Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products
Abstract
:1. Introduction
2. Functional Compounds: Definition, Importance and Trends
2.1. Organic Acids
2.2. Phenolic Compounds and Other Antioxidants
2.3. Oligosaccharides
2.4. Pectins
2.5. Bioactive Peptides
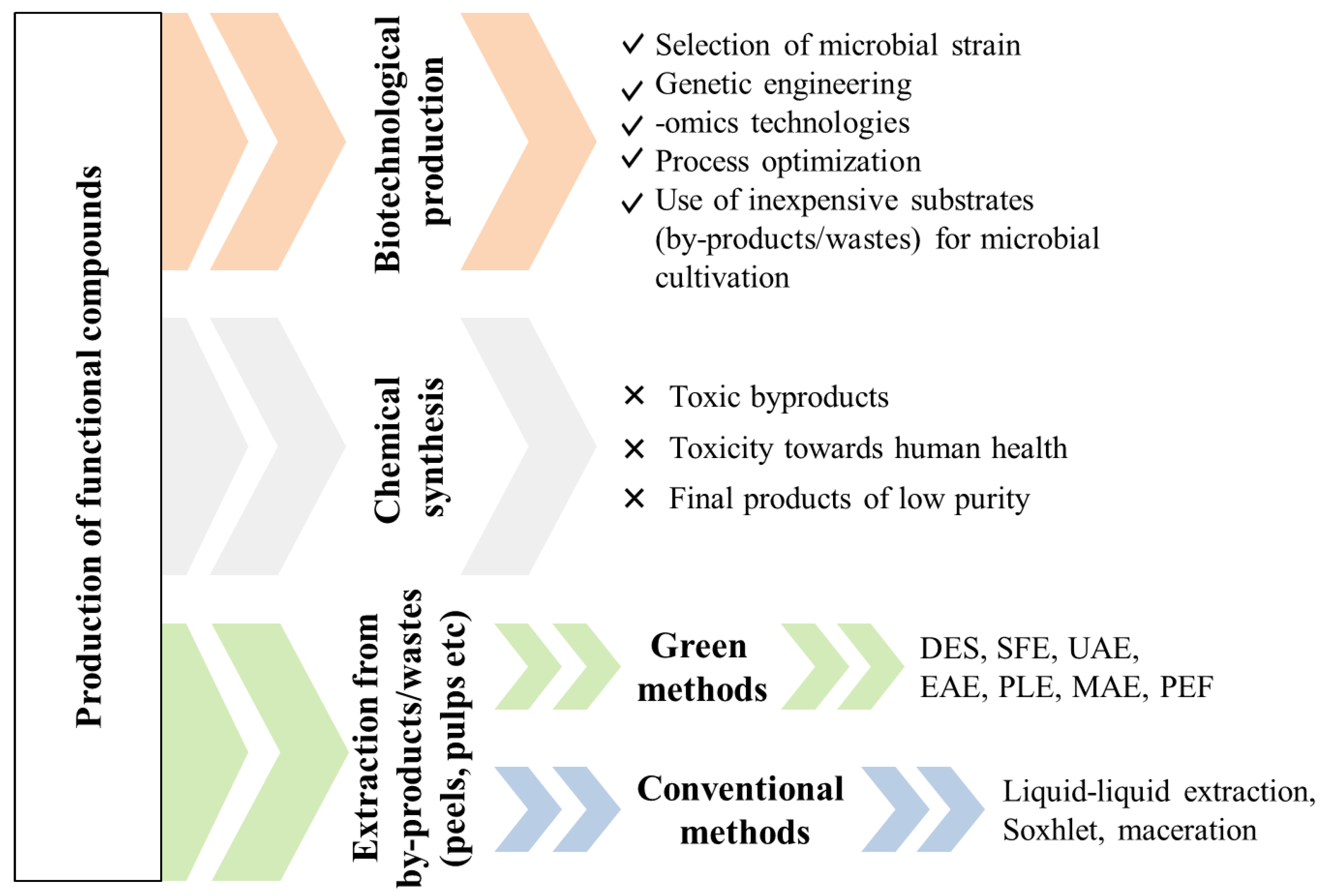
3. Chemical/Conventional Production of Functional Compounds
4. Strategies for the Production of Clean and Sustainable Compounds
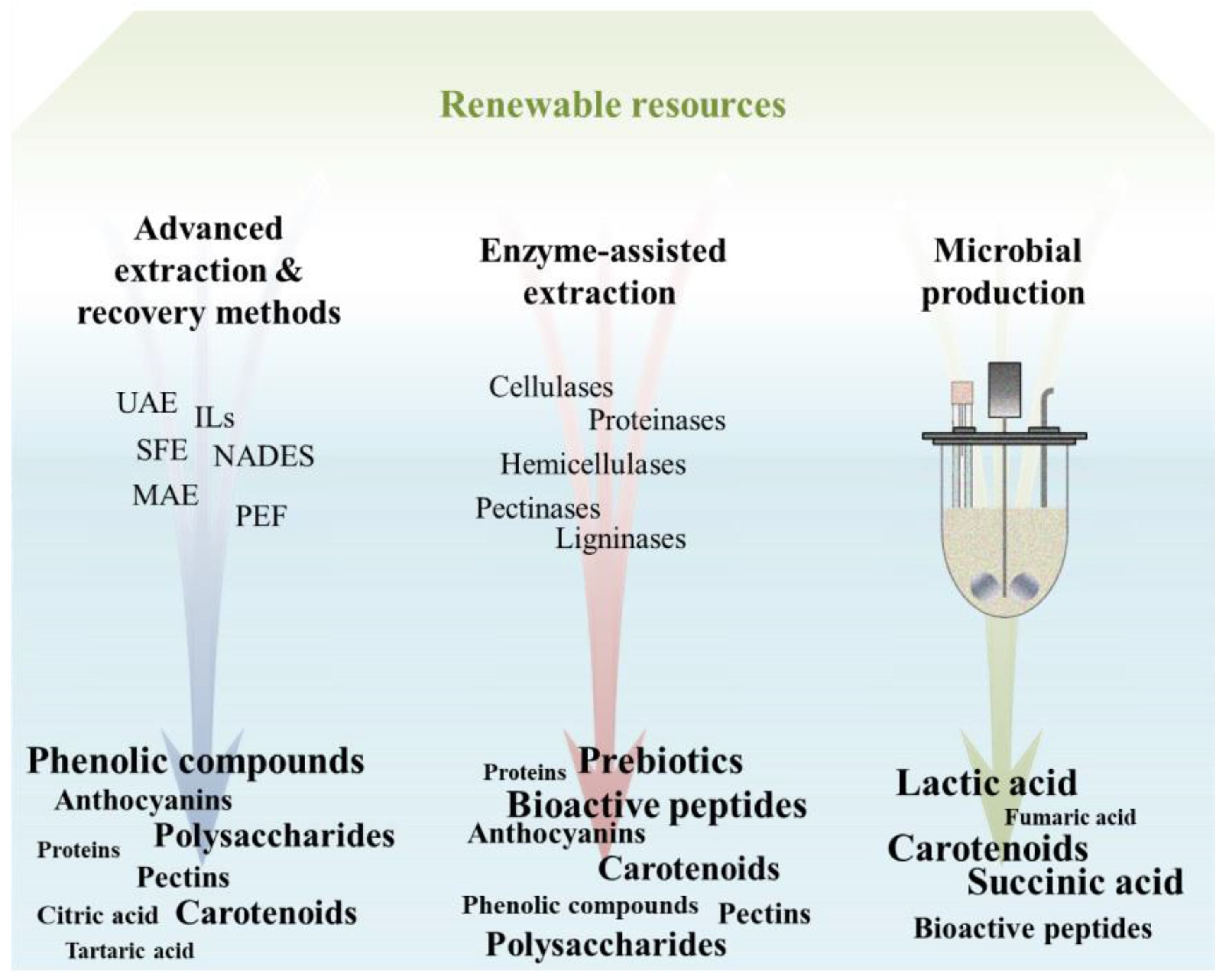
4.1. Advanced Extraction and Recovery Methods
| Raw Material | Method | Results | Reference |
|---|---|---|---|
| Crustacean waste streams | MW pretreatment with EOH at 140 °C, 300 W for 90 s and subsequent SFE extraction at 500 bar, 40 °C, 13 wt% EOH as co-solvent | Astaxanthin extraction yield 4.44 ± 0.41 μg/g dry residues | [94] |
| Passion fruit peels | Olive oil in combination with UAE at 47 °C, 100 W, for 39 min, and solid-to-oil ratio 1–30 g/100 mL | Carotenoids: 1241.95 μg/100 g peels (extraction efficiency of 91.4%) | [95] |
| Carrot juice processing waste | Flaxseed oil in combination with MAE at 165 W, for 9.39 min, solid-to-oil ratio 1:8.06 g/g | Carotenoids’ extraction efficiency 77.48% | [96] |
| Tomato processing by-products | PEF with 10 g sample at 5 kV/cm and 5 kJ/Kg and then extracted with ethyl lactate at 1:40 solid-to-liquid ratio | Lycopene: 9930 mg/kg DW | [97] |
| Bactris gasipaes fruit pulp | Aqueous solution of 140 mM of the IL [N1, 1, 1, 10]Br at 0.15 solid-to-liquid ratio for 8.2 min | Carotenoids: 88.7 ± 0.9 μg/g dried biomass | [58] |
| Shrimp waste | ILs-in-water microemulsion of [P4448]Br/(TX-100 + n-butanol)/water in combination with UAE (50 W, 60 min) | Astaxanthin extraction efficiency: 32.47 μg/g | [98] |
| Sunflower petals and florets | NADES composed of D,L-menthol: D,L-lactic acid (1:2), under (0.93:1 v/v solvent-to-water ratio and 0.15:10 w/v sunflower:liquid ratio under stirring at 200 rpm for 2 h, at 40 °C after enzymatic pretreatment with Viscozyme (0.58% v/v) | Carotenoids: 1449 mg/100 g biomass | [99] |
