrocoll. 2019, 89, 813–820. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Acevedo, N.C. Effects of pre-heating soybean protein isolate and transglutaminase treatments on the properties of egg-soybean protein isolate composite gels. Food Chem. 2020, 318, 126421. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Cui, B. Fabrication, characterization and emulsifying properties of potato starch/soy protein complexes in acidic conditions. Food Hydrocoll. 2021, 115, 106600. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Wang, B.; Yang, W.; Luo, S.; Zhao, Y.; Jiang, S.; Mu, D.; Zheng, Z. Formation of macromolecules in wheat gluten/starch mixtures during twin-screw extrusion: Effect of different additives. J. Sci. Food Agric. 2017, 97, 5131–5138. [Google Scholar] [CrossRef]
- Tanger, C.; Andlinger, D.J.; Brummer-Rolf, A.; Engel, J.; Kulozik, U. Quantification of protein-protein interactions in highly denatured whey and potato protein gels. MethodsX 2021, 8, 101243. [Google Scholar] [CrossRef]
- Tanger, C.; Utz, F.; Spaccasassi, A.; Kreissl, J.; Dombrowski, J.; Dawid, C.; Kulozik, U. Influence of Pea and Potato Protein Microparticles on Texture and Sensory Properties in a Fat-Reduced Model Milk Dessert. ACS Food Sci. Technol. 2021, 2, 169–179. [Google Scholar] [CrossRef]
- Qi, G.; Venkateshan, K.; Mo, X.; Zhang, L.; Sun, X.S. Physicochemical properties of soy protein: Effects of subunit composition. J. Agric. Food Chem. 2011, 59, 9958–9964. [Google Scholar] [CrossRef] [PubMed]
- Delahaije, R.J.; Wierenga, P.A.; Giuseppin, M.L.; Gruppen, H. Comparison of heat-induced aggregation of globular proteins. J. Agric. Food Chem. 2015, 63, 5257–5265. [Google Scholar] [CrossRef] [PubMed]
- Sorgentini, D.A.; Wagner, J.R.; Anon, M.C. Effects of Thermal Treatment of Soy Protein Isolate on the Characteristics and Structure-Function Relationship of Soluble and Insoluble Fractions. J. Agric. Food Chem. 2002, 43, 2471–2479. [Google Scholar] [CrossRef]
- Akbari, N.; Mohammadzadeh Milani, J.; Biparva, P. Functional and conformational properties of proteolytic enzyme-modified potato protein isolate. J. Sci. Food Agric. 2020, 100, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Zhang, X.; Guo, Y.; Shi, J.; Shen, S.; Han, D.; Dou, H. Asymmetrical flow field-flow fractionation combined with electrophoresis: A new approach for studying thermal aggregation behavior of soy protein isolate. Food Hydrocolloids 2021, 119, 106857. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Rampp, L.; Tanger, C.; Kulozik, U. Viscoelasticity and Protein Interactions of Hybrid Gels Produced from Potato and Whey Protein Isolates. ACS Food Sci. Technol. 2021, 1, 1304–1315. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Myofibrillar Protein Structure and Gel Properties of Trichiurus Haumela Surimi Subjected to High Pressure or High Pressure Synergistic Heat. Food Bioprocess Technol. 2020, 13, 589–598. [Google Scholar] [CrossRef]
- Chen, B.; Guo, J.; Xie, Y.; Zhou, K.; Li, P.; Xu, B. Modulating the aggregation of myofibrillar protein to alleviate the textural deterioration of protein gels at high temperature: The effect of hydrophobic interactions. Food Chem. 2021, 341, 128274. [Google Scholar] [CrossRef]
- Ai, M.; Zhou, Q.; Guo, S.; Ling, Z.; Zhou, L.; Fan, H.; Cao, Y.; Jiang, A. Effects of tea polyphenol and Ca(OH)2 on the intermolecular forces and mechanical, rheological, and microstructural characteristics of duck egg white gel. Food Hydrocoll. 2019, 94, 11–19. [Google Scholar] [CrossRef]
