The Genetic Architecture of Meat Quality Traits in a Crossbred Commercial Pig Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Samples and Meat Quality Traits Phenotyping
2.3. Genotyping and Quality Control
2.4. Population Structure and Linkage Disequilibrium Estimation
2.5. Genome-Wide Association Study
2.6. Estimation of Heritability and Phenotypic Variance
2.7. Functional Candidate Genes Search
3. Results and Discussion
3.1. Phenotype Statistic and Heritability Estimation
3.2. Assessment of Population Structure and Linkage Disequilibrium Decay
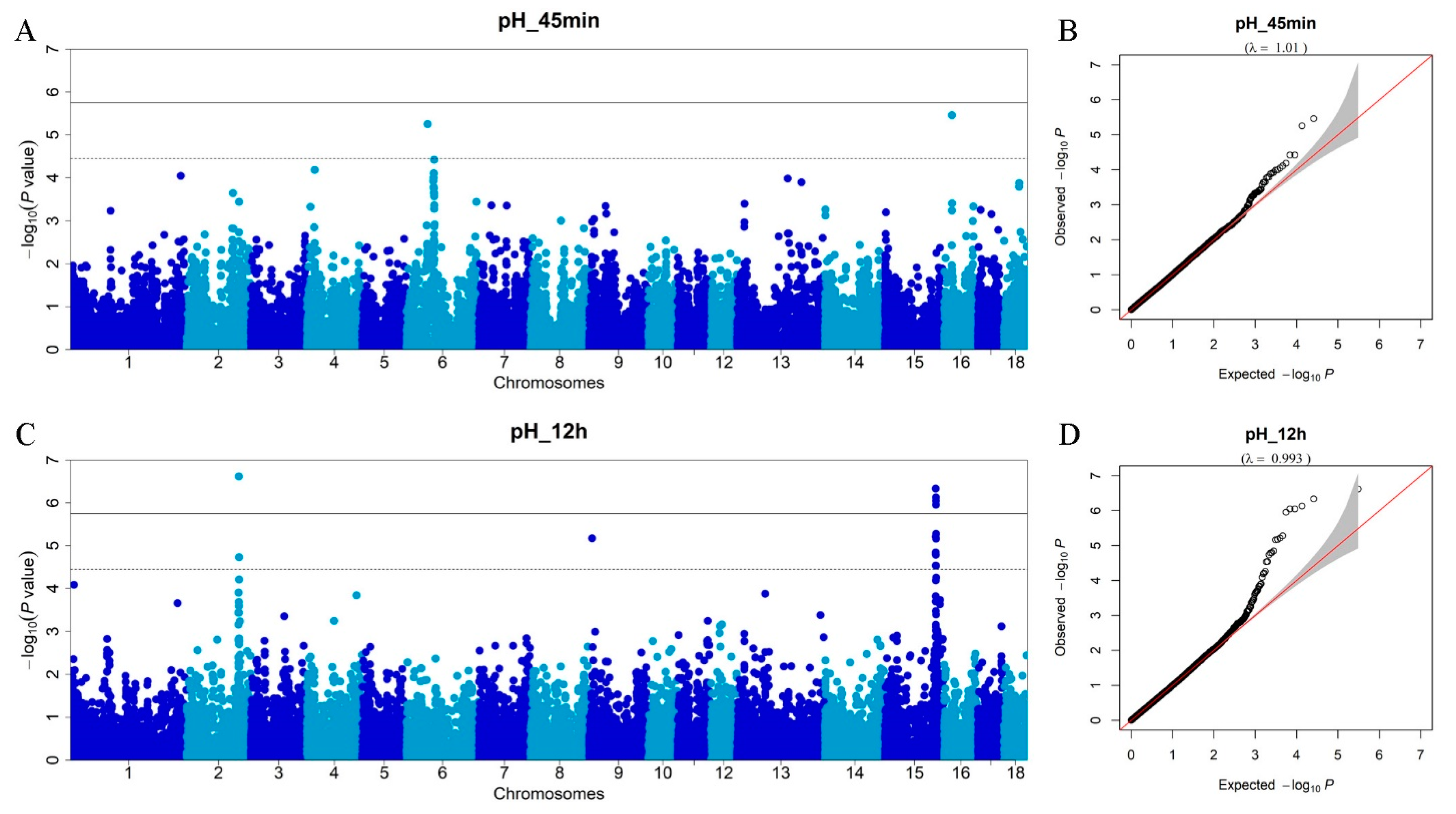
3.3. Genome-Wide Association Studies for Meat pH
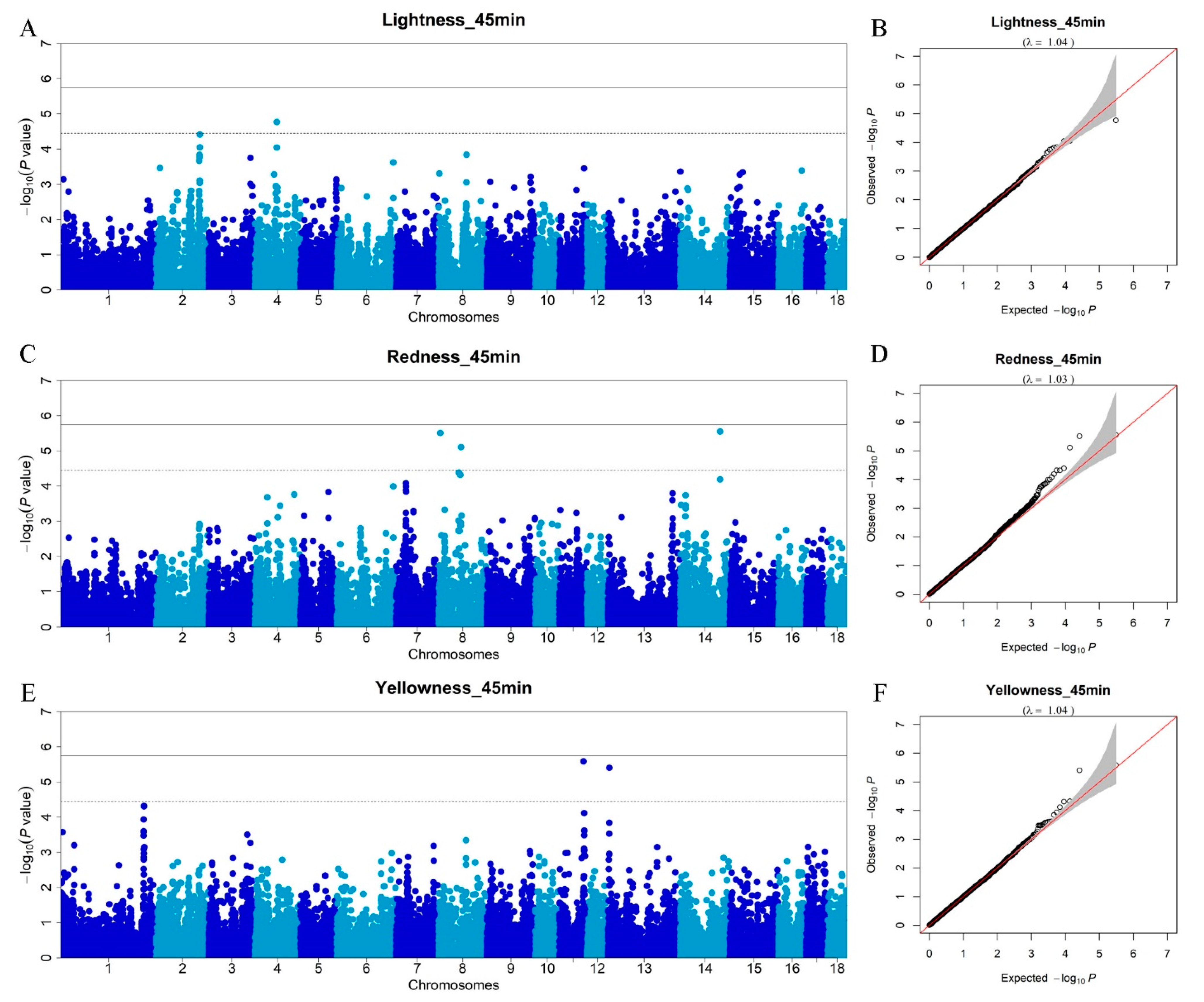
3.4. Genome-Wide Association Studies for Meat Color Traits
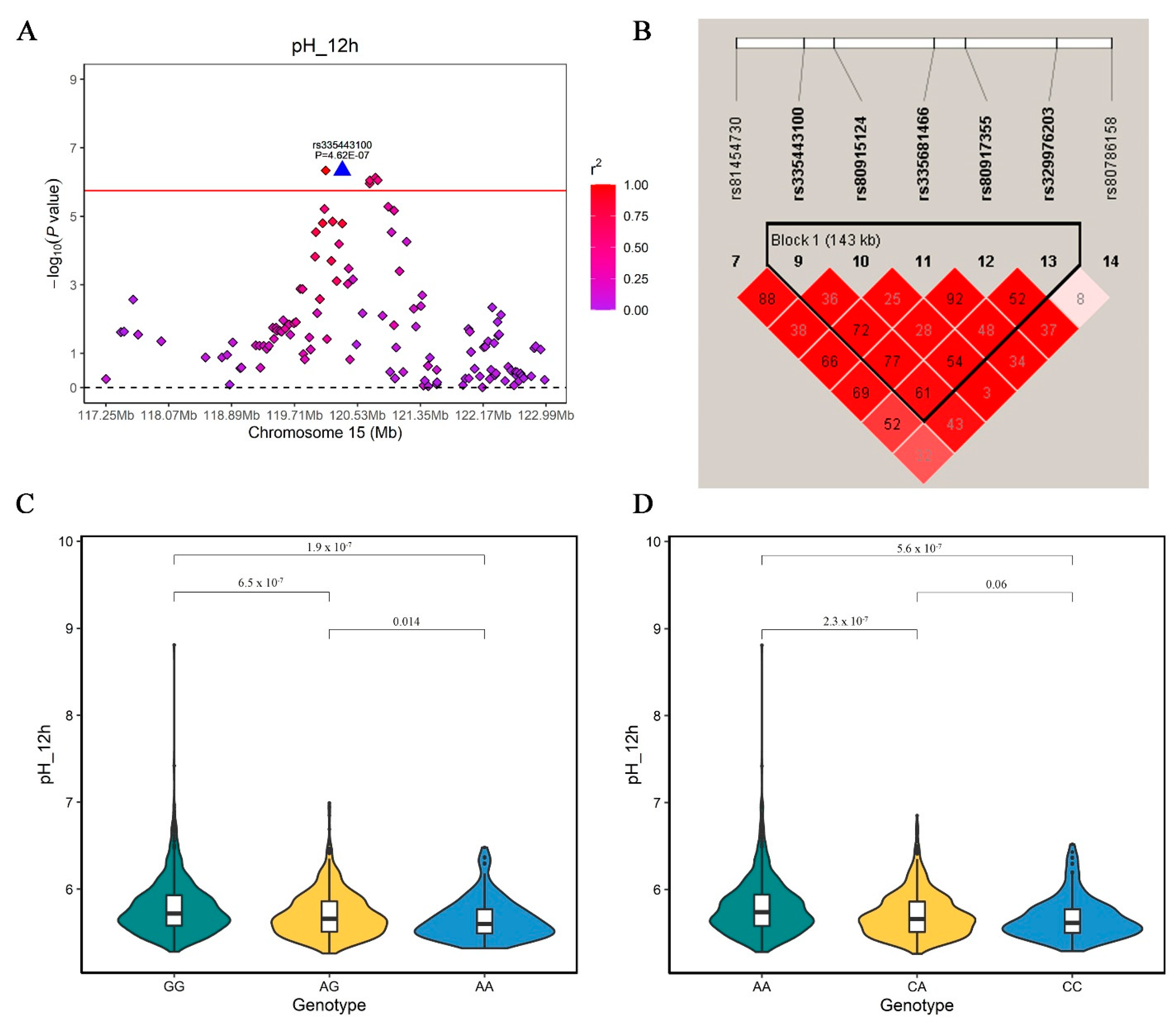
3.5. Constant QTL for Meat pH on SSC15 Detected by GWAS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, L.; Gan, M.; Chen, L.; Zhao, Y.; Niu, L.; Tang, G.; Jiang, Y.; Zhang, T.; Zhang, S.; Zhu, L. miR-152 targets pyruvate kinase to regulate the glycolytic activity of pig skeletal muscles and affects pork quality. Meat Sci. 2022, 185, 108707. [Google Scholar] [CrossRef]
- Hamoen, J.R.; Vollebregt, H.M.; van der Sman, R.G. Prediction of the time evolution of pH in meat. Food Chem. 2013, 141, 2363–2372. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Yang, F.X.; Hao, J.P.; Hou, Z.C. Genomic selection for meat quality traits in Pekin duck. Anim. Genet. 2022, 53, 94–100. [Google Scholar] [CrossRef]
- Lopes, F.B.; Baldi, F.; Passafaro, T.L.; Brunes, L.C.; Costa, M.F.O.; Eifert, E.C.; Narciso, M.G.; Rosa, G.J.M.; Lobo, R.B.; Magnabosco, C.U. Genome-enabled prediction of meat and carcass traits using Bayesian regression, single-step genomic best linear unbiased prediction and blending methods in Nelore cattle. Animal 2021, 15, 100006. [Google Scholar] [CrossRef]
