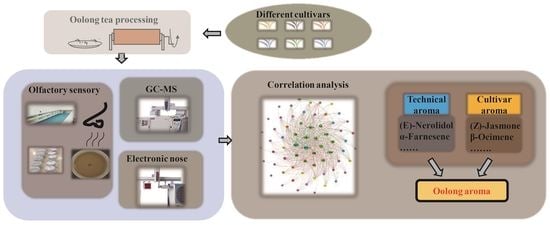
Study on the Suitability of Tea Cultivars for Processing Oolong Tea from the Perspective of Aroma Based on Olfactory Sensory, Electronic Nose, and GC-MS Data Correlation Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Manufacturing Process of Oolong Tea
2.2. Aroma Quality Assessment by Experts
2.3. Acceptance Assessment by Consumers
2.4. Electronic Nose Detection
2.5. Extraction and Analysis of Volatile Compounds in Oolong Tea
2.6. Data Processing
3. Results and Discussion
3.1. Olfactory Sensory Evaluation of Different Oolong Tea Samples
3.2. Electronic Nose Detection of Different Oolong Tea Samples
3.3. Volatile Detection of Different Oolong Tea Samples
3.4. Association Analysis and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Zeng, T.; Zhao, S.; Zhu, Y.; Feng, C.; Zhan, J.; Li, S.; Ho, C.; Gosslau, A. Multifunctional Health-Promoting Effects of Oolong Tea and Its Products. Food Sci. Hum. Wellness 2022, 11, 512–523. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, X.; Su, X.; Yang, Z. Chinese Oolong Tea: An Aromatic Beverage Produced under Multiple Stresses. Trends Food Sci. Technol. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Hu, C.-J.; Li, D.; Ma, Y.; Zhang, W.; Lin, C.; Zheng, X.; Liang, Y.; Lu, J. Formation Mechanism of the Oolong Tea Characteristic Aroma During Bruising and Withering Treatment. Food Chem. 2018, 269, 202–211. [Google Scholar] [CrossRef]
- Yang, W.; He, W.; Zhang, J.; Zhu, X. Discussion on Specific Character of Processing Suitability to Oolong Tea Varieties. J. Tea Sci. 1993, 13, 93–99. [Google Scholar]
- Lin, C.; Song, Z.; Chen, J.; Wang, L.; Yu, W.; You, Z. Profiling the Functional Constituents in Camellia Sinensis Shoots Desired for Making Oolong Teas. Acta Tea Sinica 2018, 59, 193–198. [Google Scholar]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea Aroma Formation from Six Model Manufacturing Processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; de Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory Characteristics of Wholegrain and Bran-Rich Cereal Foods—A review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef]
- Karagül-Yüceer, Y.; Coggins, P.C.; Wilson, J.C.; White, C.H. Carbonated Yogurt—Sensory Properties and Consumer Acceptance. J. Dairy Sci. 1999, 82, 1394–1398. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of Electronic Nose (E-Nose) and Electronic Tongue (E-Tongue) in Food Quality-Related Properties Determination: A Review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Banerjee, M.B.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. Black Tea Classification Employing Feature Fusion of E-Nose and E-Tongue Responses. J. Food Eng. 2019, 244, 55–63. [Google Scholar] [CrossRef]
- Shunbo, Y.; Li, D.; Li, S.; Yang, H.; Zhao, Z. Gc-Ms Metabolite and Transcriptome Analyses Reveal the Differences of Volatile Synthesis and Gene Expression Profiling between Two Apple Varieties. Int. J. Mol. Sci. 2022, 23, 2939. [Google Scholar]
- Marek, G.; Dobrzański, B.; Malaga-Toboła, U.; Tabor, S.; Combrzyński, M.; Ćwikła, D.; Strobel, W.R.; Oniszczuk, A.; Karami, H.; Darvishi, Y.; et al. Impact of Coffee Bean Roasting on the Content of Pyridines Determined by Analysis of Volatile Organic Compounds. Molecules 2022, 27, 1559. [Google Scholar]
- Rusinek, R.; Kmiecik, D.; Gawrysiak-Witulska, M.; Malaga-Toboła, U.; Tabor, S.; Findura, P.; Siger, A.; Gancarz, M. Identification of the Olfactory Profile of Rapeseed Oil as a Function of Heating Time and Ratio of Volume and Surface Area of Contact with Oxygen Using an Electronic Nose. Sensors 2021, 21, 303. [Google Scholar] [CrossRef]