| Brown crab shell residues | NADES composed of menthol: myristic acid (8:1), NADES-to-waste ratio of 1: 0.25 (w/w) under stirring at 60 rpm, temperature 60 °C, 2 h extraction time | Astaxanthin:9.3 ± 0.8 μg/g dry residue | [100] |
| Shrimp residues | Solvent extraction with DES: 5% (m/v) choline chloride: Glycerol, 10 min incubation using UA at 50/60 Hz and 1:10 solid-to-liquid ratio | Total carotenoids: 737.69 μg/g; Astaxanthin content: 32.71 μg/g | [101] |
| Fermented shrimp waste | CO2-SFE with EOH at 300 bar, 60 °C and flow 6 mL/min | Astaxanthin: 0.52 μg/g | [102] |
| Shrimp wastes | UAE (30 s ON and 30 s OFF pulse sequence interval, 54.43% amplitude) and NADES (choline chloride:lactic acid at molar ratio 1:1.02) and 10% water, 1:10 solid-to-solvent ratio, 39.23 min extraction time | Astaxanthin: 68.98 ± 1.22 μg ASX/g | [103] |
| Pumpkin pulp | Canola oil as extraction solvent at 1:10 solid-to-oil ratio, at 60 °C, under stirring at 250 rpm and 21.8 min extraction time | Total carotenoids: 373.2 μg β-carotene equivalents/g dried pumpkin pulp | [104] |
| Beetroot waste | DES composed of chloride hexahydrate and urea (2:1) | Total betalain: 3.99 ± 0.26 mg/g | [105] |
| Raw Material | Method | Results | Reference |
|---|---|---|---|
| Corn stover | UAE (40 kHz, 360 W, 30 min) and hydrothermal pretreatment at 215 °C, for 1.5 h, followed by enzymatic hydrolysis with cellulase (15 FPU/g) with 2% solids at 50 °C | XOS yield: 80.40% (21.68% X2–X4, 58.72& X > 4) | [111] |
| Caragana korshinskii | Liquid hot water pretreatment, 160 °C, for 120 min with 4 g/L aqueous choline chloride and 1:20 solid-to-liquid ratio | XOS yield 36.59% | [112]) |
| Food processing wastes | UAE at 300 W, ambient temperature | galactolipids | [113] |
| Finger citron pomace | EA (0.25 mg/mL pectinase, at 45 °C, pH 4.5, 2 h) and UAE (1:50 solid-to-liquid ratio, pH = 1, 90 °C for 1.5 h) | Pectic oligosaccharides yield of 64.5% with 94.07% DPPH antioxidant activity value | [114] |