- Magalhaes, A.F.B.; Schenkel, F.S.; Garcia, D.A.; Gordo, D.G.M.; Tonussi, R.L.; Espigolan, R.; Silva, R.M.O.; Braz, C.U.; Fernandes Junior, G.A.; Baldi, F.; et al. Genomic selection for meat quality traits in Nelore cattle. Meat Sci. 2019, 148, 32–37. [Google Scholar] [CrossRef]
- Khanal, P.; Maltecca, C.; Schwab, C.; Gray, K.; Tiezzi, F. Genetic parameters of meat quality, carcass composition, and growth traits in commercial swine. J. Anim. Sci. 2019, 97, 3669–3683. [Google Scholar] [CrossRef]
- Miar, Y.; Plastow, G.S.; Moore, S.S.; Manafiazar, G.; Charagu, P.; Kemp, R.A.; Van Haandel, B.; Huisman, A.E.; Zhang, C.Y.; McKay, R.M.; et al. Genetic and phenotypic parameters for carcass and meat quality traits in commercial crossbred pigs. J. Anim. Sci. 2014, 92, 2869–2884. [Google Scholar] [CrossRef] [Green Version]
- Davoli, R.; Braglia, S. Molecular approaches in pig breeding to improve meat quality. Brief. Funct. Genom. Proteom. 2007, 6, 313–321. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Prim. 2021, 1, 59. [Google Scholar] [CrossRef]
- Hu, Z.L.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2022, 50, D956–D961. [Google Scholar] [CrossRef]
- Otto, G.; Roehe, R.; Looft, H.; Thoelking, L.; Knap, P.W.; Rothschild, M.F.; Plastow, G.S.; Kalm, E. Associations of DNA markers with meat quality traits in pigs with emphasis on drip loss. Meat Sci. 2007, 75, 185–195. [Google Scholar] [CrossRef]
- Ciobanu, D.; Bastiaansen, J.; Malek, M.; Helm, J.; Woollard, J.; Plastow, G.; Rothschild, M. Evidence for new alleles in the protein kinase adenosine monophosphate-activated gamma(3)-subunit gene associated with low glycogen content in pig skeletal muscle and improved meat quality. Genetics 2001, 159, 1151–1162. [Google Scholar] [CrossRef]
- Milan, D.; Jeon, J.T.; Looft, C.; Amarger, V.; Robic, A.; Thelander, M.; Rogel-Gaillard, C.; Paul, S.; Iannuccelli, N.; Rask, L.; et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 2000, 288, 1248–1251. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Zhou, L.; Ren, J.; Liu, X.; Zhang, H.; Yang, B.; Zhang, Z.; Ma, H.; Xie, X.; et al. A splice mutation in the PHKG1 gene causes high glycogen content and low meat quality in pig skeletal muscle. PLoS Genet. 2014, 10, e1004710. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhou, L.; Xie, X.; Wu, Z.; Xiong, X.; Zhang, Z.; Yang, J.; Xiao, S.; Zhou, M.; Ma, J.; et al. Muscle glycogen level and occurrence of acid meat in commercial hybrid pigs are regulated by two low-frequency causal variants with large effects and multiple common variants with small effects. Genet. Sel. Evol. GSE 2019, 51, 46. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Liu, X.; Zhou, L.; Yang, J.; Yang, B.; Ma, H.; Xie, X.; Huang, Y.; Fang, S.; Xiao, S.; et al. Genome-wide association analysis reveals genetic loci and candidate genes for meat quality traits in Chinese Laiwu pigs. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2015, 26, 181–190. [Google Scholar] [CrossRef]
- Cho, I.C.; Yoo, C.K.; Lee, J.B.; Jung, E.J.; Han, S.H.; Lee, S.S.; Ko, M.S.; Lim, H.T.; Park, H.B. Genome-wide QTL analysis of meat quality-related traits in a large F2 intercross between Landrace and Korean native pigs. Genet. Sel. Evol. GSE 2015, 47, 7. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhou, L.; Zhang, J.; Liu, X.; Zhang, Y.; Cai, L.; Zhang, W.; Cui, L.; Yang, J.; Ji, J.; et al. A large-scale comparison of meat quality and intramuscular fatty acid composition among three Chinese indigenous pig breeds. Meat Sci. 2020, 168, 108182. [Google Scholar] [CrossRef]
- Ozsensoy, Y.; Sahin, S. Comparison of different DNA isolation methods and use of dodecyle trimethyl ammonium bromide (DTAB) for the isolation of DNA from meat products. J. Adv. Vet. Anim. Res. 2016, 3, 368–374. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Ai, H.; Huang, L.; Ren, J. Genetic diversity, linkage disequilibrium and selection signatures in chinese and Western pigs revealed by genome-wide SNP markers. PLoS ONE 2013, 8, e56001. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Zhou, L.; Huang, Y.; Zheng, M.; Liu, X.; Zhang, Y.; Huang, C.; Peng, S.; Zeng, Q.; Zhong, L.; et al. A whole-genome sequence based association study on pork eating quality traits and cooking loss in a specially designed heterogeneous F6 pig population. Meat Sci. 2018, 146, 160–167. [Google Scholar] [CrossRef]
- Unlu, G.; Gamazon, E.R.; Qi, X.; Levic, D.S.; Bastarache, L.; Denny, J.C.; Roden, D.M.; Mayzus, I.; Breyer, M.; Zhong, X.; et al. GRIK5 Genetically Regulated Expression Associated with Eye and Vascular Phenomes: Discovery through Iteration among Biobanks, Electronic Health Records, and Zebrafish. Am. J. Hum. Genet. 2019, 104, 503–519. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Gandin, V.; Sasaki, M.; McCracken, S.; Li, W.; Silvester, J.L.; Elia, A.J.; Wang, F.; Wakutani, Y.; Alexandrova, R.; et al. Largen: A molecular regulator of mammalian cell size control. Mol. Cell 2014, 53, 904–915. [Google Scholar] [CrossRef] [Green Version]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S.; Ami, G.O.H.; Web Presence Working Group. AmiGO: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Srikanth, K.; Won, S.; Son, J.H.; Park, J.E.; Park, W.; Chai, H.H.; Lim, D. Haplotype-Based Genome-Wide Association Study and Identification of Candidate Genes Associated with Carcass Traits in Hanwoo Cattle. Genes 2020, 11, 551. [Google Scholar] [CrossRef]
- Mateescu, R.G.; Garrick, D.J.; Reecy, J.M. Network Analysis Reveals Putative Genes Affecting Meat Quality in Angus Cattle. Front. Genet. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.A. Applied and Emerging Methods for Meat Tenderization: A Comparative Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 841–859. [Google Scholar] [CrossRef] [Green Version]
- Jonnalagadda, V.G.; Ram Raju, A.V.; Pittala, S.; Shaik, A.; Selkar, N.A. The prelude on novel receptor and ligand targets involved in the treatment of diabetes mellitus. Adv. Pharm. Bull. 2014, 4, 209–217. [Google Scholar]