- Hermansson, A.M. Soy protein gelation. J. Am. Oil Chem. Soc. 1986, 63, 658–666. [Google Scholar] [CrossRef]
- Chen, H.; Gan, J.; Ji, A.; Song, S.; Yin, L. Development of double network gels based on soy protein isolate and sugar beet pectin induced by thermal treatment and laccase catalysis. Food Chem. 2019, 292, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Li, Y.; Han, J.; Liu, Q.; Kong, B. Gelation and rheological properties of myofibrillar proteins influenced by the addition of soybean protein isolates subjected to an acidic pH treatment combined with a mild heating. Food Hydrocoll. 2017, 70, 269–276. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Hussain, S.; Alamri, M.S.; Abdo Qasem, A.A.; Ibraheem, M.A.; Alhazmi, M.I. Dynamic rheological properties of corn starch-date syrup gels. Int. J. Food Sci. Technol. 2019, 56, 927–936. [Google Scholar] [CrossRef]
- Renkema, J.M.; Gruppen, H.; van Vliet, T. Influence of pH and ionic strength on heat-induced formation and rheological properties of soy protein gels in relation to denaturation and their protein compositions. J. Agric. Food Chem. 2002, 50, 6064–6071. [Google Scholar] [CrossRef] [PubMed]
- Løkra, S.; Helland, M.H.; Claussen, I.C.; Strætkvern, K.O.; Egelandsdal, B. Chemical characterization and functional properties of a potato protein concentrate prepared by large-scale expanded bed adsorption chromatography. LWT-Food Sci. Technol. 2008, 41, 1089–1099. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Tang, T.; Li, J.; Gu, L.; Chang, C.; Zhang, M.; Yang, Y.; Su, Y. Gel properties of soy protein isolate-potato protein-egg white composite gel: Study on rheological properties, microstructure, and digestibility. Food Hydrocoll. 2022, 135, 108223. [Google Scholar] [CrossRef]
- Ikeda, S.; Foegeding, E.A.; Hagiwara, T. Rheological Study on the Fractal Nature of the Protein Gel Structure. Langmuir 1999, 15, 8584–8589. [Google Scholar] [CrossRef]
- Guldiken, B.; Stobbs, J.; Nickerson, M. Heat induced gelation of pulse protein networks. Food Chem. 2021, 350, 129158. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C. The preparation and rheology characterization of long chain branching polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]
- Wu, C.; Yan, X.; Wang, T.; Ma, W.; Xu, X.; Du, M. A self-sorted gel network formed by heating a mixture of soy and cod proteins. Food Funct. 2019, 10, 5140–5151. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, Y.; Zhang, L.; Zhang, Z.; Chi, Y.; Chi, Y. Changes in egg yolk gelation behaviour and mechanisms during freezing. LWT-Food Sci. Technol. 2021, 151, 112223. [Google Scholar] [CrossRef]
- Zhu, Y.; Tao, H.; Janaswamy, S.; Zou, F.; Cui, B.; Guo, L. The functionality of laccase- or peroxidase-treated potato flour: Role of interactions between protein and protein/starch. Food Chem. 2021, 341, 128082. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Z.; Cai, Q.; Peng, B. Effects of high hydrostatic pressure on structural and physical properties of nisin-SPI film. Int. J. Biol. Macromol. 2018, 111, 976–982. [Google Scholar] [CrossRef]
- Gui, Y.; Li, J.; Zhu, Y.; Guo, L. Roles of four enzyme crosslinks on structural, thermal and gel properties of potato proteins. LWT-Food Sci. Technol. 2020, 123, 109116. [Google Scholar] [CrossRef]