| Satsuma mandarin orange peels | Pressurized CO2 at 90 °C for 90 min and at pressures of 1–3 MPa with 40% solids | Pectic oligosaccharides with a yield of 3.8% and 94% DE | [109] |
| Artichoke by-products | UAE (pulsed mode, 2 s on/1 s off, 30% amplitude) with 13.9% Celluclast 1.5 L, 6 h extraction time, 6.5% w/v solid-to-liquid ratio, 50 °C, under constant stirring condition at 200 rpm | 10.9% pectin yield | [115] |
| Walnut processing waste | UAE at 200 W, for 10 min, at pH 1.5 with citric acid and 15 v/w liquid-to-solid ratio | Pectin extraction yield 12.78 ± 0.83% | [116] |
| Fenugreek seeds | High voltage atmospheric cold plasma with air at 80 kV for 30 min, followed by soaking at 5% NaCl, pH adjustment to 3 with acetic acid, 50 °C for 24 h, intermitted shaking at 250–300 rpm | 122% increase in extraction yield of galactomannans | [117] |
| Raw Material | Method | Results | Reference |
|---|---|---|---|
| Mixture of bioactive compounds | |||
| Capsicum annuum industrial waste | CO2-SFE, at 40 °C and 250 bar with EOH as modifier | Extraction yield: 10.08% TPC: 15.77 ± 0.51 mg GAE/g extract; TFC: 0.60 ± 0.02 mg RE/g extract β-carotene: 1.26 ± 0.01 mg/g extract; lycopene: 0.49 ± 0.02 mg/g extract | [120] |
| Orange peel waste | NADES composed of choline chloride and ethylene glycol | D-limonene: 0.5 mg/g; Polyphenols: 45.7 mg/g; Proteins: 7.7 mg/g | [121] |
| Melon peels | DES (sodium acetate: urea: water at molar ratio 1:3:1.6), 90 °C for 10 min | Oligosaccharides: 8.84 g/100 g (90% oligogalacturonides); TPC: 4.35 mg GAE/g; Protein: 2.87 g/100 g; Pectin: 7.95 g/100 g (49.44% galacturonic acid content) | [122] |
| Tomato peel waste | HHPE for pectin recovery with nitric acid at 80 °C, 300 MPa system pressure, 1:20 solid-to-liquid ratio for 30 min, and UAE for polyphenols extraction with 70% EOH, 400 W, 30 kHz, 95% amplitude, 1:50 solid-to-liquid ratio and 15 min extraction time | Pectin extraction yield: 9.1%; Total phenolics: 1625.7 mg/100 g | [123] |
