Effects of Dual-Frequency Ultrasound-Assisted Thawing Technology on Thawing Rate, Quality Properties, and Microstructure of Large Yellow Croaker (Pseudosciaena crocea)
Abstract
:1. Introduction
2. Materials and Methods
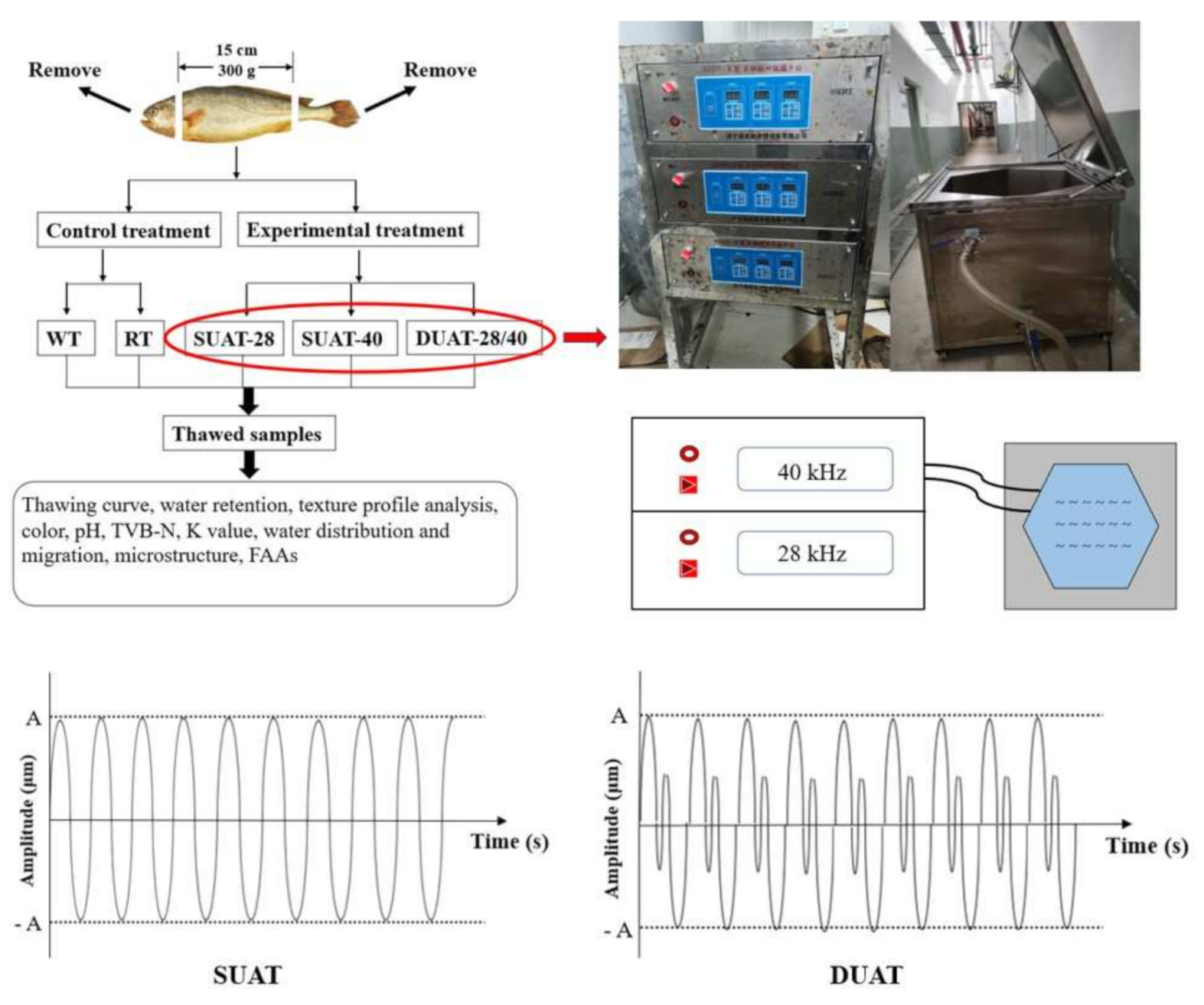
2.1. Preparation of Fish Samples
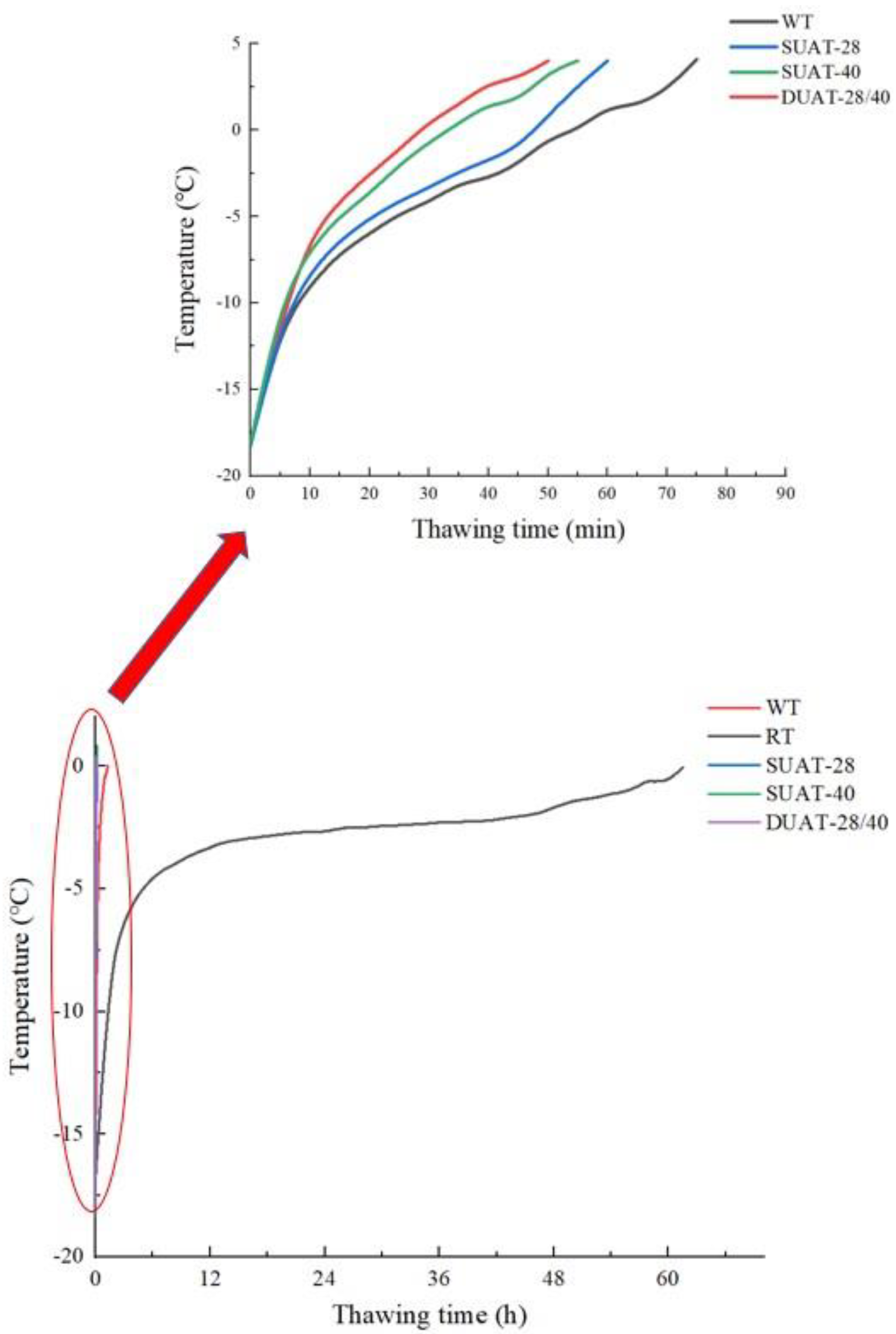
2.2. Thawing Curve
2.3. Determination of Water Retention
2.3.1. Thawing Loss
2.3.2. Water-Holding Capacity
2.3.3. Cooking Loss
2.4. Determination of the Texture Profile Analysis (TPA)
2.5. Determination of Color
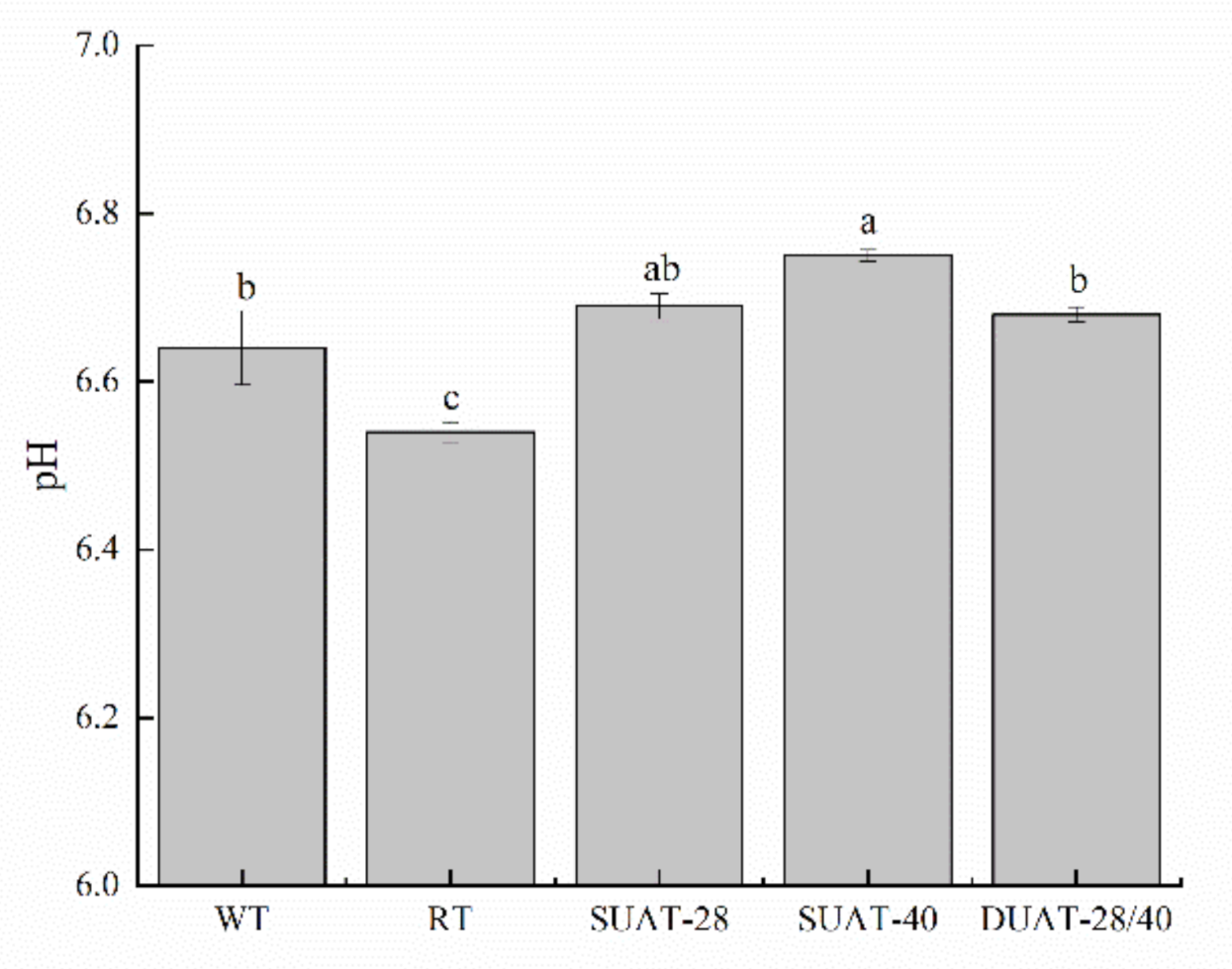
2.6. Determination of the pH Value
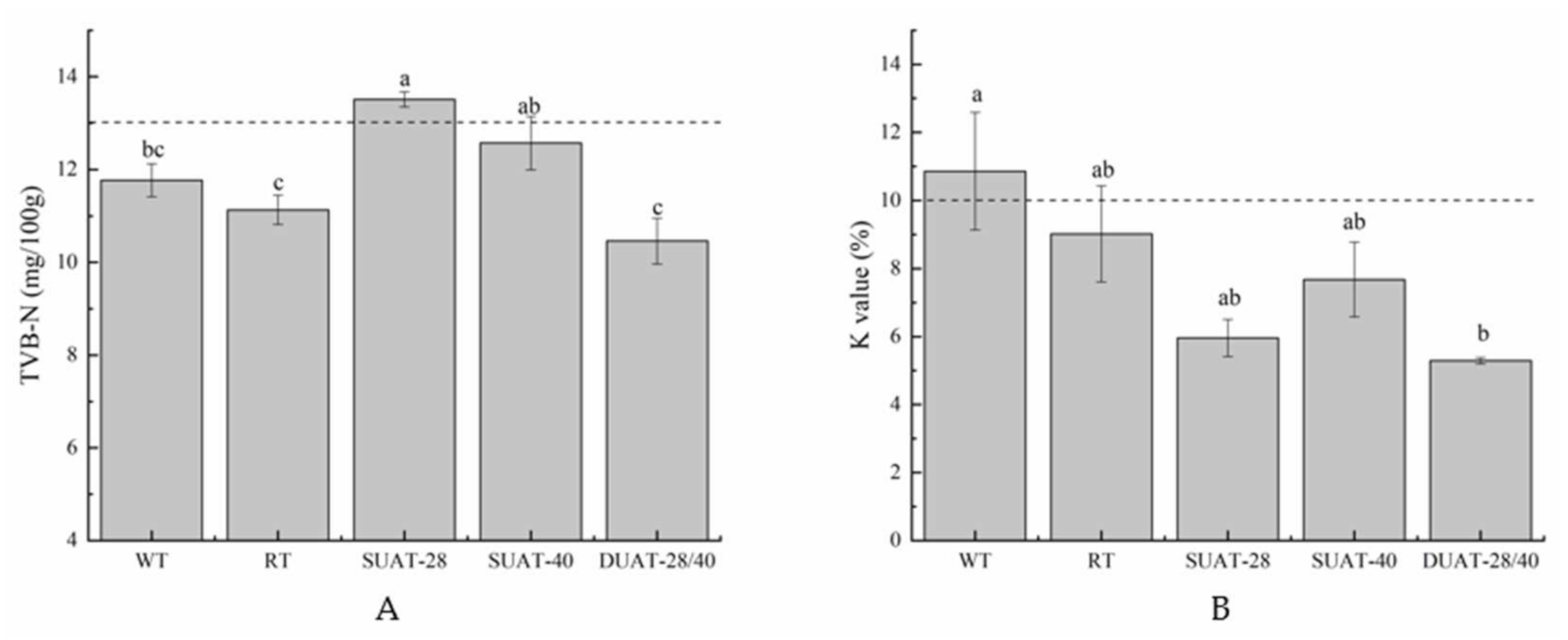
2.7. Determination of TVB-N
2.8. Determination of the K Value
2.9. Water Distribution and Migration
2.10. Scanning Electron Microscope (SEM)
2.11. Free Amino Acids (FAAs) Determination
2.12. Statistical Analysis
3. Results and Discussion
3.1. Thawing Rates and Curves
3.2. Water Retention
3.3. Texture and Color
3.4. pH Value
3.5. Analysis of TVB-N and the K Value
3.6. Analysis via LF-NMR
3.7. Microstructure of the Fish Muscle
3.8. Analysis of Free Amino Acids (FAAs)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.-Y.; Yan, J.-K.; Rashid, M.T.; Ding, Y.; Chikari, F.; Huang, S.; Ma, H. Dual-frequency sequential ultrasound thawing for improving the quality of quick-frozen small yellow croaker and its possible mechanisms. Innov. Food Sci. Emerg. Technol. 2021, 68, 102614. [Google Scholar] [CrossRef]
- Li, D.; Qin, N.; Zhang, L.; Li, Q.; Prinyawiwatkul, W.; Luo, Y. Degradation of adenosine triphosphate, water loss and textural changes in frozen common carp (Cyprinus carpio) fillets during storage at different temperatures. Int. J. Refrig. 2019, 98, 294–301. [Google Scholar] [CrossRef]
- Jun, M.; Xuan, M.; Jing, X. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, S.; Pu, Y.; Muhammad, A.I.; Hang, M.; Liu, D.; Ye, T. Ultrasound-assisted thawing of mango pulp: Effect on thawing rate, sensory, and nutritional properties. Food Chem. 2019, 286, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Sun, P.; Jia, J.-Z.; Cai, L.-Y.; Li, J.-R.; Lv, Y.