Response Surface Modeling and Optimization of Enzymolysis Parameters for the In Vitro Antidiabetic Activities of Peanut Protein Hydrolysates Prepared Using Two Proteases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Design for Optimization
2.3. Response Parameters Analysis
2.3.1. Degree of Hydrolysis Determination
2.3.2. α-Amylase Inhibition Assay
2.3.3. α-Glucosidase Inhibition Assay
2.4. Protein Patterns by SDS-PAGE
2.5. Antioxidant Activity
2.5.1. DPPH Radical-Scavenging Activity (DPPH-RSA)
2.5.2. ABTS Radical Cation Scavenging Activity (ABTS-RSA)
2.6. Data Analysis
3. Results and Discussion
3.1. Optimization of Enzymolysis Conditions
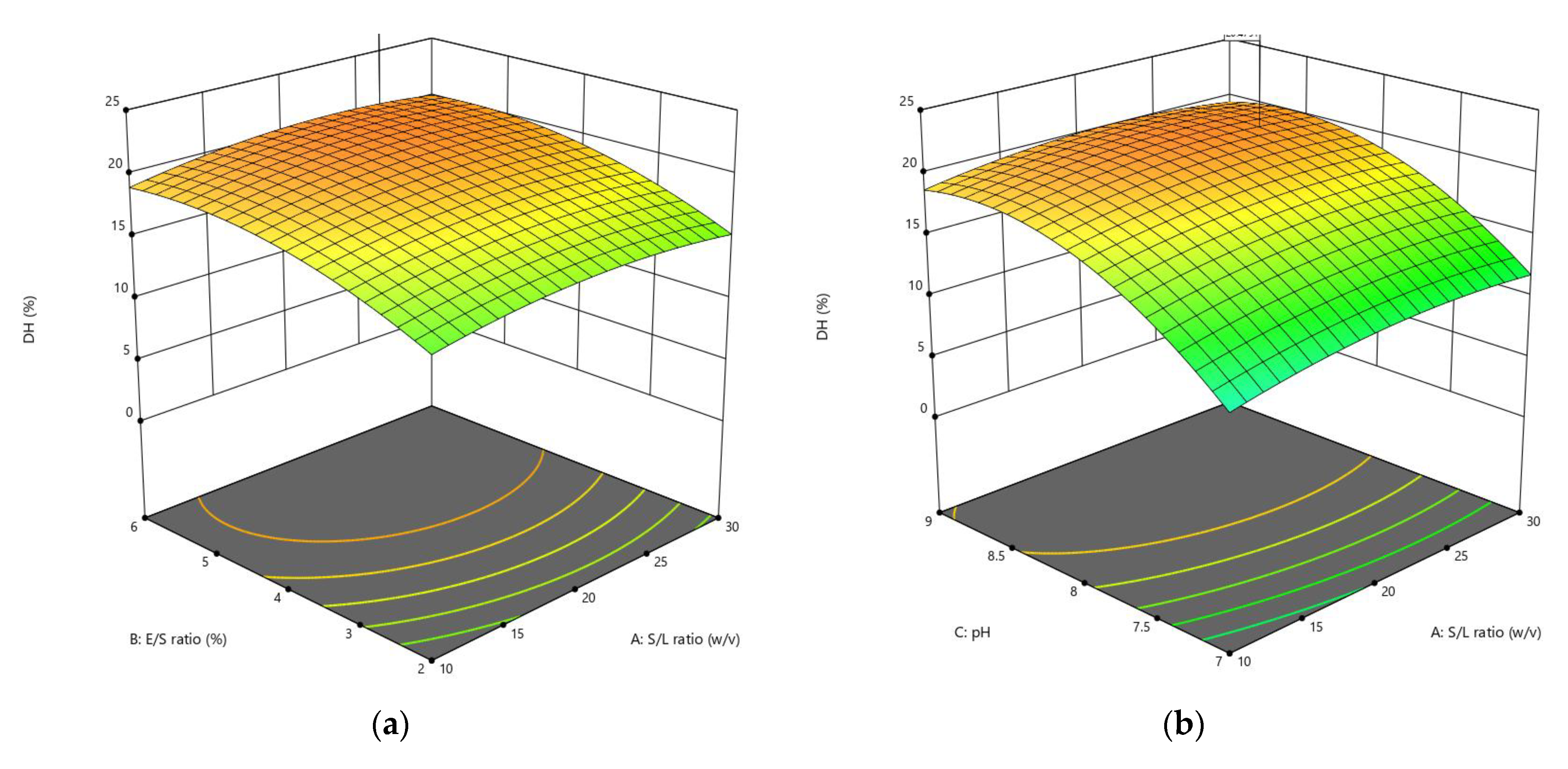
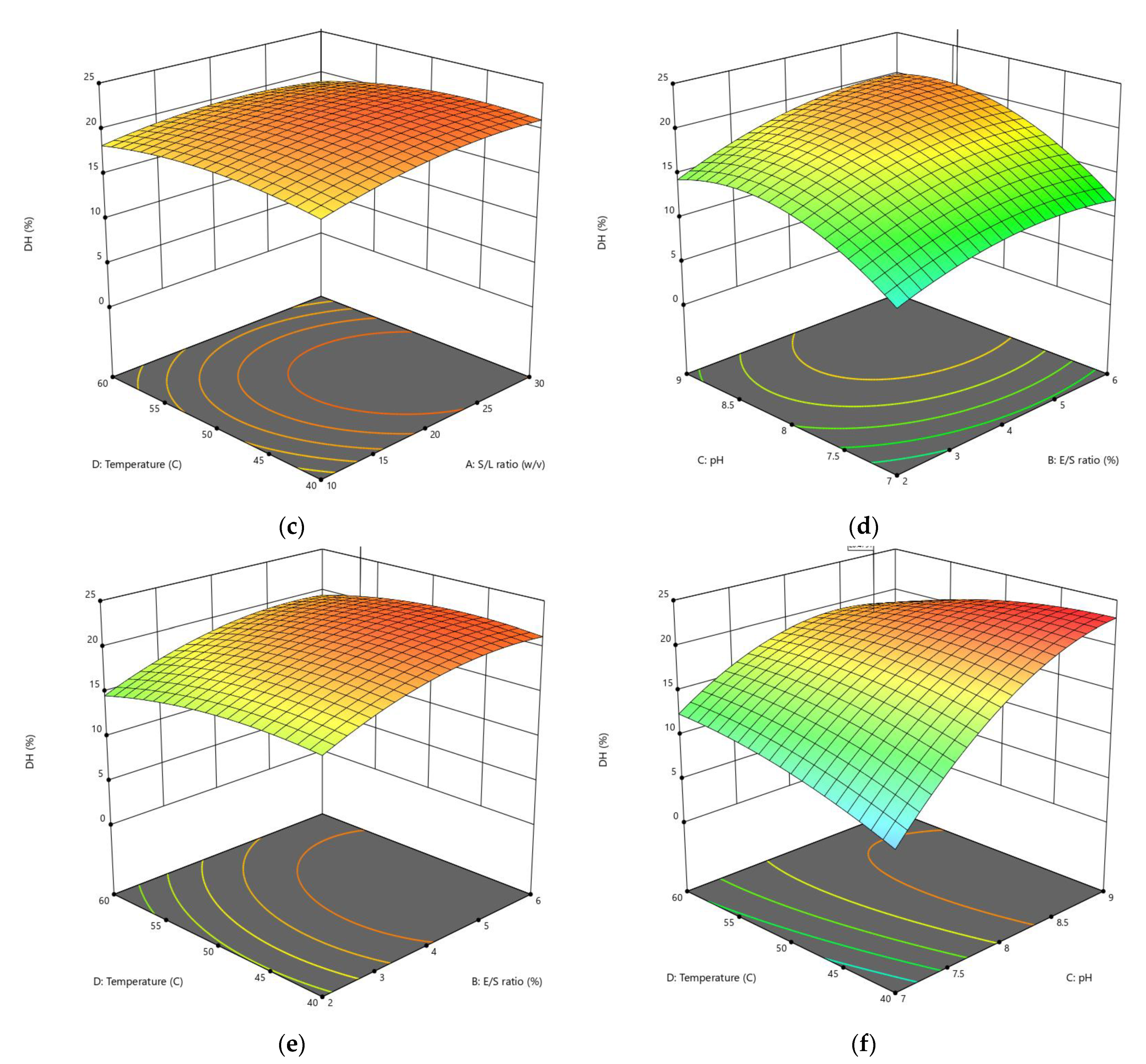
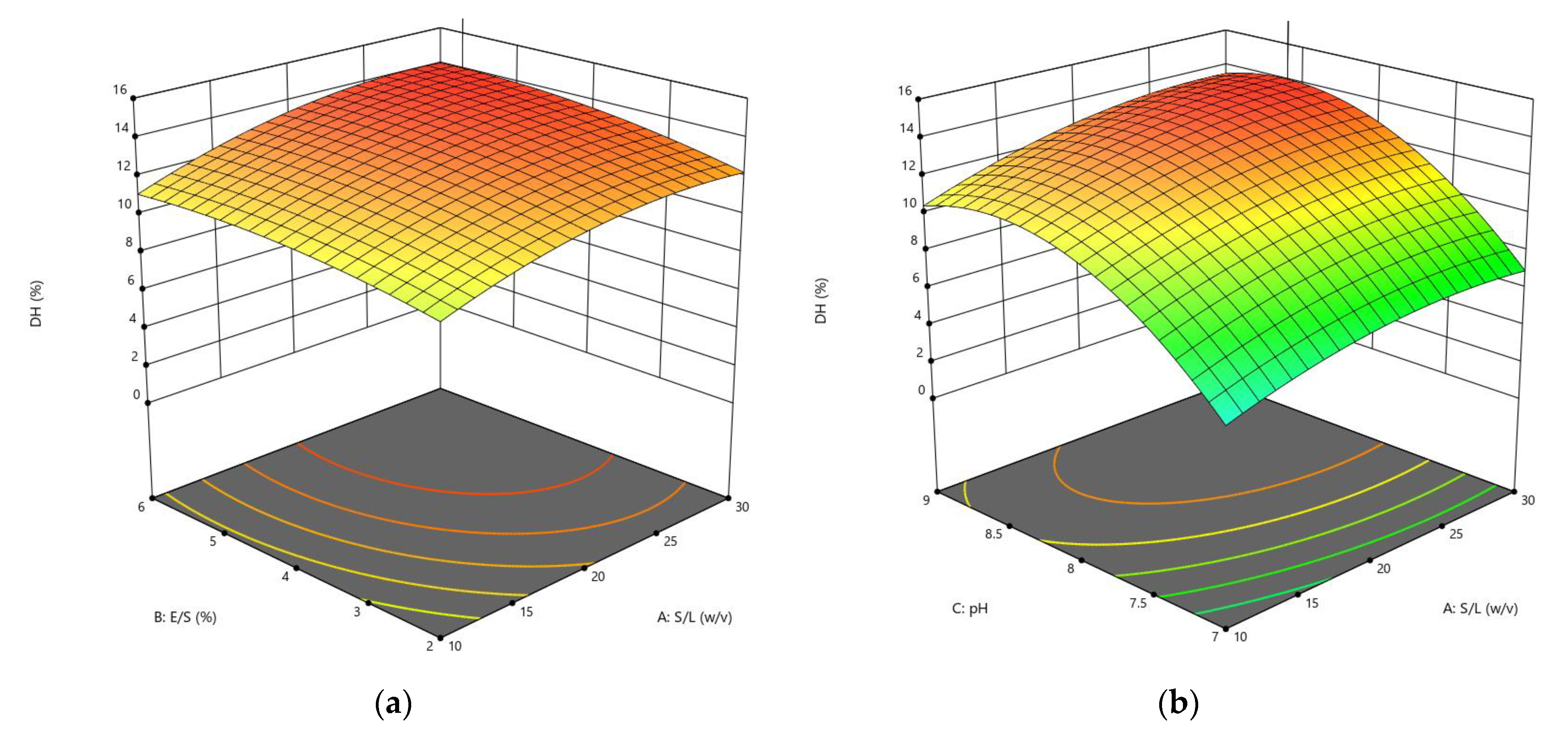
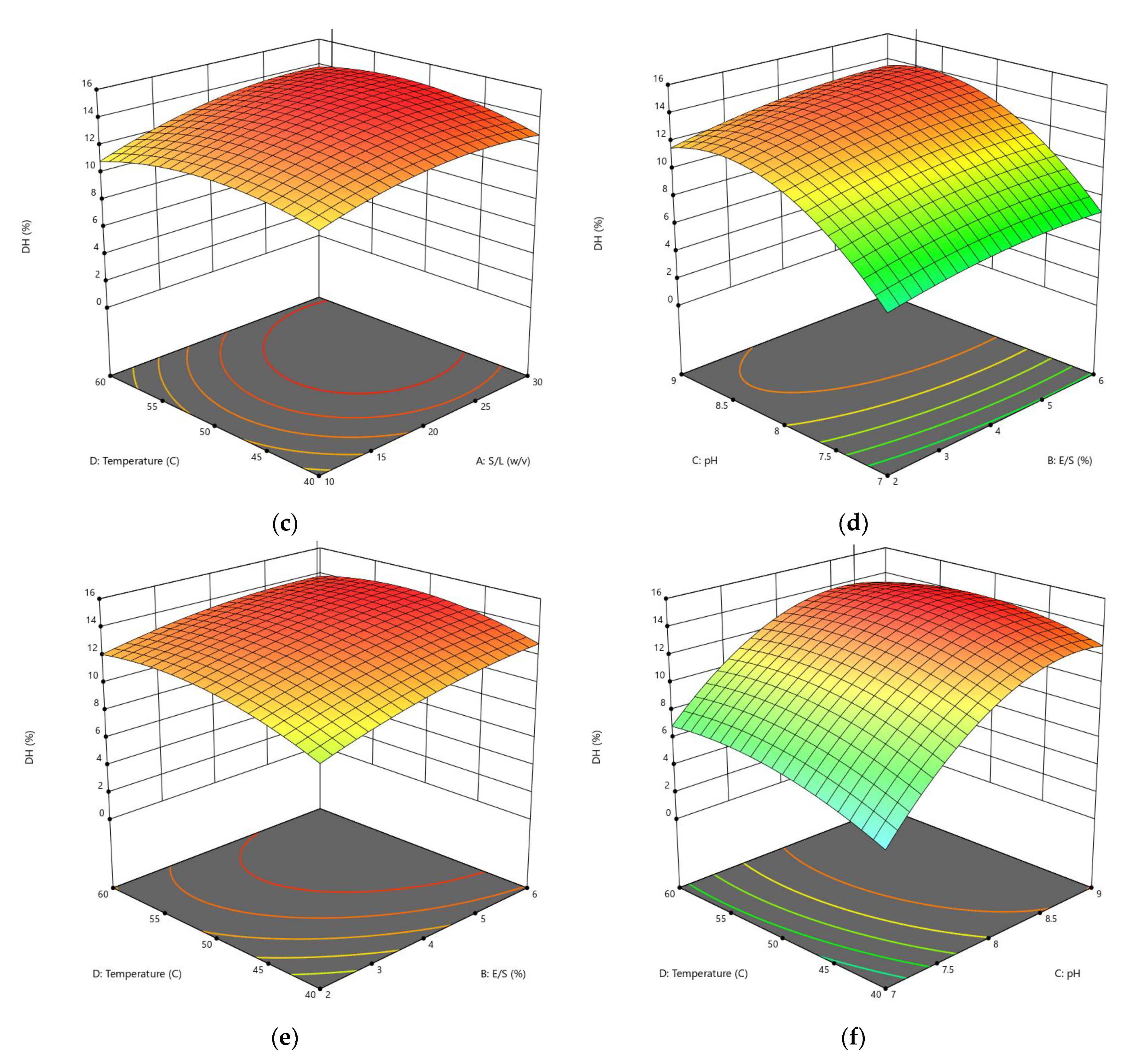
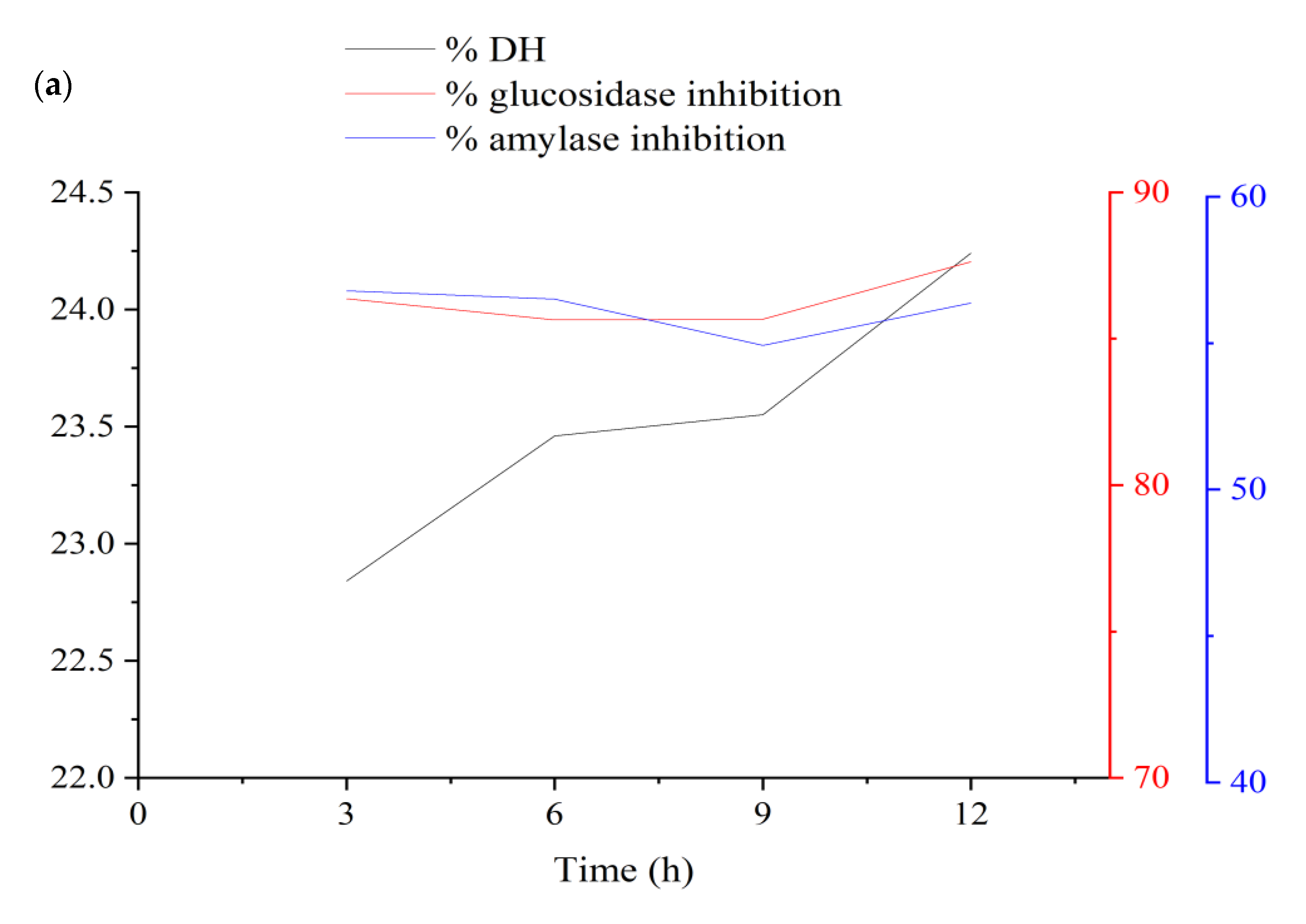
3.1.1. Influences of Parameters on the DH
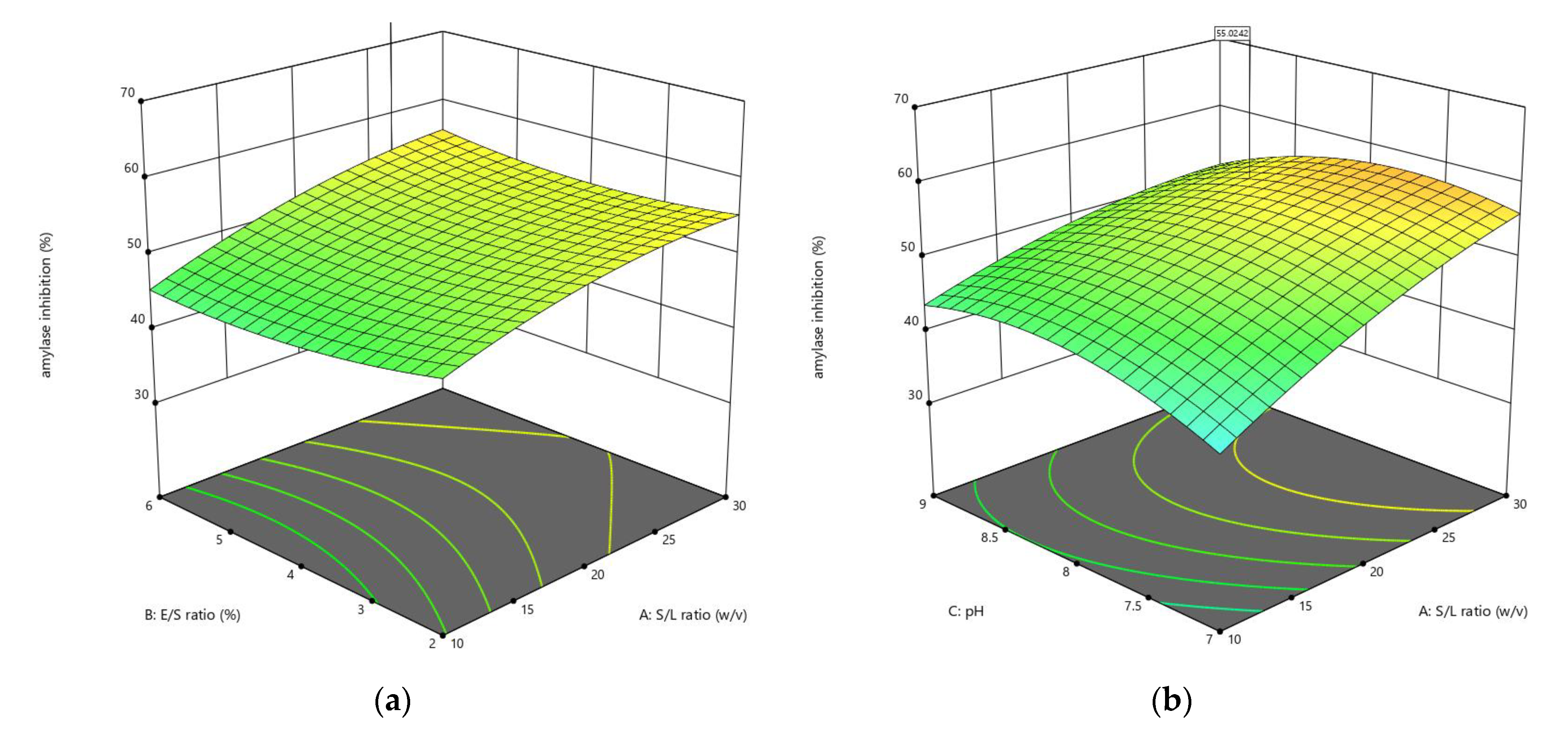
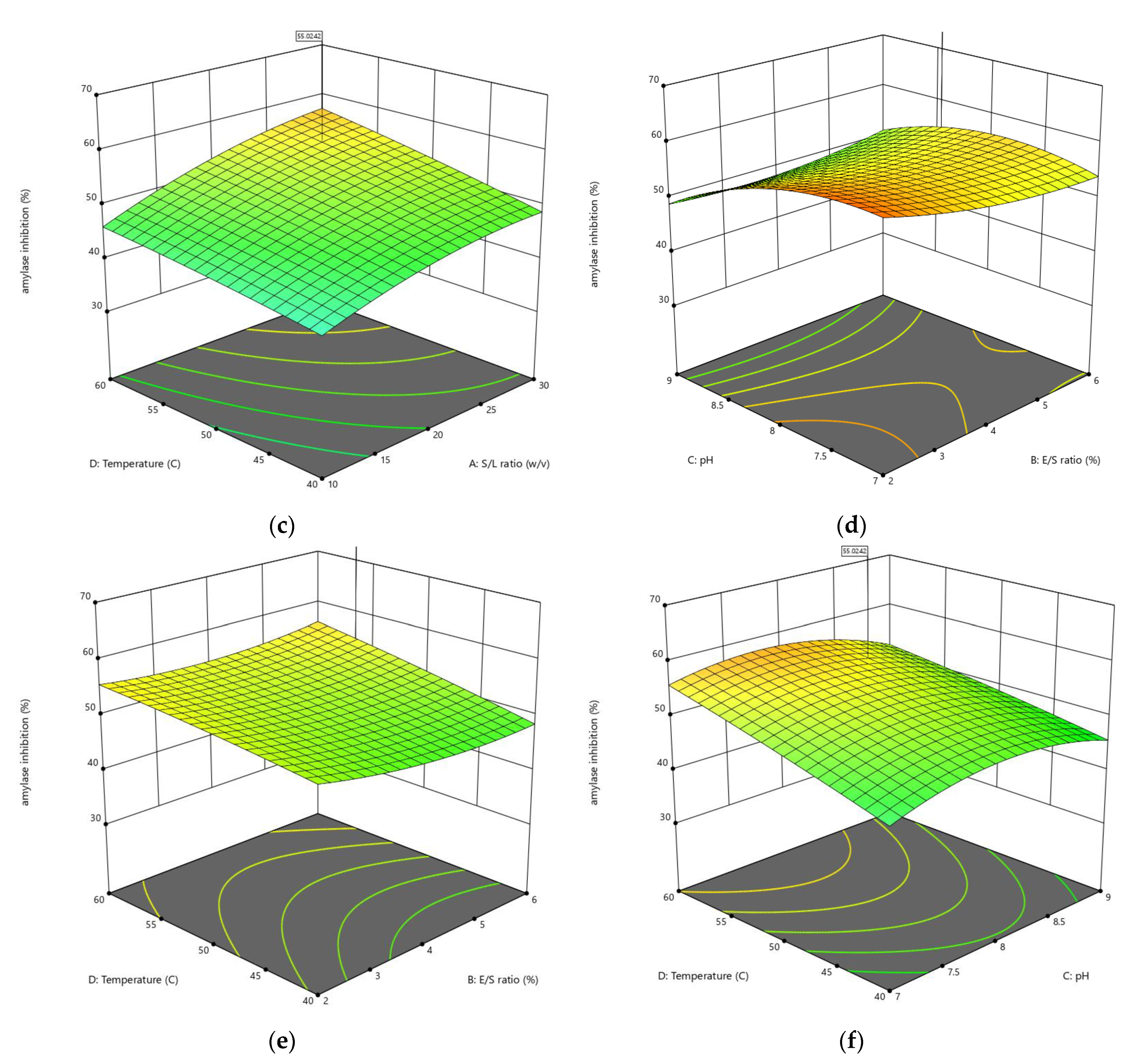
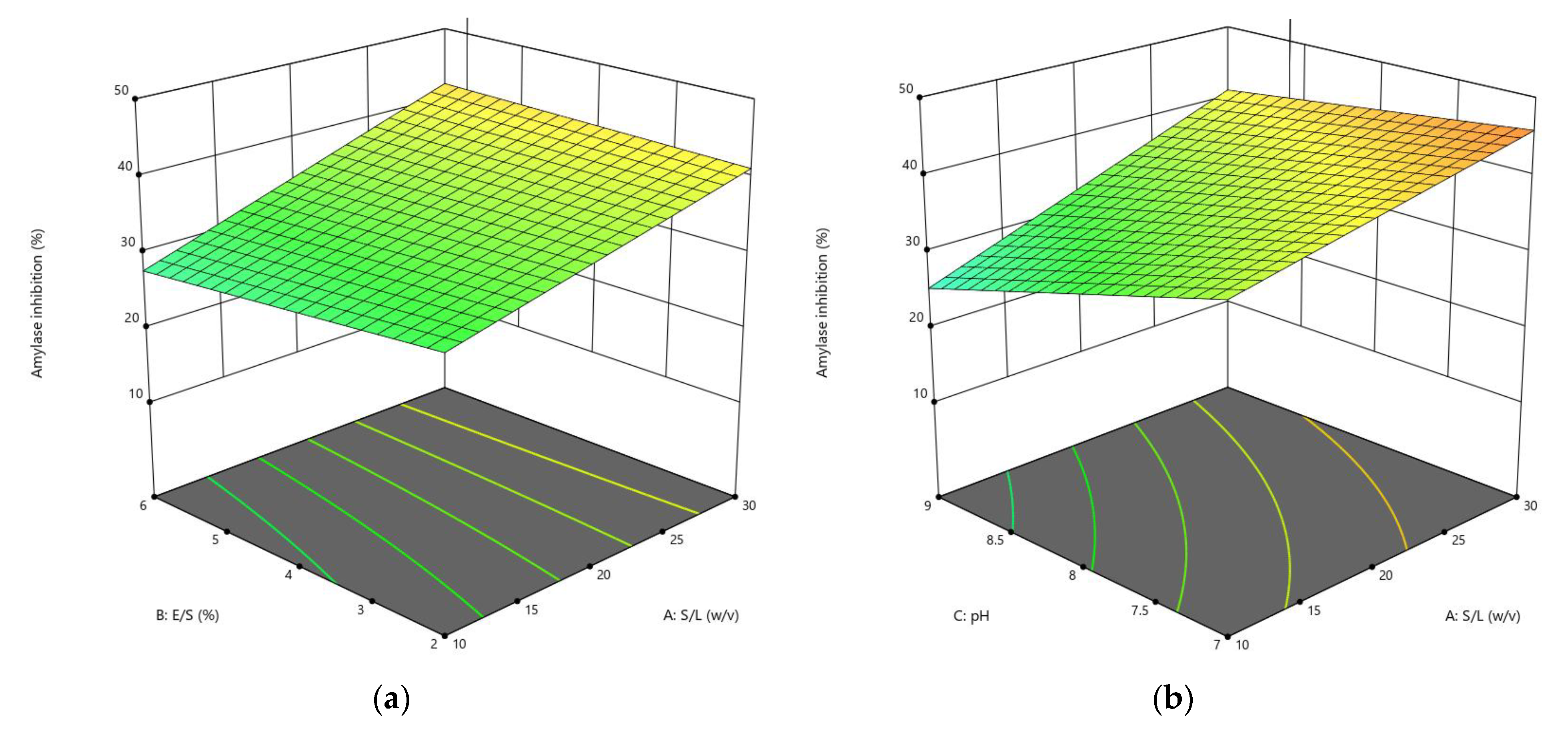
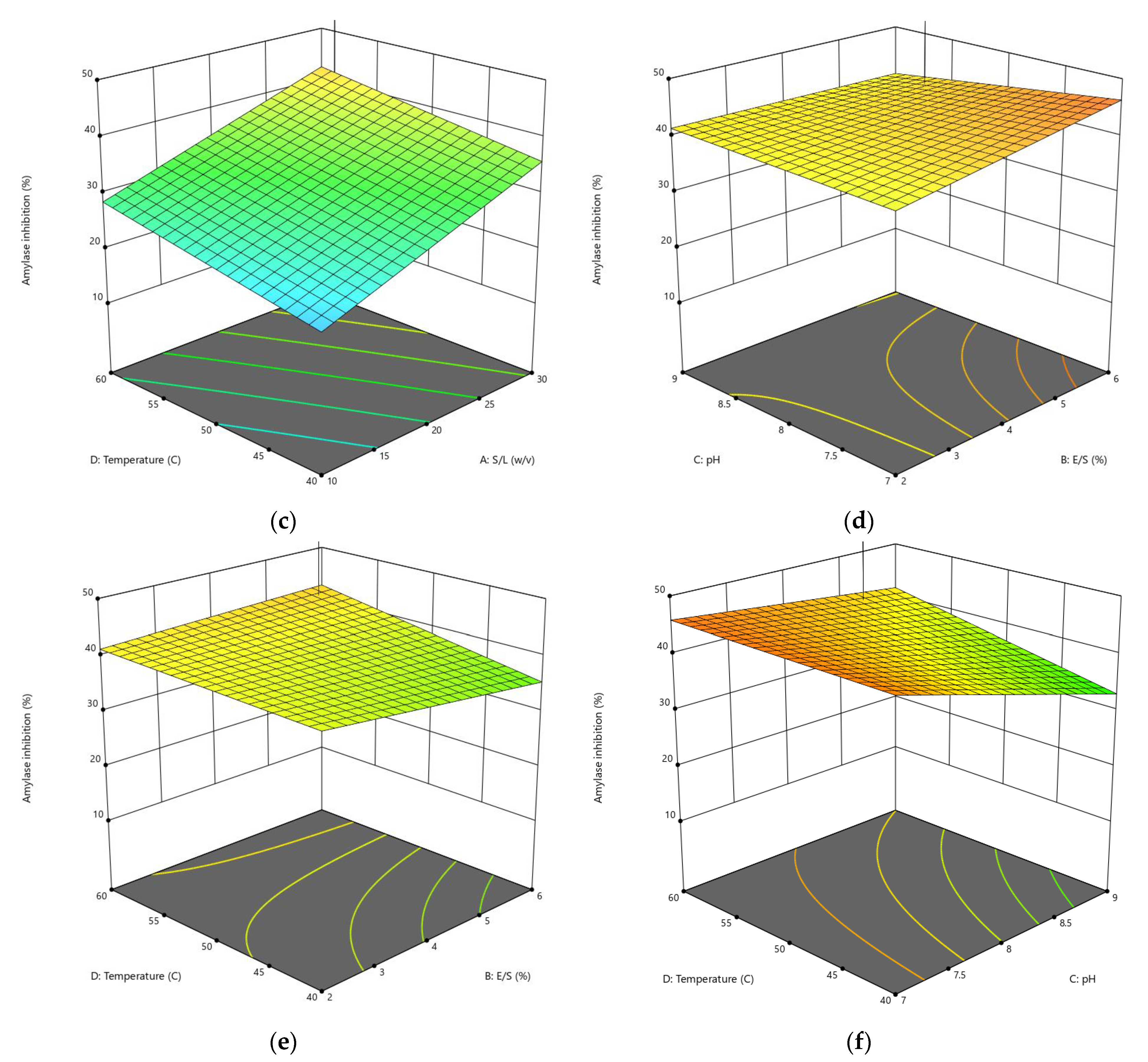
3.1.2. Influences of Parameters on α-Amylase Inhibition
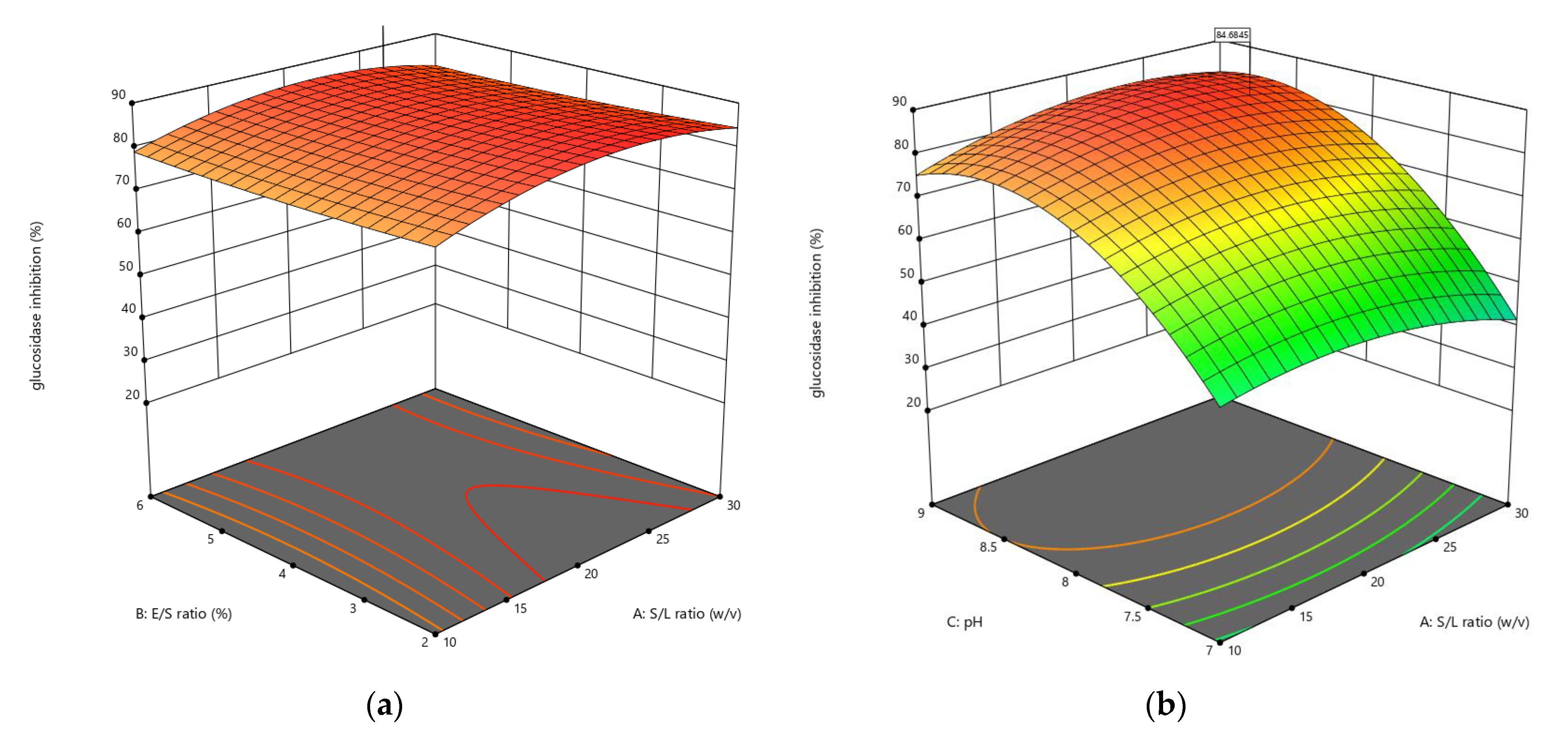
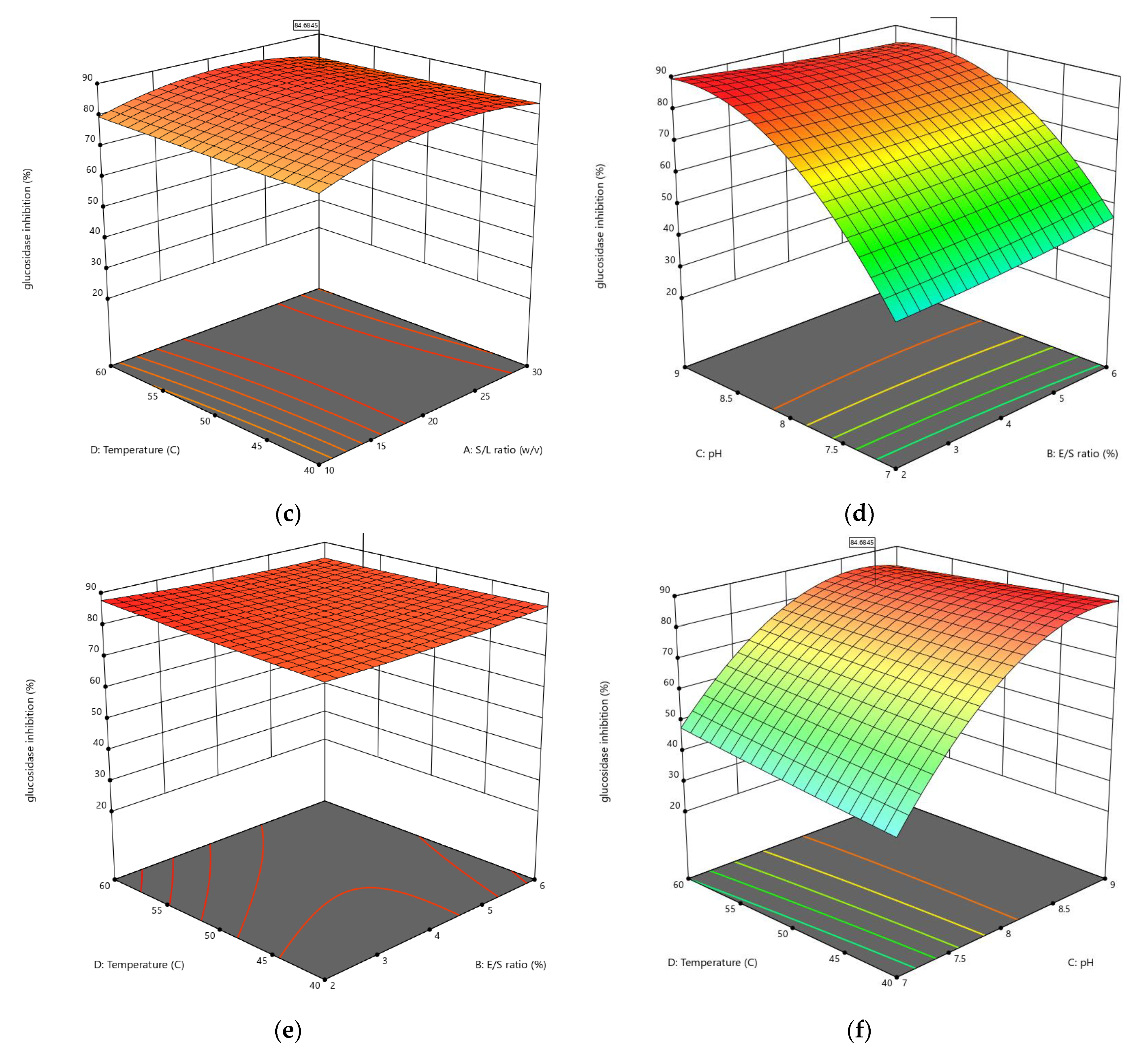
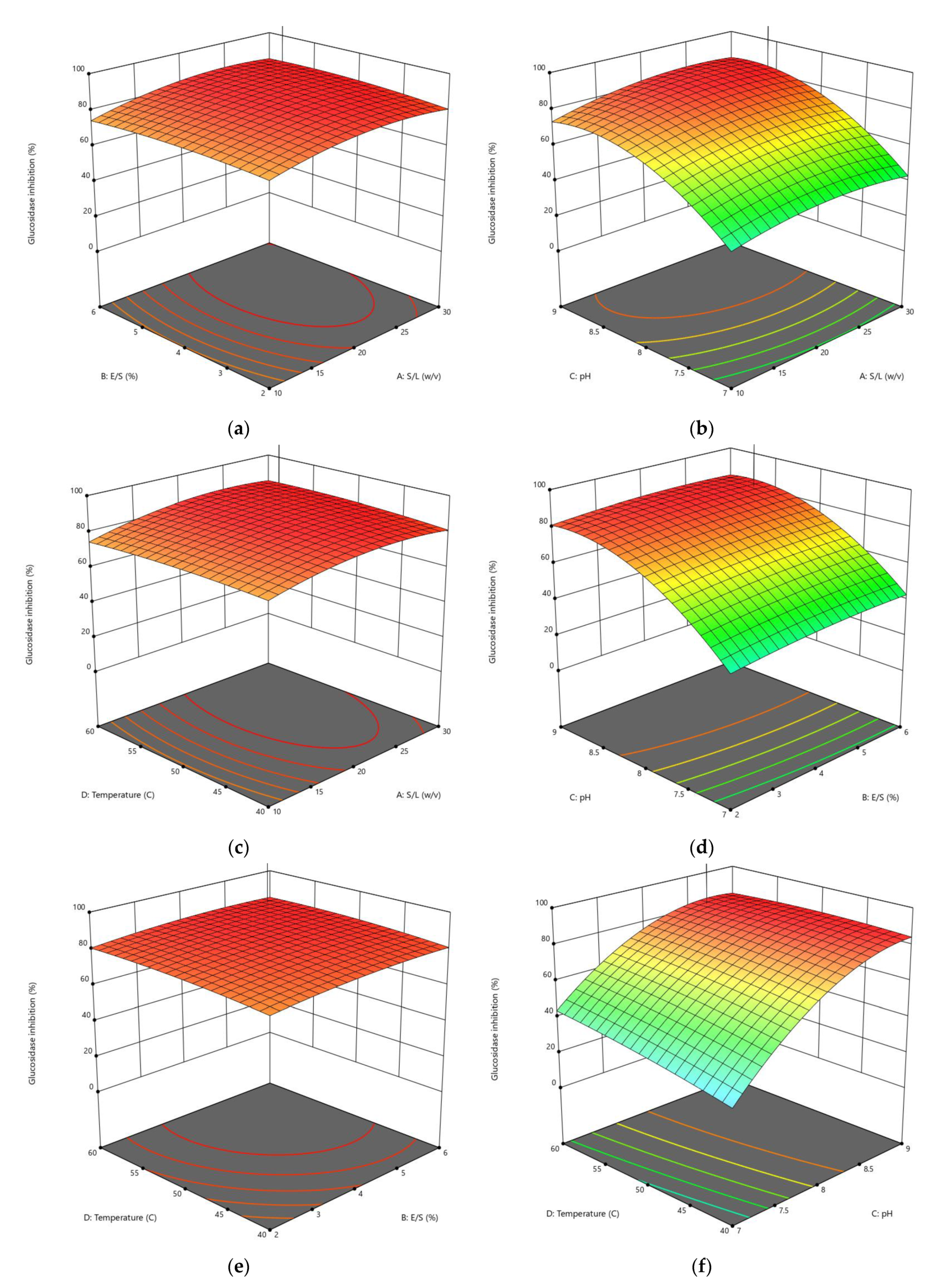
3.1.3. Influences of Parameters on α-Glucosidase Inhibition
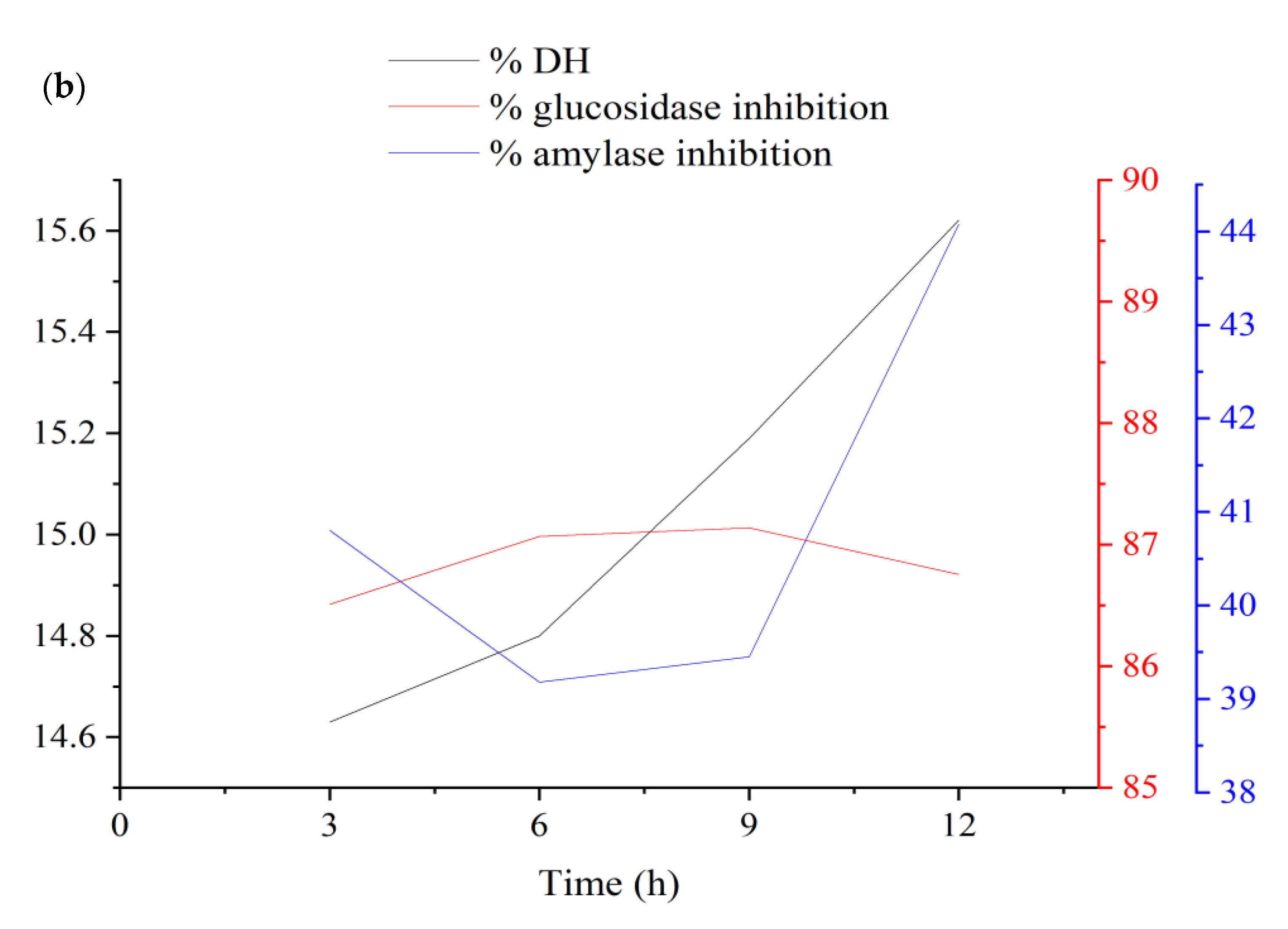
3.1.4. Verification and Optimization
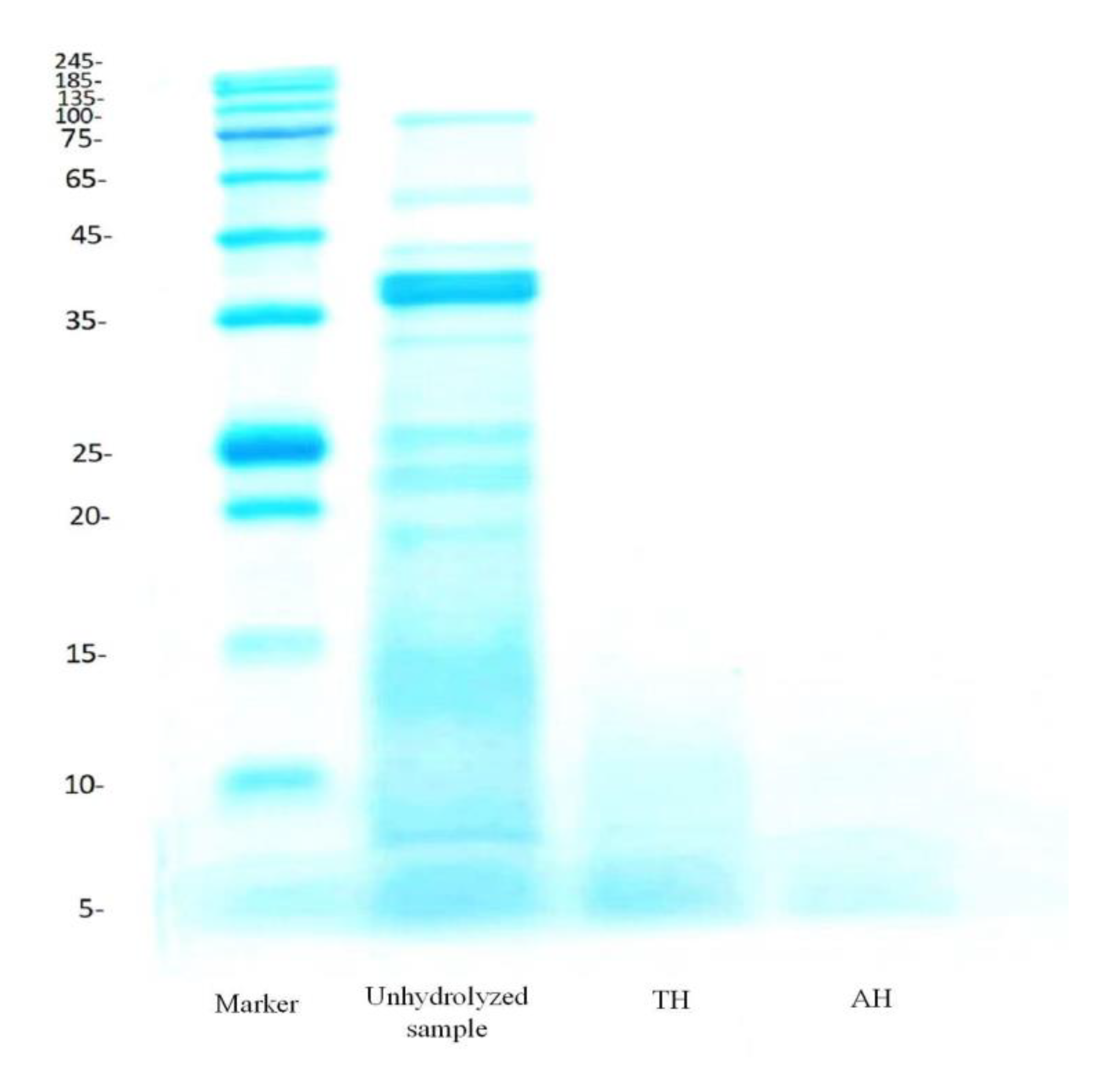
3.2. Protein Patterns by SDS-PAGE
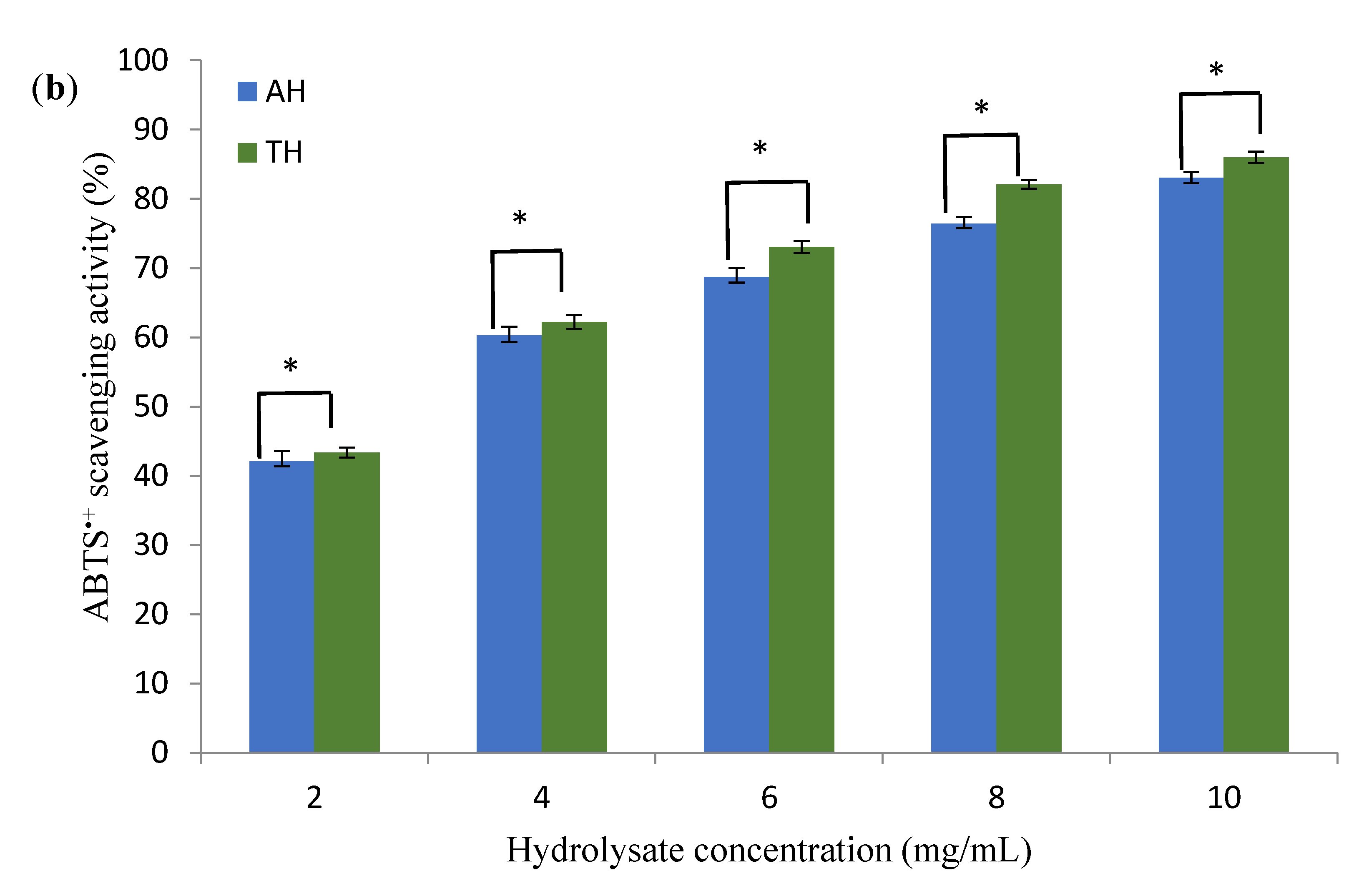
3.3. Antioxidant Activity of Hydrolysates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marthandam Asokan, S.; Wang, T.; Su, W.-T.; Lin, W.-T. Antidiabetic effects of a short peptide of potato protein hydrolysate in STZ-induced diabetic mice. Nutrients 2019, 11, 779. [Google Scholar] [CrossRef] [Green Version]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 9059. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Tomita, T. Apoptosis of pancreatic β-cells in Type 1 diabetes. Bosn. J. Basic Med. Sci. 2017, 17, 183. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; de Mejia, E.G.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef]
- Qaseem, A.; Barry, M.J.; Humphrey, L.L.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians; Fitterman, N.; Horwitch, C.; Kansagara, D.; McLean, R.M.; Wilt, T.J. Oral pharmacologic treatment of type 2 diabetes mellitus: A clinical practice guideline update from the American College of Physicians. Ann. Intern. Med. 2017, 166, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. In vitro α-glucosidase, angiotensin converting enzyme and dipeptidyl peptidase-IV inhibitory properties of brewers’ spent grain protein hydrolysates. Food Res. Int. 2014, 56, 100–107. [Google Scholar] [CrossRef]
- Karimi, A.; Azizi, M.H.; Ahmadi Gavlighi, H. Fractionation of hydrolysate from corn germ protein by ultrafiltration: In vitro antidiabetic and antioxidant activity. Food Sci. Nutr. 2020, 8, 2395–2405. [Google Scholar] [CrossRef]
- Ramadhan, A.H.; Nawas, T.; Zhang, X.; Pembe, W.M.; Xia, W.; Xu, Y. Purification and identification of a novel antidiabetic peptide from Chinese giant salamander (Andrias davidianus) protein hydrolysate against α-amylase and α-glucosidase. Int. J. Food Prop. 2017, 20, S3360–S3372. [Google Scholar] [CrossRef] [Green Version]
- Noman, A.; Wang, Y.; Zhang, C.; Abed, S.M. Antioxidant Activity of Hybrid Sturgeon (Huso dauricus × Acipenser schrenckii) Protein Hydrolysate Prepared Using Bromelain, Its Fractions and Purified Peptides. Pol. J. Food Nutr. Sci. 2022, 72, 79–89. [Google Scholar] [CrossRef]