| Black mulberry pomace | MAE at 700 W, 300 s irradiation time, pH equal to 1.42 adjusted with citric acid and 20 mL/g liquid-to-solid ratio | 10.95% pectin yield; 12.11% phenolics yield | [124] |
| Barley and canola straws | Subcritical water and pressurized 20% EOH at 180 °C, at 50 bar | TPC: 45.4 ± 1.8 mg GAE/g barley straw & 52.9 ± 2.0 mg GAE/g canola straw; Carbohydrates: 527.6 ± 0.5 mg GR/g barley straw and 442.7 ± 14.8 mg GE/g canola straw | [125] |
| Protein | |||
| Kitchen wastes | MW and PHWE, 1:30 w/w solid to liquid ratio, 800 rpm stirring, 850 W, 160 °C | Protein: 8.27 ± 0.13 g/100 g extract; TPC: 1.34 ± 0.02 g/100 g; Trolox equivalent antioxidant capacity: 0.96 ± 0.07 g/100 g | [126] |
| Brewer’s spent grain (BSG), | MAE at 110 °C, 10 min extraction time, 0.5 M NaOH | Protein extraction: 14.6 kg/100 kg | [127] |
| Bamboo shoots (TBS) and processing wastes (basal bamboo shoot (BBS) and sheat) | NADES synthesized by choline chloride and levulinic acid (molar ratio 6), at 80 °C, 50 min extraction time, 40% water content, 30 mg/L and 70 mg/mL solid-to-liquid ratio for TBS and BBS | Protein extraction yields: TBS: 39.16 ± 1.22 mg/g; BBS: 15.46 ± 0.30 mg/g; Sheath: 9.54 ± 0.17 mg/g | [128] |
| Shellfish waste streams (brown carb shell residues) | SubWE at 200 °C at 1:15 solid-to-liquid ratio, 15 min | Protein: 8.5 g/100 dry residue | [110] |
4.2. Enzymatic Recovery
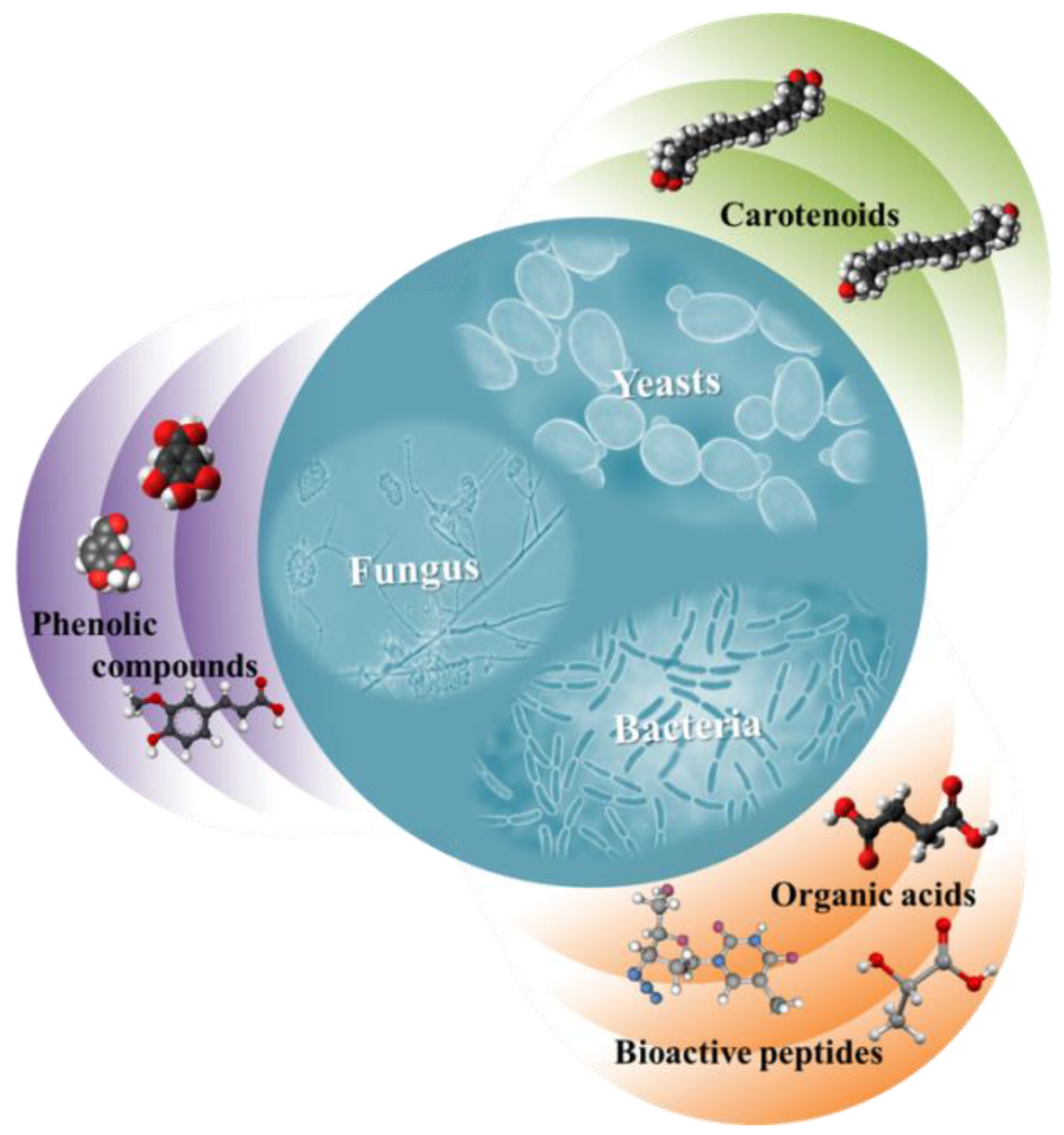
4.3. Microbial Production
5. Trends in the Application of Sustainable Functional Compounds toward the Development of Clean Label Foods
5.1. Applications as Food Additives and Preservatives
5.2. Applications as Health-Promoting Agents
5.3. Case Studies of Clean Label Products Using Sustainable Functional Compounds
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forbes, H.; Quested, T.; O’Connor, C. Food Waste Index Report 2021; Publisher UNEP—UN Environment Programme: Nairobi, Kenya, 2021; pp. 3–94. [Google Scholar]
- FAO. 2021. Available online: https://www.fao.org/platform-food-loss-waste/resources/detail/en/c/1378978/ (accessed on 30 June 2022).