- Schaeren-Wiemers, N.; Bonnet, A.; Erb, M.; Erne, B.; Bartsch, U.; Kern, F.; Mantei, N.; Sherman, D.; Suter, U. The raft-associated protein MAL is required for maintenance of proper axon--glia interactions in the central nervous system. J. Cell Biol. 2004, 166, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Xuan, M.F.; Luo, Z.B.; Wang, J.X.; Jin, S.S.; Yin, X.J.; Kang, J.D. Baicalin improves IVM of pig oocytes and subsequent preimplantation embryo development by inhibiting apoptosis. Reprod. Fertil. Dev. 2019, 31, 983–992. [Google Scholar] [CrossRef]
- Knudson, C.B.; Knudson, W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 2001, 12, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Hou, L.; Zhou, W.; Wang, B.; Han, P.; Gao, C.; Niu, P.; Zhang, Z.; Li, Q.; Huang, R.; et al. Genome-Wide Association Study and FST Analysis Reveal Four Quantitative Trait Loci and Six Candidate Genes for Meat Color in Pigs. Front. Genet. 2022, 13, 768710. [Google Scholar] [CrossRef]
- Verardo, L.L.; Sevon-Aimonen, M.L.; Serenius, T.; Hietakangas, V.; Uimari, P. Whole-genome association analysis of pork meat pH revealed three significant regions and several potential genes in Finnish Yorkshire pigs. BMC Genet. 2017, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Piórkowska, K.; Tyra, M.; Żukowski, K.; Ropka-Molik, K. The SSC15 QTL-Rich Region Mutations Affecting Intramuscular Fat and Production Traits in Pigs. Ann. Anim. Sci. 2020, 20, 425–444. [Google Scholar] [CrossRef]
- Bernau, K.; Torr, E.E.; Evans, M.D.; Aoki, J.K.; Ngam, C.R.; Sandbo, N. Tensin 1 Is Essential for Myofibroblast Differentiation and Extracellular Matrix Formation. Am. J. Respir. Cell Mol. Biol. 2017, 56, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Ye, B.; Liu, B.; Hao, L.; Zhu, X.; Yang, L.; Wang, S.; Xia, P.; Du, Y.; Meng, S.; Huang, G.; et al. Klf4 glutamylation is required for cell reprogramming and early embryonic development in mice. Nat. Commun. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]