-F. Effect of low frequency ultrasound thawing method on the quality characteristics of Peru squid (Dosidicus gigas). Food Sci. Technol. Int. 2019, 25, 171–181. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Muhammad, A.I.; Song, L.; Guo, M.; Liu, D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020, 320, 126614. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Yang, L.; Ma, G.; Ma, J.; Yu, Q.L.; Han, L. Ultrasound-assisted thawing of frozen white yak meat: Effects on thawing rate, meat quality, nutrients, and microstructure. Ultrason. Sonochem. 2021, 70, 105345. [Google Scholar] [CrossRef]
- Kang, D.C.; Zou, Y.H.; Cheng, Y.P.; Xing, L.J.; Zhou, G.H.; Zhang, W.G. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016, 33, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Sun, P.; Ma, Y.; Cai, L.; Li, J.-R. Effect of ultrasonic thawing on the water-holding capacity, physicochemical properties and structure of frozen tuna (Thunnus tonggol) myofibrillar proteins. J. Sci. Food Agric. 2019, 99, 5083–5091. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.; Xia, X.; Kong, B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Zhang, M.; Rao, Z. Effect of ultrasound-assisted thawing on gelling and 3D printing properties of silver carp surimi. Food Res. Int. 2021, 145, 110405. [Google Scholar] [CrossRef]
- Bian, C.; Cheng, H.; Yu, H.; Mei, J.; Xie, J. Effect of multi-frequency ultrasound assisted thawing on the quality of large yellow croaker (Larimichthys crocea). Ultrason. Sonochem. 2022, 82, 105907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Yan, J.-K.; Ding, Y.; Rashid, M.T.; Ma, H. Effect of sweep frequency ultrasound and fixed frequency ultrasound thawing on gelling properties of myofibrillar protein from quick-frozen small yellow croaker and its possible mechanisms. LWT 2021, 150, 111922. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Tayyab Rashid, M.; Yan, J.-K.; Ma, H. Effect of multi-frequency ultrasound thawing on the structure and rheological properties of myofibrillar proteins from small yellow croaker. Ultrason. Sonochem. 2021, 70, 105352. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sun, F.; Xia, X.; Xu, H.; Kong, B. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019, 51, 281–291. [Google Scholar] [CrossRef]