- Manzanares, P.; Gandía, M.; Garrigues, S.; Marcos, J.F. Improving health-promoting effects of food-derived bioactive peptides through rational design and oral delivery strategies. Nutrients 2019, 11, 2545. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Li, J.; Jiang, G.; Li, H.; Zhao, M.; Jiang, Y. Effects of combined high pressure and enzymatic treatments on physicochemical and antioxidant properties of peanut proteins. Food Sci. Nutr. 2019, 7, 1417–1425. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: Barking, UK, 1986. [Google Scholar]
- Zhang, L.; Song, C.; Chang, J.; Wang, Z.; Meng, X. Optimization of protein hydrolysates production from defatted peanut meal based on physicochemical characteristics and sensory analysis. LWT 2022, 163, 113572. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Dinparast, L.; Zengin, G.; Sarikurkcu, C.; Bahadori, S.; Asghari, B.; Movahhedin, N. Functional components, antidiabetic, anti-Alzheimer’s disease, and antioxidant activities of Salvia syriaca L. Int. J. Food Prop. 2017, 20, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.-R.; Chi, C.-F.; Zhang, Q.-H.; Luo, H.-Y. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 2012, 36, 240–250. [Google Scholar] [CrossRef]
- Liu, F.-F.; Li, Y.-Q.; Sun, G.-J.; Wang, C.-Y.; Liang, Y.; Zhao, X.-Z.; He, J.-X.; Mo, H.-Z. Influence of ultrasound treatment on the physicochemical and antioxidant properties of mung bean protein hydrolysate. Ultrason. Sonochem. 2022, 84, 105964. [Google Scholar] [CrossRef]
- Bahari, A.N.; Saari, N.; Salim, N.; Ashari, S.E. Response factorial design analysis on papain-generated hydrolysates from Actinopyga lecanora for determination of antioxidant and antityrosinase activities. Molecules 2020, 25, 2663. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- See, S.; Hoo, L.; Babji, A. Optimization of enzymatic hydrolysis of Salmon (Salmo salar) skin by Alcalase. Int. Food Res. J. 2011, 18, 1359–1365. [Google Scholar]