- Li, R.; Fu, N.; Wu, Z.; Wang, Y.; Wang, Y. Protein aggregation in foam fractionation of bovine serum albumin: Effect of protein concentration. Biochem. Eng. J. 2015, 103, 234–241. [Google Scholar] [CrossRef]
- Li, X.; Ji, N.; Li, M.; Zhang, S.; Xiong, L.; Sun, Q. Morphology and Structural Properties of Novel Short Linear Glucan/Protein Hybrid Nanoparticles and Their Influence on the Rheological Properties of Starch Gel. J. Agric. Food Chem. 2017, 65, 7955–7965. [Google Scholar] [CrossRef]
- Mach, H.; Middaugh, C.R. Simultaneous monitoring of the environment of tryptophan, tyrosine, and phenylalanine residues in proteins by near-ultraviolet second-derivative spectroscopy. Anal. Biochem. 1994, 222, 323–331. [Google Scholar] [CrossRef]
- Bosch Ojeda, C.; Sanchez Rojas, F. Recent developments in derivative ultraviolet/visible absorption spectrophotometry. Anal. Chim. Acta 2004, 518, 1–24. [Google Scholar] [CrossRef]
- Li, T.; Bu, G.; Xi, G. Effects of heat treatment on the antigenicity, antigen epitopes, and structural properties of beta-conglycinin. Food Chem. 2021, 346, 128962. [Google Scholar] [CrossRef]
- Du, X.; Zhao, M.; Pan, N.; Wang, S.; Xia, X.; Zhang, D. Tracking aggregation behaviour and gel properties induced by structural alterations in myofibrillar protein in mirror carp (Cyprinus carpio) under the synergistic effects of pH and heating. Food Chem. 2021, 362, 130222. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, H.; Wu, D.; Lin, D.; Qin, W.; Meng, D.; Yang, R.; Zhang, Q. Rheological and textural properties of acid-induced soybean protein isolate gel in the presence of soybean protein isolate hydrolysates or their glycosylated products. Food Chem. 2021, 360, 129991. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.-J.; Lai, K.-M.; Hsu, K.-C. A Comparative Study on Physical Properties and Chemical Interactions of Gels from Tilapia Meat Pastes Induced by Heat and Pressure. J. Texture Stud. 2010, 41, 153–170. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Effect of soybean protein isolate and egg white mixture on gelation of chicken myofibrillar proteins under salt/-free conditions. LWT-Food Sci. Technol. 2021, 149, 111871. [Google Scholar] [CrossRef]









| SPI/PP (w/w) | Hardness (N) | Springiness | WHC (%) |
|---|---|---|---|
| 3/0 | 1.53 ± 0.01 a | 0.83 ± 0.01 d | 98.33 ± 0.01 c |
| 2/1 | 3.62 ± 0.01 c | 0.75 ± 0.01 b | 98.30 ± 0.22 c |
| 1/1 | 3.18 ± 0.02 b | 0.67 ± 0.01 e | 91.84 ± 0.10 b |
| 1/2 | 4.45 ± 0.02 d | 0.61 ± 0.02 a | 91.84 ± 0.89 b |
| 0/3 | 14.53 ± 0.03 e | 0.53 ± 0.02 c | 76.42 ± 0.97 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Gu, X.; Lü, M.; Huang, Y.; Zhu, Y.; Sun, Y.; Zhu, X. Structural Analysis and Study of Gel Properties of Thermally-Induced Soybean Isolate–Potato Protein Gel System. Foods 2022, 11, 3562. https://doi.org/10.3390/foods11223562
Wang F, Gu X, Lü M, Huang Y, Zhu Y, Sun Y, Zhu X. Structural Analysis and Study of Gel Properties of Thermally-Induced Soybean Isolate–Potato Protein Gel System. Foods. 2022; 11(22):3562. https://doi.org/10.3390/foods11223562
Chicago/Turabian StyleWang, Fengqiujie, Xuelian Gu, Mingshou Lü, Yuyang Huang, Ying Zhu, Ying Sun, and Xiuqing Zhu. 2022. "Structural Analysis and Study of Gel Properties of Thermally-Induced Soybean Isolate–Potato Protein Gel System" Foods 11, no. 22: 3562. https://doi.org/10.3390/foods11223562