- Tan, M.; Ye, J.; Chu, Y.; Xie, J. The effects of ice crystal on water properties and protein stability of large yellow croaker (Pseudosciaena crocea). Int. J. Refrig. 2021, 130, 242–252. [Google Scholar] [CrossRef]
- Jinfeng, W.; Wenhui, Y.; Jing, X. Effect of glazing with different materials on the quality of tuna during frozen storage. Foods 2020, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. Impact of different air blast freezing conditions on the physicochemical quality of albacore (Thunnus alalunga) pretreated by high pressure processing. LWT 2021, 145, 111538. [Google Scholar] [CrossRef]
- Lv, Y.; Xie, J. Effects of Freeze–Thaw Cycles on Water Migration, Microstructure and Protein Oxidation in Cuttlefish. Foods 2021, 10, 2576. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Tan, M.; Bian, C.; Xie, J. Effect of ultrasonic thawing on the physicochemical properties, freshness, and protein-related properties of frozen large yellow croaker (Pseudosciaena crocea). J. Food Sci. 2021, 79, 105787. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Tan, M.; Yi, Z.; Ding, Z.; Yang, D.; Xie, J. Shelf-Life Prediction of Glazed Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage Based on Arrhenius Model and Long-Short-Term Memory Neural Networks Model. Fishes 2021, 6, 39. [Google Scholar] [CrossRef]
- Li, N.; Zhan, X. Identification of clinical trait-related lncRNA and mRNA biomarkers with weighted gene co-expression network analysis as useful tool for personalized medicine in ovarian cancer. EPMA J. 2019, 10, 273–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Cheng, H.; Yu, H.; Mei, J.; Xie, J. Quality enhancement of large yellow croaker (Pseudosciaena crocea) during frozen (−18 °C) storage by spiral freezing. CyTA J. Food 2021, 19, 710–720. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Wang, J.; Cai, L.; Regenstein, J.; Ruan, Y.; Li, X. Effect of magnetic nanoparticles plus microwave or far-infrared thawing on protein conformation changes and moisture migration of red seabream (Pagrus major) fillets. Food Chem. 2018, 266, 498–507. [Google Scholar] [CrossRef]