- Gao, R.; Shen, Y.; Shu, W.; Bai, F.; Jin, W.; Wang, J.; Yuan, L. Optimization of enzymatic conditions of sturgeon muscles and their anti-inflammatory potential. J. Food Qual. 2020, 2020, 9698134. [Google Scholar] [CrossRef]
- Ktari, N.; Fakhfakh, N.; Balti, R.; Ben Khaled, H.; Nasri, M.; Bougatef, A. Effect of degree of hydrolysis and protease type on the antioxidant activity of protein hydrolysates from cuttlefish (Sepia officinalis) by-products. J. Aquat. Food Prod. Technol. 2013, 22, 436–448. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.; Yang, R.; Zhao, W. Identification of bioactive peptides with α-amylase inhibitory potential from enzymatic protein hydrolysates of red seaweed (Porphyra spp.). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Famuwagun, A.A.; Alashi, A.M.; Gbadamosi, O.S.; Taiwo, K.A.; Oyedele, D.; Adebooye, O.C.; Aluko, R.E. Antioxidant and enzymes inhibitory properties of Amaranth leaf protein hydrolyzates and ultrafiltration peptide fractions. J. Food Biochem. 2021, 45, e13396. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro activities of α-amylase, α-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Fadimu, G.J.; Farahnaky, A.; Gill, H.; Truong, T. Influence of ultrasonic pretreatment on structural properties and biological activities of lupin protein hydrolysate. Int. J. Food Sci. Technol. 2022, 57, 1729–1738. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; de Mejia, E.G. Optimization, identification, and comparison of peptides from germinated chickpea (Cicer arietinum) protein hydrolysates using either papain or ficin and their relationship with markers of type 2 diabetes. Food Chem. 2022, 374, 131717. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Yu, Y.; Liu, B.; Liu, J.; Chen, F. Novel peptides derived from egg white protein inhibiting alpha-glucosidase. Food Chem. 2011, 129, 1376–1382. [Google Scholar] [CrossRef]
- Zaky, A.A.; Liu, Y.; Han, P.; Ma, A.; Jia, Y. Effect of flavorzyme digestion on the antioxidant capacities of ultra-filtrated rice bran protein hydrolyzates. J. Food Process. Preserv. 2020, 44, e14551. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.-T.; Rawdkuen, S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Sila, A.; Przybylski, R.; Nedjar-Arroume, N.; Makhlouf, I.; Blecker, C.; Attia, H.; Dhulster, P.; Bougatef, A.; Besbes, S. Purification and identification of novel antioxidant peptides from enzymatic hydrolysate of chickpea (Cicer arietinum L.) protein concentrate. J. Funct. Foods 2015, 12, 516–525. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B.; Andreu, D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019, 32, 100450. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Purification and characterization of antioxidative peptides derived from chicken skin gelatin hydrolysate. Food Hydrocoll. 2018, 85, 311–320. [Google Scholar] [CrossRef]
- Latorres, J.; Rios, D.; Saggiomo, G.; Wasielesky, W.; Prentice-Hernandez, C. Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeus vannamei). J. Food Sci. Technol. 2018, 55, 721–729. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Coded Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Solid/liquid ratio (w/v, X1) | 1:10 | 1:20 | 1:30 |
| Enzyme/substrate ratio (w/w, X2) | 2 | 4 | 6 |
| pH (X3) | 7 | 8 | 9 |
| Temperature (X4) | 40 | 50 | 60 |