- Zabaniotou, A.; Kamaterou, P.; Kachrimanidou, V.; Vlysidis, A.; Koutinas, A. Taking a reflexive TRL3-4 approach to sustainable use of sunflower meal for the transition from a mono-process pathway to a cascade biorefinery in the context of Circular Bioeconomy. J. Clean. Prod. 2018, 172, 4119–4129. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel approaches for the recovery of natural pigments with potential health effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- Guindani, C.; Podestá, R.; Block, J.M.; Rossi, M.J.; Mezzomo, N.; Ferreira, S.R.S. Valorization of chia (Salvia hispanica) seed cake by means of supercritical fluid extraction. J. Supercrit. Fluids 2016, 112, 67–75. [Google Scholar] [CrossRef]
- Sepehr, A.; Bahari Kashani, R.; Esmaeili, M.; Safari, O.; Rombenso, A. Effects of extruded, milled, and whole flaxseed (Linum usitatissimum) on egg performance, lipid components, and fatty acids concentrations in yolk and blood, and antioxidant system of commercial laying hens. Anim. Feed Sci. Technol. 2021, 276, 114877. [Google Scholar] [CrossRef]
- El-Bahr, S.M.; Shousha, S.; Alfattah, M.A.; Al-Sultan, S.; Khattab, W.; Sabeq, I.I.; Ahmed-Farid, O.; El-Garhy, O.; Albusadah, K.A.; Alhojaily, S.; et al. Enrichment of broiler chickens’ meat with dietary linseed oil and lysine mixtures: Influence on nutritional value, carcass characteristics and oxidative stress biomarkers. Foods 2021, 10, 618. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (By-Products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef]
- Long, S.F.; Kang, S.; Wang, Q.Q.; Xu, Y.T.; Pan, L.; Hu, J.X.; Li, M.; Piao, X.S. Dietary supplementation with DHA-rich microalgae improves performance, serum composition, carcass trait, antioxidant status, and fatty acid profile of broilers. Poult. Sci. 2018, 97, 1881–1890. [Google Scholar] [CrossRef]
- John, R.; Singla, A. Functional Foods: Components, health benefits, challenges, and major projects. DRC Sustain. Future J. Environ. Agric. Energy 2021, 2, 61–72. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Hebbar, A.; Selvaraj, S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environ. Sci. Pollut. Res. 2022, 29, 35518–35541. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef]
- Ren, Y.; Perez, T.I.; Zuidhof, M.J.; Renema, R.A.; Wu, J. Oxidative stability of omega-3 polyunsaturated fatty acids enriched eggs. J. Agric. Food Chem. 2013, 61, 11595–11602. [Google Scholar] [CrossRef]
- Martirosyan, D.; Miller, E. Bioactive compounds: The key to functional foods. Bioact. Compd. Health Dis. 2018, 1, 36–39. [Google Scholar] [CrossRef]
- Statista. Size of the Functional Food Market Worldwide from 2019 to 2027. Available online: https://www.statista.com/statistics/1264165/global-functional-food-market-size/#:~:text=In2019%2C (accessed on 31 July 2022).
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Khan, S.H.; Iqbal, J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Wei, X.; Bottoms, K.A.; Stein, H.H.; Blavi, L.; Bradley, C.L.; Bergstrom, J.; Knapp, J.; Story, R.; Maxwell, C.; Tsai, T.; et al. Dietary organic acids modulate gut microbiota and improve growth performance of nursery pigs. Microorganisms 2021, 9, 110. [Google Scholar] [CrossRef]
- Galli, G.M.; Aniecevski, E.; Petrolli, T.G.; da Rosa, G.; Boiago, M.M.; Simões, C.A.D.P.; Wagner, R.; Copetti, P.M.; Morsch, V.M.; Araujo, D.N.; et al. Growth performance and meat quality of broilers fed with microencapsulated organic acids. Anim. Feed Sci. Technol. 2021, 271, 114706. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, B.; Zhang, H. Butyric and citric acids and their salts in poultry nutrition: Effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, T.A.; Al-Homidan, I.H. Organic acids and their potential role for modulating the gastrointestinal tract, antioxidative status, immune response, and performance in poultry. Worlds Poult. Sci. J. 2022, 78, 83–101. [Google Scholar] [CrossRef]
- Ma, J.; Mahfuz, S.; Wang, J.; Piao, X. Effect of dietary supplementation with mixed organic acids on immune function, antioxidative characteristics, digestive enzymes activity, and intestinal health in broiler chickens. Front. Nutr. 2021, 8, 673316. [Google Scholar] [CrossRef] [PubMed]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.S.; Lee, J.H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits—A review of recent advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef] [PubMed]
- Catenza, K.F.; Donkor, K.K. Recent approaches for the quantitative analysis of functional oligosaccharides used in the food industry: A review. Food Chem. 2021, 355, 129416. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.; Sharma, A.; Bachheti, R.K.; Chandel, A.K. Lignocellulose derived functional oligosaccharides: Production, properties, and health benefits. Prep. Biochem. Biotechnol. 2019, 49, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.; Illanes, A.; Guerrero, C. Enzymatic production of prebiotic oligosaccharides. Curr. Opin. Food Sci. 2021, 37, 160–170. [Google Scholar] [CrossRef]