- Jia, G.; Liu, H.; Nirasawa, S.; Liu, H. Effects of high-voltage electrostatic field treatment on the thawing rate and post-thawing quality of frozen rabbit meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 348–356. [Google Scholar] [CrossRef]
- Jiang, Q.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in quality properties and tissue histology of lightly salted tuna meat subjected to multiple freeze-thaw cycles. Food Chem. 2019, 293, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, S.; Oladejo, A.O.; Wang, Y.; Huang, S.; Zhou, C.; Ye, X.; Ma, H.; Duan, Y. Effects of ultrasound-assisted alpha-amylase degradation treatment with multiple modes on the extraction of rice protein. Ultrason. Sonochem. 2018, 40, 890–899. [Google Scholar] [CrossRef]
- Amiri, A.; Mousakhani-Ganjeh, A.; Shafiekhani, S.; Mandal, R.; Singh, A.P.; Kenari, R.E. Effect of high voltage electrostatic field thawing on the functional and physicochemical properties of myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2019, 56, 102191. [Google Scholar] [CrossRef]
- Peng-cheng, Z.; Jing, X. Effect of different thawing methods on the quality of mackerel (Pneumatophorus japonicus). Food Sci. Biotechnol. 2021, 30, 1213–1223. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of freezing under electrostatic field on the quality of lamb meat. Innov. Food Sci. Emerg. Technol. 2016, 37, 68–73. [Google Scholar] [CrossRef]
- Mousakhani-Ganjeh, A.; Hamdami, N.; Soltanizadeh, N. Impact of high voltage electric field thawing on the quality of frozen tuna fish (Thunnus albacares). J. Food Eng. 2015, 156, 39–44. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yan, J.K.; Ding, Y.; Ma, H. Effects of ultrasound on the thawing of quick-frozen small yellow croaker (Larimichthys polyactis) based on TMT-labeled quantitative proteomic. Food Chem. 2022, 366, 130600. [Google Scholar] [CrossRef]
- Sun, Q.; Kong, B.; Liu, S.; Zheng, O.; Zhang, C. Ultrasound-assisted thawing accelerates the thawing of common carp (Cyprinus carpio) and improves its muscle quality. LWT 2021, 141, 111080. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 degrees C. Food Chem. 2013, 140, 105–114. [Google Scholar] [CrossRef]