| Source | Sum of Squares | Degree of Freedom | Mean of Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Alcalase | |||||
| DH | |||||
| Model | 985.28 | 14 | 70.38 | 11.20 | <0.0001 |
| Residual | 94.22 | 15 | 6.28 | ||
| Pure error | 0.1070 | 5 | 0.0214 | ||
| Lack of fit | 94.11 | 10 | 9.41 | 439.77 | <0.0001 |
| Total | 1079.50 | 29 | |||
| R2 | 0.9127 | Adj. R2 | 0.8313 | ||
| α-Amylase inhibition | |||||
| Model | 1101.75 | 14 | 78.70 | 5.81 | 0.0008 |
| Residual | 203.05 | 15 | 13.54 | ||
| Pure error | 11.92 | 5 | 2.38 | ||
| Lack of fit | 191.12 | 10 | 19.11 | 8.02 | 0.0165 |
| Total | 1304.79 | 29 | |||
| R2 | 0.8444 | Adj. R2 | 0.6991 | ||
| α-Glucosidase inhibition | |||||
| Model | 12933.34 | 14 | 923.81 | 41.00 | <0.0001 |
| Residual | 338.01 | 15 | 22.53 | ||
| Pure error | 3.98 | 5 | 0.7954 | ||
| Lack of fit | 334.04 | 10 | 33.40 | 42.00 | 0.0003 |
| Total | 13271.36 | 29 | |||
| R2 | 0.9745 | Adj. R2 | 0.9508 | ||
| Trypsin | |||||
| DH | |||||
| Model | 433.92 | 14 | 30.99 | 27.31 | <0.0001 |
| Residual | 17.02 | 15 | 1.13 | ||
| Pure error | 0.0000 | 5 | 0.0000 | ||
| Lack of fit | 17.02 | 10 | 1.70 | ||
| Total | 450.94 | 29 | |||
| R2 | 0.9623 | Adj. R2 | 0.9270 | ||
| α-Amylase inhibition | |||||
| Model | 1229.94 | 10 | 122.99 | 9.19 | <0.0001 |
| Residual | 254.33 | 19 | 13.39 | ||
| Pure error | 0.0000 | 5 | 0.0000 | ||
| Lack of fit | 254.33 | 14 | 18.17 | ||
| Total | 1484.27 | 29 | |||
| R2 | 0.8287 | Adj. R2 | 0.7385 | ||
| α-Glucosidase inhibition | |||||
| Model | 14264.56 | 14 | 1018.90 | 46.72 | <0.0001 |
| Residual | 327.15 | 15 | 21.81 | ||
| Pure error | 0.6474 | 5 | 0.1295 | ||
| Lack of fit | 326.50 | 10 | 32.65 | 252.17 | <0.0001 |
| Total | 14591.71 | 29 | |||
| R2 | 0.9776 | Adj. R2 | 0.9567 | ||