- Ziyin, Y.; Baldermann, S.; Watanabe, N. Recent Studies of the Volatile Compounds in Tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar]
- Yang, Y.; Liang, Y. Clonal Tea Cultivars of China, 1st ed.; Shanghai Science and Technology Press: Shanghai, China, 2014. [Google Scholar]
- Xia, T. Manufacture of Tea, 3rd ed.; China Agriculture Press: Beijing, China, 2014; Chapter 9. [Google Scholar]
- Li, Y.; He, C.; Yu, X.; Zhou, J.; Ntezimana, B.; Yu, Z.; Chen, Y.; Ni, D. Study on Improving Aroma Quality of Summer-Autumn Black Tea by Red-Light Irradiation During Withering. LWT 2022, 154, 112597. [Google Scholar] [CrossRef]
- Wang, C.; Lv, S.; Wu, Y.; Gao, X.; Li, J.; Zhang, W.; Meng, Q. Oolong Tea Made from Tea Plants from Different Locations in Yunnan and Fujian, China Showed Similar Aroma but Different Taste Characteristics. SpringerPlus 2016, 5, 576. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, C.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; Lin, Z. Enantiomeric and Quantitative Analysis of Volatile Terpenoids in Different Teas (Camellia sinensis). J. Chromatogr. A 2017, 1490, 177–190. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma Formation and Dynamic Changes During White Tea Processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef]
- Ho, C.; Zheng, X.; Li, S. Tea Aroma Formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of Aroma-Active Volatiles in Oolong Tea Infusions Using Gc–Olfactometry, Gc–Fpd, and Gc–Ms. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Gui, J.; Fu, X.; Zhou, Y.; Katsuno, T.; Mei, X.; Deng, R.; Xu, X.; Zhang, L.; Dong, F.; Watanabe, N.; et al. Does Enzymatic Hydrolysis of Glycosidically Bound Volatile Compounds Really Contribute to the Formation of Volatile Compounds During the Oolong Tea Manufacturing Process? J. Agric. Food Chem. 2015, 63, 6905–6914. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.; Shao, C.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of Key Odorants Responsible for Chestnut-Like Aroma Quality of Green Teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Schwab, W.; Ho, C.; Song, C.; Wan, X. Characterization of the Aroma Profiles of Oolong Tea Made from Three Tea Cultivars by Both Gc–Ms and Gc-Ims. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Fu, Y.; Wang, J.; Zhang, L.; Wang, F.; Yin, J.; Xu, Y. Sensory and Chemical Characteristics of Tieguanyin Oolong Tea after Roasting. Food Chem. X 2021, 12, 100178. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The Family of Terpene Synthases in Plants: A Mid-Size Family of Genes for Specialized Metabolism That Is Highly Diversified Throughout the Kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant Terpenes: Defense Responses, Phylogenetic Analysis, Regulation and Clinical Applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Zeng, L.; Liao, Y.; Li, J.; Zhou, Y.; Tang, J.; Dong, F.; Yang, Z. A-Farnesene and Ocimene Induce Metabolite Changes by Volatile Signaling in Neighboring Tea (Camellia sinensis) Plants. Plant Sci. 2017, 264, 29–36. [Google Scholar] [CrossRef]
- Chen, S.; Xie, P.; Li, Y.; Wang, X.; Liu, H.; Wang, S.; Han, W.; Wu, R.; Li, X.; Guan, Y.; et al. New Insights into Stress-Induced Β-Ocimene Biosynthesis in Tea (Camellia sinensis) Leaves During Oolong Tea Processing. J. Agric. Food Chem. 2021, 69, 11656–11664. [Google Scholar] [CrossRef]