- Cropotova, J.; Tappi, S.; Genovese, J.; Rocculi, P.; Dalla Rosa, M.; Rustad, T. The combined effect of pulsed electric field treatment and brine salting on changes in the oxidative stability of lipids and proteins and color characteristics of sea bass (Dicentrarchus labrax). Heliyon 2021, 7, e05947. [Google Scholar] [CrossRef]
- Stadnik, J.; Dolatowski, Z.J. Influence of sonication on Warner-Bratzler shear force, colour and myoglobin of beef (m. semimembranosus). Eur. Food Res. Technol. 2011, 233, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhao, X.; Zhang, C.; Xia, X.; Sun, F.; Kong, B. Ultrasound-assisted immersion freezing accelerates the freezing process and improves the quality of common carp (Cyprinus carpio) at different power levels. LWT 2019, 108, 106–112. [Google Scholar] [CrossRef]
- Diao, Y.; Cheng, X.; Wang, L.; Xia, W. Effects of immersion freezing methods on water holding capacity, ice crystals and water migration in grass carp during frozen storage. Int. J. Refrig. 2021, 131, 581–591. [Google Scholar] [CrossRef]
- Zhang, M.; Haili, N.; Chen, Q.; Xia, X.; Kong, B. Influence of ultrasound-assisted immersion freezing on the freezing rate and quality of porcine longissimus muscles. Meat Sci 2018, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Chunlin, H.; Jing, X. The effect of multiple freeze-thaw cycles on the microstructure and quality of Trachurus murphyi. Foods 2021, 10, 1350. [Google Scholar] [CrossRef]
- Agyekum, A.A.; Kutsanedzie, F.Y.H.; Annavaram, V.; Mintah, B.K.; Asare, E.K.; Wang, B. FT-NIR coupled chemometric methods rapid prediction of K-value in fish. Vib. Spectrosc. 2020, 108, 103044. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Mingtang, T.; Jing, X. Exploring the effect of dehydration on water migrating property and protein changes of large yellow croaker (Pseudosciaena crocea) during frozen storage. Foods 2021, 10, 784. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Kawai, K.; Suzuki, T. Effects of Freezing Temperature and Water Activity on Microstructure, Color, and Protein Conformation of Freeze-Dried Bluefin Tuna (Thunnus orientalis). Food Bioprocess Technol. 2015, 8, 916–925. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Xie, J. Evaluation of water dynamics and protein changes in bigeye tuna (Thunnus obesus) during cold storage. LWT 2019, 108, 289–296. [Google Scholar] [CrossRef]