- Muñoz-Labraador, A.; Lebrón-Aguilar, R.; Quintanilla-López, J.; Plácido, G.-I.; Azcarate, S.M.; Kolida, S.; Kachrimanidou, V.; Garcia-Cañas, V.; Methven, L.; Rastall, R.A.; et al. Prebiotic potential of a new sweetener based on galactooligosaccharides and modified mogrosides. J. Agric. Food Chem. 2022, 70, 9048–9056. [Google Scholar] [CrossRef]
- Zhang, S.; Waterhouse, G.I.N.; Xu, F.; He, Z.; Du, Y.; Lian, Y.; Wu, P.; Sun-Waterhouse, D. Recent advances in utilization of pectins in biomedical applications: A review focusing on molecular structure-directing health-promoting properties. Crit. Rev. Food Sci. Nutr. 2021, 1–34. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Wang, S.; Sun-Waterhouse, D.; Neil Waterhouse, G.I.; Zheng, L.; Su, G.; Zhao, M. Effects of food-derived bioactive peptides on cognitive deficits and memory decline in neurodegenerative diseases: A review. Trends Food Sci. Technol. 2021, 116, 712–732. [Google Scholar] [CrossRef]
- Valenzuela Zamudio, F.; Segura Campos, M.R. Amaranth, quinoa and chia bioactive peptides: A comprehensive review on three ancient grains and their potential role in management and prevention of Type 2 diabetes. Crit. Rev. Food Sci. Nutr. 2022, 62, 2707–2721. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Ali, M.A.; Kamal, M.M.; Rahman, M.H.; Siddiqui, M.N.; Haque, M.A.; Saha, K.K.; Rahman, M.A. Functional dairy products as a source of bioactive peptides and probiotics: Current trends and future prospectives. J. Food Sci. Technol. 2022, 59, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, G.; Liao, W.; Gong, S.; Xiao, J.; Bai, J.; Wendy Hsiao, W.L.; Li, N.; Wu, J.L. Discovery of the bioactive peptides secreted by Bifidobacterium using integrated MCX coupled with LC–MS and feature-based molecular networking. Food Chem. 2021, 347, 129008. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.H.; Dong, Y.; Sun, Y.F.; Mei, X.S.; Ma, X.S.; Shi, J.; Yang, Q.L.; Ji, Y.R.; Zhang, Z.H.; Sun, H.N.; et al. Anticancer property of Hemp Bioactive Peptides in Hep3B liver cancer cells through Akt/GSK3β/β-catenin signaling pathway. Food Sci. Nutr. 2021, 9, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, P.G.; Cavanhi, V.A.F.; Costa, J.A.V.; Colla, L.M. Bioactive peptides and proteases: Characteristics, applications and the simultaneous production in solid-state fermentation. Biocatal. Biotransform. 2021, 39, 360–377. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef]
- Rodríguez García, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6446–6466. [Google Scholar] [CrossRef]
- Llano, T.; Alexandri, M.; Koutinas, A.; Gardeli, C.; Papapostolou, H.; Coz, A.; Quijorna, N.; Andres, A.; Komaitis, M. Liquid-liquid extraction of phenolic compounds from spent sulphite liquor. Waste Biomass Valorization 2015, 6, 1149–1159. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene-properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, C.; Wang, C.; Liu, C.; Yuan, Y.; Wang, B.; Wu, G.; Han, Y.; Zhao, Y.; Wu, Z.; et al. The emulsification properties of alkaline-extracted polysaccharide conjugates from Apocynum venetum L. tea residues. Food Hydrocoll. 2022, 124, 107315. [Google Scholar] [CrossRef]
- Chen, R.; Luo, S.; Wang, C.; Bai, H.; Lu, J.; Tian, L.; Gao, M.; Wu, J.; Bai, C.; Sun, H. Effects of ultra-high pressure enzyme extraction on characteristics and functional properties of red pitaya (Hylocereus polyrhizus) peel pectic polysaccharides. Food Hydrocoll. 2021, 121, 107016. [Google Scholar] [CrossRef]
- Wu, D.; Chen, S.; Ye, X.; Zheng, X.; Ahmadi, S.; Hu, W.; Yu, C.; Cheng, H.; Linhardt, R.J.; Chen, J. Enzyme-extracted raspberry pectin exhibits a high-branched structure and enhanced anti-inflammatory properties than hot acid-extracted pectin. Food Chem. 2022, 383, 132387. [Google Scholar] [CrossRef] [PubMed]
- De Souza Mesquita, L.M.; Martins, M.; Maricato, É.; Nunes, C.; Quinteiro, P.S.G.N.; Dias, A.C.R.V.; Coutinho, J.A.P.; Pisani, L.P.; De Rosso, V.V.; Ventura, S.P.M. Ionic liquid-mediated recovery of carotenoids from the Bactris gasipaes fruit waste and their application in food-packaging chitosan films. ACS Sustain. Chem. Eng. 2020, 8, 4085–4095. [Google Scholar] [CrossRef]
- Alexandri, M.; López-Gómez, J.P.; Olszewska-Widdrat, A.; Venus, J. Valorising agro-industrial wastes within the circular bioeconomy concept: The case of defatted rice bran with emphasis on bioconversion strategies. Fermentation 2020, 6, 42. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Ko, M.J.; Nam, H.H.; Chung, M.S. Subcritical water extraction of bioactive compounds from Orostachys japonicus A. Berger (Crassulaceae). Sci. Rep. 2020, 10, 10890. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Ran, X.L.; Zhang, M.; Wang, Y.; Adhikari, B. Novel technologies applied for recovery and value addition of high value compounds from plant byproducts: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 450–461. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green extraction of bioactive compounds from plant biomass and their application in meat as natural antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Žlabur, J.; Voća, S.; Brnčić, M.; Rimac-Brnčić, S. New Trends in Food Technology for Green Recovery of Bioactive Compounds from Plant Materials. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–36. ISBN 9780128115008. [Google Scholar]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Da Silva, R.F.; Carneiro, C.N.; Cheila, C.B.; de Sousa, C.B.D.C.; Gomez, F.J.V.; Espino, M.; Boiteux, J.; Fernández, M.D.L.Á.; Silva, M.F.; Dias, F.D.S. Sustainable extraction bioactive compounds procedures in medicinal plants based on the principles of green analytical chemistry: A review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The effect of different pressurized fluids on the extraction of anthocyanins and total phenolics from cranberry pomace. J. Supercrit. Fluids 2021, 175, 105279. [Google Scholar] [CrossRef]
- Chien, W.J.; Saputri, D.S.; Lin, H.Y. Valorization of Taiwan’s Citrus depressa Hayata peels as a source of nobiletin and tangeretin using simple ultrasonic-assisted extraction. Curr. Res. Food Sci. 2022, 5, 278–287. [Google Scholar] [CrossRef]
- Kim, H.S.; Ko, M.J.; Park, C.H.; Chung, M.S. Application of pulsed electric field as a pre-treatment for subcritical water extraction of quercetin from onion skin. Foods 2022, 11, 1069. [Google Scholar] [CrossRef]
- Symes, A.; Shavandi, A.; Bekhit, A.E.D.A. Effects of ionic liquids and pulsed electric fields on the extraction of antioxidants from green asparagus roots. Int. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Kupnik, K.; Leitgeb, M.; Primožič, M.; Postružnik, V.; Kotnik, P.; Kučuk, N.; Knez, Ž.; Marevci, M.K. Supercritical fluid and conventional extractions of high value-added compounds from pomegranate peels waste: Production, quantification and antimicrobial activity of bioactive constituents. Plants 2022, 11, 928. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of waste mango peels for extraction of polyphenolic antioxidants by ultrasound-assisted natural deep eutectic solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Le, N.T.; Hoang, N.T.; Van, V.T.T.; Nguyen, T.P.D.; Chau, N.H.T.; Le, N.T.N.; Le, H.B.T.; Phung, H.T.; Nguyen, H.T.; Nguyen, H.M. Extraction of curcumin from turmeric residue (Curcuma longa L.) using deep eutectic solvents and surfactant solvents. Anal. Methods 2022, 14, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S. Utilization of pomegranate peel waste: Natural deep eutectic solvents as a green strategy to recover valuable phenolic compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Ciardi, M.; Ianni, F.; Sardella, R.; Di Bona, S.; Cossignani, L.; Germani, R.; Tiecco, M.; Clementi, C. Effective and selective extraction of quercetin from onion (Allium cepa L.) skin waste using water dilutions of acid-based deep eutectic solvents. Materials 2021, 14, 6465. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; Da Costa, S.C.; Madrona, G.S. Uvaia (Eugenia pyriformis Cambess) residue as a source of antioxidants: An approach to ecofriendly extraction. LWT—Food Sci. Technol. 2021, 138, 110785. [Google Scholar] [CrossRef]
- Carvalho Gualberto, N.; Santos de Oliveira, C.; Pedreira Nogueira, J.; Silva de Jesus, M.; Caroline Santos Araujo, H.; Rajan, M.; Terezinha Santos Leite Neta, M.; Narain, N. Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extracti. Food Res. Int. 2021, 147, 110538. [Google Scholar] [CrossRef]
- Ahmad Shiekh, K.; Odunayo Olatunde, O.; Zhang, B.; Huda, N.; Benjakul, S. Pulsed electric field assisted process for extraction of bioactive compounds from custard apple (Annona squamosa) leaves. Food Chem. 2021, 359, 129976. [Google Scholar] [CrossRef]
- Buelvas-Puello, L.M.; Franco-Arnedo, G.; Martínez-Correa, H.A.; Ballesteros-Vivas, D.; Sánchez-Camargo, A.D.P.; Miranda-Lasprilla, D.; Narváez-Cuenca, C.E.; Parada-Alfonso, F. Supercritical fluid extraction of phenolic compounds from mango (Mangifera indica L.) seed kernels and their application as an antioxidant in an edible oil. Molecules 2021, 26, 7516. [Google Scholar] [CrossRef]
- González-Rivera, J.; Mero, A.; Husanu, E.; Mezzetta, A.; Ferrari, C.; D’Andrea, F.; Bramanti, E.; Pomelli, C.S.; Guazzelli, L. Combining acid-based deep eutectic solvents and microwave irradiation for improved chestnut shell waste valorization. Green Chem. 2021, 23, 10101–10115. [Google Scholar] [CrossRef]
- Sanz, V.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Valorisation of Camellia sinensis branches as a raw product with green technology extraction methods. Curr. Res. Food Sci. 2020, 2, 20–24. [Google Scholar] [CrossRef]
- Hirondart, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Bily, A.; Chemat, F. Comparison between pressurized liquid extraction and conventional Soxhlet extraction for rosemary antioxidants, yield, composition, and environmental footprint. Foods 2020, 9, 584. [Google Scholar] [CrossRef]