- Su, D.; He, J.; Zhou, Y.; Li, Y.; Zhou, H. Aroma Effects of Key Volatile Compounds in Keemun Black Tea at Different Grades: Hs-Spme-Gc-Ms, Sensory Evaluation, and Chemometrics. Food Chem. 2022, 373, 131587. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Z.; Zhao, C.; Kong, H.; Lu, X.; Xu, G. A Comparative Study of Volatile Components in Green, Oolong and Black Teas by Using Comprehensive Two-Dimensional Gas Chromatography–Time-of-Flight Mass Spectrometry and Multivariate Data Analysis. J. Chromatogr. A 2013, 1313, 245–252. [Google Scholar] [CrossRef]
- Cai, X.; Mai, R.; Zou, J.; Zhang, H.; Zeng, X.; Zheng, R.; Wang, C. Analysis of Aroma-Active Compounds in Three Sweet Osmanthus (Osmanthus fragrans) Cultivars by Gc-Olfactometry and Gc-Ms. J. Zhejiang Univ.-Sci. B 2014, 15, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Jasmonate-Induced Defenses: A Tale of Intelligence, Collaborators and Rascals. Trends Plant Sci. 2011, 16, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Oliveira, J.N.; da Costa, J.G.; Loza-Reyes, E.; Bleicher, E.; Santana, A.E.G.; Caulfield, J.C.; Mayon, P.; Dewhirst, S.Y.; Bruce, T.J.A.; et al. Aphid Antixenosis in Cotton Is Activated by the Natural Plant Defence Elicitor Cis-Jasmone. Phytochemistry 2012, 78, 81–88. [Google Scholar] [CrossRef] [PubMed]
- David, Y.C.; Ylagan, J.B.; Gonzales, H.A.; Chan, J.M.P.; Mondragon, J.M.S.; Tavera, M.A.A.; Redillas, M.C.F.R. Volatile Organic Compound Profiling of Var. Grown under Different Concentrations of Nitrogen. Hell. Plant Prot. J. 2021, 14, 77–88. [Google Scholar]
- Hirao, T.; Okazawa, A.; Harada, K.; Kobayashi, A.; Muranaka, T.; Hirata, K. Green Leaf Volatiles Enhance Methyl Jasmonate Response in Arabidopsis. J. Biosci. Bioeng. 2012, 114, 540–545. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the Key Aroma Compounds in the Beverage Prepared from Darjeeling Black Tea: Quantitative Differences between Tea Leaves and Infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The Tomato Carotenoid Cleavage Dioxygenase 1 Genes Contribute to the Formation of the Flavor Volatiles Β-Ionone, Pseudoionone, and Geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma Characterization of Chinese Rice Wine by Gas Chromatography–Olfactometry, Chemical Quantitative Analysis, and Aroma Reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef]
- Yang, P.; Yu, M.; Song, H.; Xu, Y.; Lin, Y.; Granvogl, M. Characterization of Key Aroma-Active Compounds in Rough and Moderate Fire Rougui Wuyi Rock Tea (Camellia sinensis) by Sensory-Directed Flavor Analysis and Elucidation of the Influences of Roasting on Aroma. J. Agric. Food Chem. 2021, 70, 267–278. [Google Scholar] [CrossRef]
- Ijichi, C.; Wakabayashi, H.; Sugiyama, S.; Hayashi, K.; Ihara, Y.; Nishijima, H.; Touhara, K.; Kondo, K. Odorant metabolism of the olfactory cleft mucus in idiopathic olfactory impairment patients and healthy volunteers. Int. Forum Allergy Rhinol. 2021, 12, 293–301. [Google Scholar] [CrossRef]
- Da Costa, N.C.; Anastasiou, T.J. Analysis of Volatiles in Limoncello Liqueur and Aging Study with Sensory. In Flavors in Noncarbonated Beverages; American Chemical Society: Washington, DC, USA, 2010; pp. 177–193. [Google Scholar]
- Tokitomo, Y.; Shimono, Y.; Kobayashi, A.; Yamanishi, T. Aroma Components of Baelfruit (Aegle Marmelos Correa). Agric. Biol. Chem. 1982, 46, 1873–1877. [Google Scholar] [CrossRef]