- Jiang, Q.; Jia, R.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in protein properties and tissue histology of tuna meat as affected by salting and subsequent freezing. Food Chem. 2019, 271, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Fei, W.; Min, Z.; Benu, A.; Jincai, S. Recent developments in novel freezing and thawing technologies applied to foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3620–3631. [Google Scholar] [CrossRef]
- Anggo, A.D.; Ma’ruf, W.F.; Swastawati, F.; Rianingsih, L. Changes of Amino and Fatty Acids in Anchovy (Stolephorus Sp) Fermented Fish Paste with Different Fermentation Periods. Procedia Environ. Sci. 2015, 23, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Xicai, Z.; Wenbo, H.; Jing, X. Effect of different packaging methods on protein oxidation and degradation of grouper (Epinephelus coioides) during refrigerated storage. Foods 2019, 8, 325. [Google Scholar] [CrossRef] [Green Version]
- Dabade, D.S.; Jacxsens, L.; Miclotte, L.; Abatih, E.; Devlieghere, F.; De Meulenaer, B. Survey of multiple biogenic amines and correlation to microbiological quality and free amino acids in foods. Food Control 2021, 120, 107497. [Google Scholar] [CrossRef]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of melanosis and microbial growth in Pacific white shrimp (Litopenaeus vannamei) using high CO2 modified atmosphere packaging. Food Chem. 2020, 312, 126114. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hirose, N.; Ohno, S.; Arakaki, M.; Wada, K. Flavor characteristics and antioxidant capacities of hihatsumodoki (Piper retrofractum Vahl) fresh fruit at three edible maturity stages. J. Food Sci. Technol. 2018, 55, 1295–1305. [Google Scholar] [CrossRef]
- Kang, D.C.; Gao, X.Q.; Ge, Q.F.; Zhou, G.H.; Zhang, W.G. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017, 38, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Kang, D.; Liu, R.; Qi, J.; Zhou, G.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochem. 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and alpha-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef]