| Run | Independent Variables | Alcalase | Trypsin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Y1 | Y2 | Y3 | Y1 | Y2 | Y3 | |
| 1 | 30 | 2 | 7 | 60 | 8.45 | 63.26 | 38.19 | 3.88 | 34.75 | 35.54 |
| 2 | 20 | 4 | 8 | 50 | 18.64 | 54.72 | 79.38 | 2.70 | 37.53 | 23.61 |
| 3 | 30 | 6 | 9 | 60 | 15.03 | 52.68 | 84.97 | 3.52 | 49.44 | 25.72 |
| 4 | 10 | 2 | 9 | 40 | 15.39 | 43.11 | 79.50 | 9.34 | 32.32 | 75.17 |
| 5 | 10 | 2 | 7 | 40 | 3.71 | 49.58 | 21.97 | 12.60 | 34.79 | 75.73 |
| 6 | 30 | 6 | 7 | 60 | 11.71 | 57.51 | 41.05 | 3.75 | 39.98 | 27.84 |
| 7 | 20 | 4 | 8 | 50 | 18.99 | 55.14 | 77.28 | 3.42 | 37.01 | 24.87 |
| 8 | 34.14 | 4 | 8 | 50 | 22.86 | 56.50 | 77.53 | 5.36 | 37.55 | 37.54 |
| 9 | 5.86 | 4 | 8 | 50 | 13.28 | 34.94 | 58.80 | 9.84 | 33.43 | 82.21 |
| 10 | 20 | 4 | 8 | 50 | 18.72 | 51.97 | 77.41 | 12.60 | 34.79 | 75.73 |
| 11 | 20 | 6.82 | 8 | 50 | 20.56 | 52.06 | 78.25 | 12.60 | 34.79 | 74.85 |
| 12 | 30 | 2 | 9 | 60 | 10.10 | 49.31 | 83.59 | 12.60 | 34.79 | 75.73 |
| 13 | 10 | 2 | 9 | 60 | 12.66 | 48.38 | 81.70 | 11.18 | 45.23 | 78.73 |
| 14 | 10 | 6 | 9 | 60 | 17.04 | 43.75 | 78.23 | 8.82 | 27.91 | 73.46 |
| 15 | 20 | 4 | 8 | 50 | 18.74 | 51.97 | 77.41 | 14.38 | 40.62 | 78.34 |
| 16 | 30 | 6 | 9 | 40 | 22.93 | 43.33 | 85.87 | 11.15 | 31.43 | 78.54 |
| 17 | 10 | 6 | 9 | 40 | 21.12 | 44.24 | 76.56 | 12.60 | 34.79 | 75.73 |
| 18 | 10 | 2 | 7 | 60 | 7.63 | 48.43 | 45.90 | 0.86 | 48.42 | 14.54 |
| 19 | 30 | 2 | 7 | 40 | 4.19 | 53.06 | 23.97 | 12.66 | 34.62 | 77.29 |
| 20 | 20 | 4 | 8 | 50 | 18.85 | 51.89 | 78.73 | 8.91 | 36.64 | 75.18 |
| 21 | 20 | 4 | 8 | 35.86 | 13.25 | 47.02 | 78.17 | 12.60 | 34.79 | 75.73 |
| 22 | 20 | 4 | 8 | 64.14 | 22.43 | 50.21 | 80.06 | 8.03 | 25.69 | 59.76 |
| 23 | 30 | 6 | 7 | 40 | 6.05 | 46.57 | 32.56 | 4.34 | 39.50 | 38.45 |