- Postma, W.; Kappers, I.; Dicke, M. Characterization of an Enzyme That Converts (3s)-(E)-Nerolidol to 4, 8-Dimethyl-1, 3 (E), 7-Nonatriene (Dmnt) in Cucumber (Cucumis Sativus L.) Upon Spider Mite (Tetranychus Urticae Koch) Infestation; Wageningen University: Wageningen, The Netherlands, 2005. [Google Scholar]
- Chen, X.; Xu, Y.; Wu, Z. High Hydrostatic Pressure and Co-Fermentation by Lactobacillus Rhamnosus and Gluconacetobacter Xylinus Improve Flavor of Yacon-Litchi-Longan Juice. Foods 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound-Assisted Extraction of Volatile Compounds from Citrus Flowers and Citrus Honey. Food Chem. 2003, 82, 575–582. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C. Rifm Fragrance Ingredient Safety Assessment, Hexyl Butyrate, Cas Registry Number 2639-63-6. Food Chem. Toxicol. 2019, 130, 110608. [Google Scholar] [CrossRef] [PubMed]
- Maraval, I.; Mestres, C.; Pernin, K.; Ribeyre, F.; Boulanger, R.; Guichard, E.; Gunata, Z. Odor-Active Compounds in Cooked Rice Cultivars from Camargue (France) Analyzed by Gc−O and Gc−Ms. J. Agric. Food Chem. 2008, 56, 5291–5298. [Google Scholar] [CrossRef] [PubMed]
- Guichard, E.; Barba, C.; Thomas-Danguin, T.; Tromelin, A. Multivariate Statistical Analysis and Odor–Taste Network to Reveal Odor–Taste Associations. J. Agric. Food Chem. 2020, 68, 10318–10328. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Smith, D.; Buchbauer, G. Aroma Compound Analysis of Eruca Sativa (Brassicaceae) Spme Headspace Leaf Samples Using Gc, Gc−Ms, and Olfactometry. J. Agric. Food Chem. 2002, 50, 4643–4646. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, L.; Cheng, X.; Gao, Y.; Zhang, X.; Yuan, X.; Xue, C.; Chen, X. Volatile Flavor Profile and Sensory Properties of Vegetable Soybean. Molecules 2022, 27, 939. [Google Scholar] [CrossRef]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.-S.; Kays, S.J. Comparison of Odor-Active Compounds from Six Distinctly Different Rice Flavor Types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [CrossRef]
- Wang, Y.; Kays, S. Contribution of Volatile Compounds to the Characteristic Aroma of Baked ‘Jewel’ Sweetpotatoes. J. Am. Soc. Hortic. Sci. 2000, 125, 638–643. [Google Scholar] [CrossRef]
- Leiser-Miller, L.B.; Kaliszewska, Z.A.; Lauterbur, M.E.; Mann, B.; Riffell, J.A.; Santana, S.E. A Fruitful Endeavor: Scent Cues and Echolocation Behavior Used by Carollia castanea to Find Fruit. Integr. Org. Biol. 2020, 2, obaa007. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, P.; Zhan, P.; Tian, H. Characterization of Key Aroma Compounds in Stewed Mutton (Goat Meat) Added with Thyme (Thymus Vulgaris L.) Based on the Combination of Instrumental Analysis and Sensory Verification. Food Chem. 2022, 371, 131111. [Google Scholar] [CrossRef]
- Janzantti, N.S.; Macoris, M.S.; Garruti, D.S.; Monteiro, M. Influence of the cultivation system in the aroma of the volatile compounds and total antioxidant activity of passion fruit. LWT Food Sci. Technol. 2012, 46, 511–518. [Google Scholar] [CrossRef]
- Wang, B.; Chen, H.; Qu, F.; Song, Y.; Di, T.; Wang, P.; Zhang, X. Identification of aroma-active components in black teas produced by six Chinese tea cultivars in high-latitude region by GC–MS and GC–O analysis. Eur. Food Res. Technol. 2021, 248, 647–657. [Google Scholar] [CrossRef]
- Ito, Y.; Sugimoto, A.; Kakuda, T.; Kubota, K. Identification of Potent Odorants in Chinese Jasmine Green Tea Scented with Flowers of Jasminum sambac. J. Agric. Food Chem. 2002, 50, 4878–4884. [Google Scholar] [CrossRef] [PubMed]