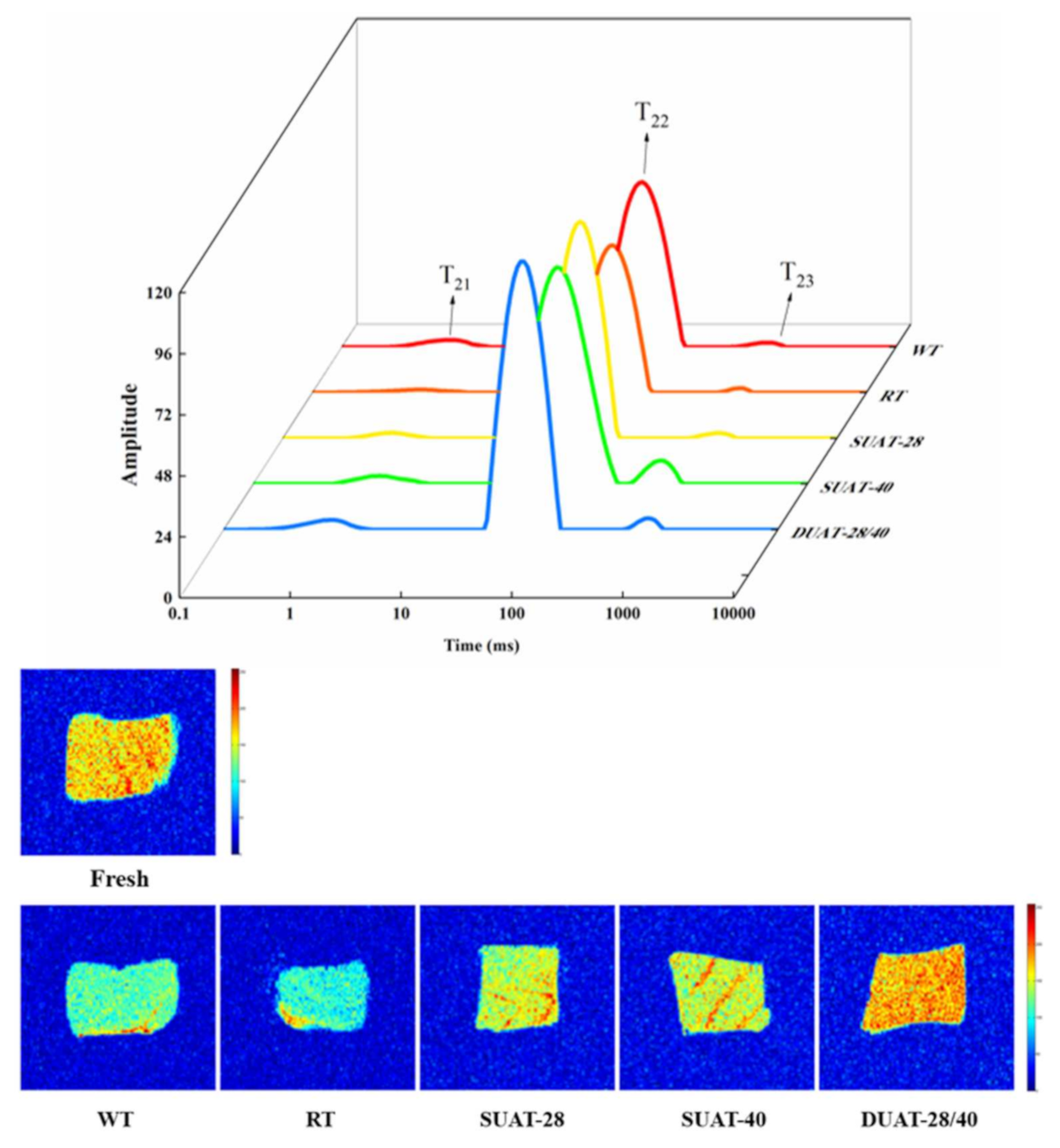
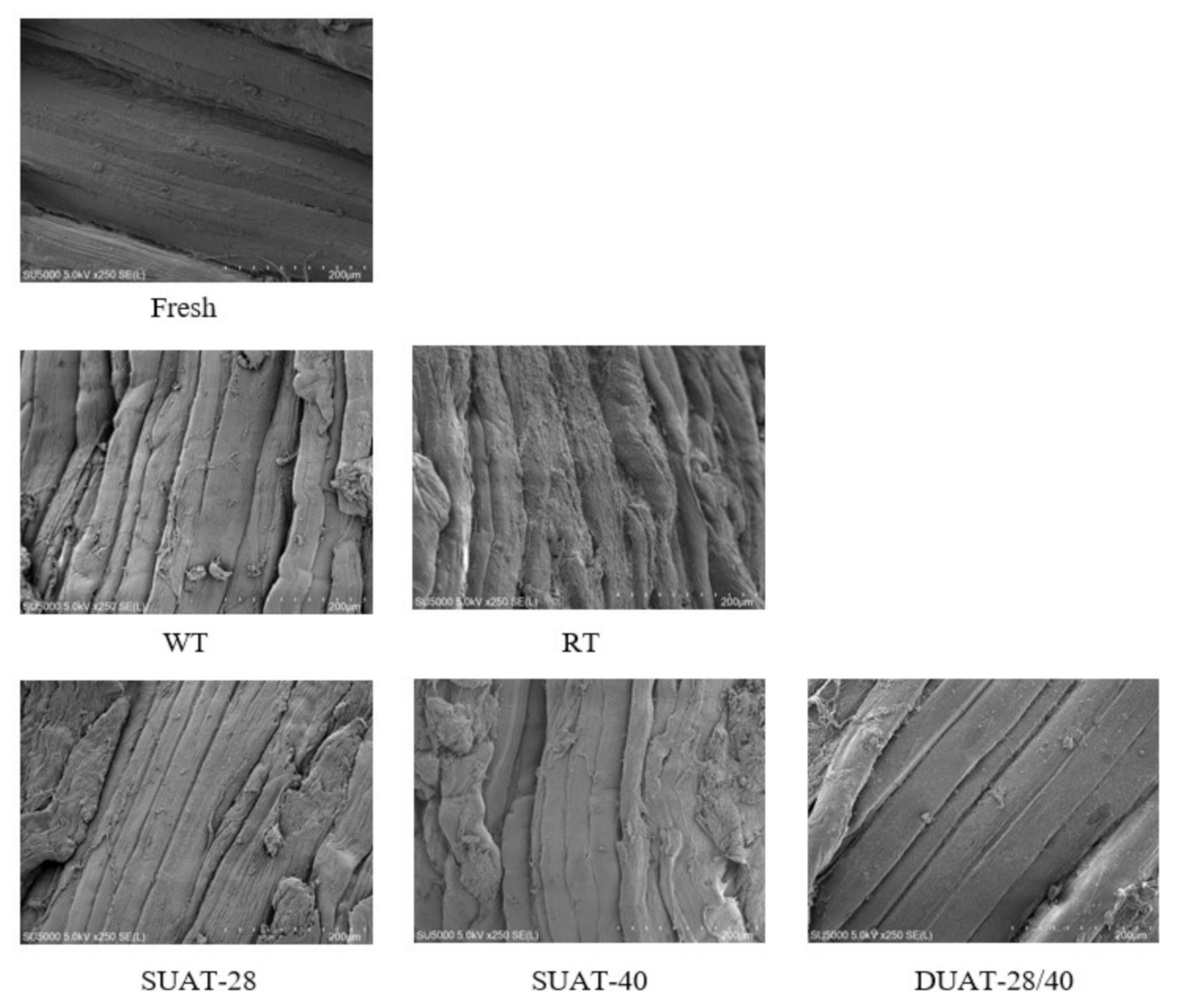

| Treatments | Thawing Loss (%) | Water-Holding Capacity (%) | Cooking Loss (%) |
|---|---|---|---|
| WT | 3.28 | 79.43 ± 3.21 a | 14.44 ± 2.48 c |
| RT | 1.97 | 75.08 ± 2.34 c | 26.88 ± 1.70 a |
| SUAT-28 | 1.97 | 79.45 ± 2.76 a | 20.52 ± 1.13 b |
| SUAT-40 | 3.22 | 77.58 ± 4.45 b | 21.42 ± 1.31 b |
| DUAT-28/40 | 1.70 | 81.49 ± 2.19 a | 16.65 ± 1.40 bc |
| Treatments | Hardness (g) | Springiness | Chewiness | Resilience | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|---|---|
| WT | 2025.48 ± 68.43 b | 0.41 ± 0.03 b | 288.13 ± 48.37 ab | 0.137 ± 0.02 ab | 47.31 ± 1.54 ab | 0.02 ± 0.19 c | −1.67 ± 0.39 c | 47.35 ± 1.53 ab |
| RT | 1891.09 ± 61.10 b | 0.44 ± 0.01 ab | 222.66 ± 27.78 bc | 0.093 ± 0.01 c | 43.39 ± 0.96 c | 0.28 ± 0.05 bc | 0.05 ± 0.22 a | 43.39 ± 0.96 c |
| SUAT-28 | 1067.77 ± 43.34 c | 0.43 ± 0.01 ab | 185.64 ± 22.73 c | 0.114 ± 0.01 bc | 45.02 ± 1.23 bc | 0.57 ± 0.01 b | −1.66 ± 0.45 c | 45.06 ± 1.21 bc |
| SUAT-40 | 1427.11 ± 24.13 c | 0.41 ± 0.01 b | 262.31 ± 12.69 abc | 0.103 ± 0.01 c | 42.95 ± 0.63 c | 0.72 ± 0.43 a | −1.68 ± 0.56 c | 43.00 ± 0.63 c |
| DUAT-28/40 | 2482.59 ± 37.21 a | 0.47 ± 0.01 a | 349.69 ± 35.53 a | 0.152 ± 0.01 a | 49.80 ± 0.75 a | 0.72 ± 0.64 a | −0.78 ± 0.89 b | 49.84 ± 0.74 a |
| Free Amino Acids | Presentation of Taste | Treatments | ||||
|---|---|---|---|---|---|---|
| WT | RT | SUAT-28 | SUAT-40 | DUAT-28/40 | ||
| Aspartic acid (Asp) | umami | 10.56 ± 0.55 | 4.19 ± 0.10 | 5.27 ± 0.01 | 9.18 ± 0.32 | 13.12 ± 0.42 |
| Threonine (Thr) | sweet | 28.59 ± 0.16 | 12.59 ± 0.25 | 14.18 ± 0.17 | 26.31 ± 1.18 | 37.05 ± 0.42 |
| Serine (Ser) | sweet | 30.39 ± 0.30 | 19.96 ± 0.30 | 16.63 ± 0.12 | 55.25 ± 2.58 | 88.15 ± 2.65 |
| Glutamic acid (Glu) | umami | 29.95 ± 0.24 | 15.95 ± 0.29 | 7.48 ± 0.09 | ND | 64.67 ± 1.27 |
| Glycine (Gly) | umami, sweet | 38.17 ± 0.51 | 25.64 ± 0.46 | 22.50 ± 0.08 | 58.23 ± 2.29 | 237.45 ± 6.58 |