| 24 | 20 | 4 | 8 | 50 | 18.59 | 51.97 | 77.41 | 11.35 | 14.50 | 78.13 |
| 25 | 20 | 4 | 6.59 | 50 | 4.90 | 44.84 | 23.97 | 12.92 | 38.55 | 81.01 |
| 26 | 10 | 6 | 7 | 40 | 5.19 | 30.53 | 43.77 | 10.79 | 26.55 | 75.23 |
| 27 | 10 | 6 | 7 | 60 | 9.90 | 43.26 | 50.03 | 12.14 | 40.44 | 84.82 |
| 28 | 30 | 2 | 9 | 40 | 17.96 | 47.67 | 87.73 | 6.35 | 49.64 | 41.47 |
| 29 | 20 | 4 | 9.41 | 50 | 21.71 | 39.16 | 76.56 | 10.20 | 39.29 | 75.22 |
| 30 | 20 | 1.17 | 8 | 50 | 12.76 | 52.80 | 81.98 | 13.72 | 32.98 | 75.71 |
| Parameters | Alcalase | Trypsin | ||
|---|---|---|---|---|
| Predicted | Actual | Predicted | Actual | |
| DH | 20.48 | 22.84 | 14.02 | 14.63 |
| α-amylase inhibition | 55.02 | 56.78 | 42.15 | 40.80 |
| α-glucosidase inhibition | 84.68 | 86.37 | 84.54 | 86.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Bukhaiti, W.Q.; Al-Dalali, S.; Noman, A.; Qiu, S.; Abed, S.M.; Qiu, S.-X. Response Surface Modeling and Optimization of Enzymolysis Parameters for the In Vitro Antidiabetic Activities of Peanut Protein Hydrolysates Prepared Using Two Proteases. Foods 2022, 11, 3303. https://doi.org/10.3390/foods11203303
AL-Bukhaiti WQ, Al-Dalali S, Noman A, Qiu S, Abed SM, Qiu S-X. Response Surface Modeling and Optimization of Enzymolysis Parameters for the In Vitro Antidiabetic Activities of Peanut Protein Hydrolysates Prepared Using Two Proteases. Foods. 2022; 11(20):3303. https://doi.org/10.3390/foods11203303
Chicago/Turabian StyleAL-Bukhaiti, Wedad Q., Sam Al-Dalali, Anwar Noman, Silin Qiu, Sherif M. Abed, and Sheng-Xiang Qiu. 2022. "Response Surface Modeling and Optimization of Enzymolysis Parameters for the In Vitro Antidiabetic Activities of Peanut Protein Hydrolysates Prepared Using Two Proteases" Foods 11, no. 20: 3303. https://doi.org/10.3390/foods11203303