- Sköld, M.; Karlberg, A.; Matura, M.; Börje, A. The Fragrance Chemical Β-Caryophyllene—Air Oxidation and Skin Sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Kumazawa, K. Characterization of the Potent Odorants Contributing to the Characteristic Aroma of Chinese Green Tea Infusions by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2014, 62, 8308–8313. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wu, M. Characterization of Key Aroma Compounds from Different Rose Essential Oils Using Gas Chromatography-Mass Spectrometry, Gas Chromatography–Olfactometry and Partial Least Squares Regression. Nat. Prod. Res. 2018, 32, 1567–1572. [Google Scholar] [CrossRef]
- Hong, K.; Xu, Z.; Wang, L.; Johnpaul, A.; Cheng, Y.; Lv, C.; Ma, C. Varietal differences in the phytochemical components’ accumulation and aroma profile of three Humulus lupulus cultivars. Food Control 2021, 132, 108499. [Google Scholar] [CrossRef]
- Qi, D.; Miao, A.; Chen, W.; Wang, W.; He, X.; Ma, C. Characterization of the volatile compounds profile of the innovative broken oolong-black tea in comparison with broken oolong and broken black tea. Food Control 2021, 129, 108197. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Du, W.; Jin, L.; Ren, P.; Ren, F.; Xie, J.C. Comparative Analysis of Aroma Compounds in Chinese Traditional Dry-Rendered Fat by Hs/Gc-Ims, Spme/Gc-Ms, and Spme/Gc-O. J. Food Compos. Anal. 2022, 107, 104378. [Google Scholar] [CrossRef]
- Sutherland, O.; Hutchins, R. Attraction of newly hatched codling moth larvae (Laspeyresia pomonella) to synthetic stereo-isomers of farnesene. J. Insect Physiol. 1973, 19, 723–727. [Google Scholar] [CrossRef]
- Miyazawa, M.; Nakashima, Y.; Nakahashi, H.; Hara, N.; Nakagawa, H.; Usami, A.; Chavasiri, W. Volatile Compounds with Characteristic Odor of Essential Oil from Magnolia obovata Leaves by Hydrodistillation and Solvent-assisted Flavor Evaporation. J. Oleo Sci. 2015, 64, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, Z.; Gao, J.; Peng, J.; Wang, Z.; Zhao, Y.; Lin, Z.; Dai, W. Metabolomics Combined with Proteomics Provides a Novel Interpretation of the Compound Differences among Chinese Tea Cultivars (Camellia sinensis var. sinensis) with Different Manufacturing Suitabilities. Food Chem. 2022, 377, 131976. [Google Scholar] [CrossRef]
- Chang, S.-T.; Wang, S.-Y.; Kuo, Y.-H. Resources and bioactive substances from Taiwania (Taiwania cryptomerioides). J. Wood Sci. 2003, 49, 0001–0004. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Li, Y.; Zhou, J.; Yu, X.; Zhang, D.; Chen, Y.; Ni, D.; Yu, Z. Study on the Suitability of Tea Cultivars for Processing Oolong Tea from the Perspective of Aroma Based on Olfactory Sensory, Electronic Nose, and GC-MS Data Correlation Analysis. Foods 2022, 11, 2880. https://doi.org/10.3390/foods11182880
He C, Li Y, Zhou J, Yu X, Zhang D, Chen Y, Ni D, Yu Z. Study on the Suitability of Tea Cultivars for Processing Oolong Tea from the Perspective of Aroma Based on Olfactory Sensory, Electronic Nose, and GC-MS Data Correlation Analysis. Foods. 2022; 11(18):2880. https://doi.org/10.3390/foods11182880
Chicago/Turabian StyleHe, Chang, Yuchuan Li, Jingtao Zhou, Xinlei Yu, De Zhang, Yuqiong Chen, Dejiang Ni, and Zhi Yu. 2022. "Study on the Suitability of Tea Cultivars for Processing Oolong Tea from the Perspective of Aroma Based on Olfactory Sensory, Electronic Nose, and GC-MS Data Correlation Analysis" Foods 11, no. 18: 2880. https://doi.org/10.3390/foods11182880