| Alanine (Ala) | umami, sweet | 80.07 ± 0.90 | 59.37 ± 1.11 | 57.93 ± 0.40 | 103.98 ± 4.59 | 137.14 ± 3.92 |
| Cysteine (Cys) | - | 2.00 ± 0.08 | 1.96 ± 0.09 | 1.43 ± 0.04 | 3.26 ± 0.17 | 6.08 ± 0.18 |
| Valine (Val) | bitter | 21.95 ± 0.29 | 10.81 ± 0.24 | 8.00 ± 0.10 | 8.86 ± 0.45 | 12.24 ± 0.27 |
| Methionine (Met) | bitter | 10.17 ± 0.10 | 7.64 ± 0.19 | 6.68 ± 0.19 | 6.08 ± 0.39 | 9.33 ± 0.16 |
| Isoleucine (Ile) | bitter | 12.76 ± 0.15 | 6.29 ± 0.11 | 4.92 ± 0.06 | 4.76 ± 0.21 | 6.72 ± 0.19 |
| Leucine (Leu) | bitter | 23.35 ± 0.25 | 11.71 ± 0.27 | 9.19 ± 0.06 | 9.56 ± 0.42 | 12.54 ± 0.25 |
| Tyrosine (Tyr) | bitter | 9.64 ± 0.04 | 6.26 ± 0.22 | 6.90 ± 0.09 | 6.21 ± 0.32 | 8.67 ± 0.24 |
| Phenylalanine (Phe) | bitter | 9.06 ± 0.16 | 6.55 ± 0.20 | 5.53 ± 0.10 | 5.34 ± 0.29 | 7.72 ± 0.25 |
| Lysine (Lys) | bitter | 29.71 ± 0.35 | 55.77 ± 1.35 | 8.46 ± 0.16 | 52.37 ± 2.82 | 171.82 ± 3.74 |
| Histidine (His) | bitter | 12.56 ± 0.19 | 13.40 ± 0.33 | 9.41 ± 0.08 | 34.10 ± 1.81 | 67.18 ± 1.57 |
| Arginine (Arg) | sweet | 0.66 ± 0.02 | 1.59 ± 0.07 | 0.04 ± 0.04 | 2.38 ± 0.18 | 2.63 ± 0.00 |
| Proline (Pro) | sweet | 15.82 ± 5.45 | 4.42 ± 1.07 | 5.13 ± 0.01 | 6.25 ± 1.04 | 33.93 ± 11.13 |
| Total | 365.40 ± 9.73 | 264.07 ± 6.66 | 189.66 ± 1.50 | 392.12 ± 19.04 | 916.44 ± 33.23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Bian, C.; Chu, Y.; Mei, J.; Xie, J. Effects of Dual-Frequency Ultrasound-Assisted Thawing Technology on Thawing Rate, Quality Properties, and Microstructure of Large Yellow Croaker (Pseudosciaena crocea). Foods 2022, 11, 226. https://doi.org/10.3390/foods11020226
Cheng H, Bian C, Chu Y, Mei J, Xie J. Effects of Dual-Frequency Ultrasound-Assisted Thawing Technology on Thawing Rate, Quality Properties, and Microstructure of Large Yellow Croaker (Pseudosciaena crocea). Foods. 2022; 11(2):226. https://doi.org/10.3390/foods11020226
Chicago/Turabian StyleCheng, Hao, Chuhan Bian, Yuanming Chu, Jun Mei, and Jing Xie. 2022. "Effects of Dual-Frequency Ultrasound-Assisted Thawing Technology on Thawing Rate, Quality Properties, and Microstructure of Large Yellow Croaker (Pseudosciaena crocea)" Foods 11, no. 2: 226. https://doi.org/10.3390/foods11020226