| Traits | N | Mean (±SD) | h2 (±SE) |
|---|---|---|---|
| pH_45min | 1480 | 6.33 ± 0.32 | 0.17 ± 0.03 |
| L*_45min | 1518 | 43.86 ± 2.42 | 0.12 ± 0.04 |
| a*_45min | 1516 | −0.71 ± 1.02 | 0.03 ± 0.03 |
| b*_45min | 1517 | 7.31 ± 1.22 | 0.05 ± 0.03 |
| pH_12h | 1498 | 5.73 ± 0.28 | 0.12 ± 0.04 |
| L*_12h | 1515 | 48.4 ± 4.05 | 0.12 ± 0.04 |
| a*_12h | 1515 | −0.46 ± 1.10 | 0.06 ± 0.03 |
| b*_12h | 1512 | 7.83 ± 1.45 | 0.03 ± 0.03 |
| Traits | Chr | SNP ID | Position (bp) | MAF | p-Value | R2 (%) a | Nearest Gene | Distance (bp) |
|---|---|---|---|---|---|---|---|---|
| pH_45min | 6 | rs81274518 | 49883373 | 0.36 | 5.56 × 10−6 | 2.36 | GRIK5 | within |
| 16 | rs81324442 | 18478368 | 0.46 | 3.46 × 10−6 | 1.27 | MTMR12 | within | |
| L*_45min | 4 | rs80971313 | 63076056 | 0.40 | 1.70 × 10−5 | 0.90 | KCNB2 | within |
| a*_45min | 8 | rs332726079 | 5403889 | 0.22 | 3.09 × 10−6 | 0.10 | STK32B | 14440 |
| 8 | rs81400902 | 64388531 | 0.28 | 7.81 × 10−6 | 0.47 | / | / | |
| 14 | rs80944912 | 113938495 | 0.11 | 2.81 × 10−6 | 0.01 | CNNM2 | within | |
| b*_45min | 11 | rs342146775 | 69898991 | 0.42 | 2.59 × 10−6 | 1.01 | NALCN | within |
| 13 | rs343103580 | 3546365 | 0.12 | 3.95 × 10−6 | 0.37 | RFTN1 | within |
| Traits | Chr | SNP ID | Position (bp) | MAF | p-Value | R2 (%)a | Nearest Gene | Distance (bp) |
|---|---|---|---|---|---|---|---|---|
| pH_12h | 2 | rs81303631 | 123600292 | 0.26 | 2.40 × 10−7 | 0.72 | FAM170A | 133583 |
| 2 | rs81295472 | 123998374 | 0.12 | 1.86 × 10−5 | 0.31 | PRR16 | 201134 | |
| 9 | rs81316230 | 6815282 | 0.23 | 6.68 × 10−6 | 0.31 | PHOX2A | 2903 | |
| 15 | rs81454672 | 119995203 | 0.30 | 2.91 × 10−5 | 1.33 | TNS1 | within | |
| 15 | rs81454730 | 120083397 | 0.29 | 1.59 × 10−5 | 1.61 | TNS1 | within | |
| 15 | rs80818610 | 120106066 | 0.48 | 6.13 × 10−6 | 1.68 | TNS1 | within | |
| 15 | rs335443100 | 120121891 | 0.30 | 4.62 × 10−7 | 1.94 | TNS1 | within | |
| 15 | rs80917355 | 120213666 | 0.35 | 1.43 × 10−5 | 1.52 | RUFY4 | 204 | |
| 15 | rs338238642 | 120337815 | 0.33 | 1.63 × 10−5 | 1.61 | GPBAR1 | within | |
| 15 | rs80816788 | 120696351 | 0.33 | 1.10 × 10−6 | 2.52 | / | / | |
| 15 | rs320130359 | 120699144 | 0.33 | 8.96 × 10−7 | 2.52 | / | / | |
| 15 | / | 120770590 | 0.32 | 7.44 × 10−7 | 2.67 | TTLL4 | 2595 | |
| 15 | / | 120801238 | 0.32 | 8.91 × 10−7 | 2.67 | TTLL4 | within | |
| 15 | rs345318543 | 120938602 | 0.35 | 5.27 × 10−6 | 2.04 | WNT10A | 172 | |
| 15 | / | 120982452 | 0.32 | 2.93 × 10−5 | 1.89 | CDK5R2 | 2793 | |
| 15 | rs81218648 | 121014341 | 0.28 | 6.87 × 10−6 | 2.46 | CRYBA2 | within | |
| L*_12h | 9 | rs81316230 | 6815282 | 0.23 | 1.38 × 10−5 | 0.40 | PHOX2A | 2903 |
| 11 | rs80993821 | 71619677 | 0.11 | 2.95 × 10−5 | 0.62 | SLC10A2 | 270898 | |
| 14 | rs80985792 | 27342214 | 0.20 | 3.32 × 10−5 | 0.98 | / | / | |
| a*_12h | 2 | rs81303631* | 123600292 | 0.26 | 9.18 × 10−5 | 0.11 | FAM170A | 133583 |
| b*_12h | 4 | rs343786555 | 17678626 | 0.08 | 3.84 × 10−7 | 0.01 | SHAS2 | 3040 |
| 5 | rs346116771 | 92003197 | 0.05 | 9.56 × 10−7 | 0.09 | EPYC | 125750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Z.; Wu, J.; Xu, C.; Ruan, D.; Qiu, Y.; Zhou, S.; Ding, R.; Quan, J.; Yang, M.; Zheng, E.; et al. The Genetic Architecture of Meat Quality Traits in a Crossbred Commercial Pig Population. Foods 2022, 11, 3143. https://doi.org/10.3390/foods11193143
Zhuang Z, Wu J, Xu C, Ruan D, Qiu Y, Zhou S, Ding R, Quan J, Yang M, Zheng E, et al. The Genetic Architecture of Meat Quality Traits in a Crossbred Commercial Pig Population. Foods. 2022; 11(19):3143. https://doi.org/10.3390/foods11193143
Chicago/Turabian StyleZhuang, Zhanwei, Jie Wu, Cineng Xu, Donglin Ruan, Yibin Qiu, Shenping Zhou, Rongrong Ding, Jianping Quan, Ming Yang, Enqin Zheng, and et al. 2022. "The Genetic Architecture of Meat Quality Traits in a Crossbred Commercial Pig Population" Foods 11, no. 19: 3143. https://doi.org/10.3390/foods11193143