- Maraulo, G.E.; dos Santos Ferreira, C.; Mazzobre, M.F. Β-Cyclodextrin enhanced ultrasound-assisted extraction as a green method to recover olive pomace bioactive compounds. J. Food Process. Preserv. 2021, 45, e15194. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-assisted extraction for recovery of polyphenolic antioxidants from ripe mango (Mangifera indica L.) peel using lactic acid/sodium acetate deep eutectic mixtures. Food Sci. Technol. Int. 2020, 26, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Gahruie, H.H.; Parastouei, K.; Mokhtarian, M.; Rostami, H.; Niakousari, M.; Mohsenpour, Z. Application of innovative processing methods for the extraction of bioactive compounds from saffron (Crocus sativus) petals. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100264. [Google Scholar] [CrossRef]
- González, M.; Barrios, S.; Budelli, E.; Pérez, N.; Lema, P.; Heinzen, H. Ultrasound assisted extraction of bioactive compounds in fresh and freeze-dried Vitis vinifera cv Tannat grape pomace. Food Bioprod. Process. 2020, 124, 378–386. [Google Scholar] [CrossRef]
- Franco, D.; Munekata, P.E.S.; Agregán, R.; Bermúdez, R.; López-Pedrouso, M.; Pateiro, M.; Lorenzo, J.M. Application of pulsed electric fields for obtaining antioxidant extracts from fish residues. Antioxidants 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT—Food Sci. Technol. 2020, 133, 109970. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Chamika, W.A.S.; Ho, T.C.; Roy, V.C.; Kiddane, A.T.; Park, J.S.; Kim, G.D.; Chun, B.S. In vitro characterization of bioactive compounds extracted from sea urchin (Stomopneustes variolaris) using green and conventional techniques. Food Chem. 2021, 361, 129866. [Google Scholar] [CrossRef]
- Phan, K.; Raes, K.; Van Speybroeck, V.; Roosen, M.; De Clerck, K.; De Meester, S. Non-food applications of natural dyes extracted from agro-food residues: A critical review. J. Clean. Prod. 2021, 301, 126920. [Google Scholar] [CrossRef]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrate Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT—Food Sci. Technol. 2020, 123, 109100. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Gao, J.; You, J.; Kang, J.; Nie, F.; Ji, H.; Liu, S. Recovery of astaxanthin from shrimp (Penaeus vannamei) waste by ultrasonic-assisted extraction using ionic liquid-in-water microemulsions. Food Chem. 2020, 325, 126850. [Google Scholar] [CrossRef]
- Ricarte, G.N.; Coelho, M.A.Z.; Marrucho, I.M.; Ribeiro, B.D. Enzyme-assisted extraction of carotenoids and phenolic compounds from sunflower wastes using green solvents. 3 Biotech 2020, 10, 405. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-based natural deep eutectic systems as efficient solvents to recover astaxanthin from brown crab shell residues. ACS Sustain. Chem. Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Padilha, C.E.A.; Damasceno, K.S.F.S.C.; Leite, P.I.P.; De Araújo, A.C.J.; Freitas, P.R.; Vieira, É.A.; Cordeiro, A.M.T.M.; De Sousa, F.C.; De Assis, C.F. Astaxanthin recovery from shrimp residue by solvent ethanol extraction using choline chloride: Glycerol deep eutectic solvent as adjuvant. J. Braz. Chem. Soc. 2021, 32, 1030–1039. [Google Scholar] [CrossRef]
- Cabanillas-Bojórquez, L.A.; Gutiérrez-Grijalva, E.P.; González-Aguilar, G.A.; López-Martinez, L.X.; Castillo-López, R.I.; Bastidas-Bastidas, P.D.J.; Heredia, J.B. Valorization of fermented shrimp waste with supercritical CO2 conditions: Extraction of astaxanthin and effect of simulated gastrointestinal digestion on its antioxidant capacity. Molecules 2021, 26, 4465. [Google Scholar] [CrossRef]
- Chandra Roy, V.; Ho, T.C.; Lee, H.J.; Park, J.S.; Nam, S.Y.; Lee, H.; Getachew, A.T.; Chun, B.S. Extraction of astaxanthin using ultrasound-assisted natural deep eutectic solvents from shrimp wastes and its application in bioactive films. J. Clean. Prod. 2021, 284, 125417. [Google Scholar] [CrossRef]
- Portillo-López, R.; Morales-Contreras, B.E.; Lozano-Guzmán, E.; Basilio-Heredia, J.; Muy-Rangel, M.D.; Ochoa-Martínez, L.A.; Rosas-Flores, W.; Morales-Castro, J. Vegetable oils as green solvents for carotenoid extraction from pumpkin (Cucurbita argyrosperma Huber) byproducts: Optimization of extraction parameters. J. Food Sci. 2021, 86, 3122–3136. [Google Scholar] [CrossRef]
- Hernández-Aguirre, O.A.; Muro, C.; Hernández-Acosta, E.; Alvarado, Y.; Díaz-Nava, M.D.C. Extraction and stabilization of betalains from beetroot (Beta vulgaris) wastes using deep eutectic solvents. Molecules 2021, 26, 6342. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, X.; Zhang, L.; Shao, P.; Wu, W.; Chen, Z.; Li, J.; Renard, C.M.G.C. An overview of carotenoid extractions using green solvents assisted by Z-isomerization. Trends Food Sci. Technol. 2022, 123, 145–160. [Google Scholar] [CrossRef]
- Sharma, M.; Hussain, S.; Shalima, T.; Aav, R.; Bhat, R. Valorization of seabuckthorn pomace to obtain bioactive carotenoids: An innovative approach of using green extraction techniques (ultrasonic and microwave-assisted extractions) synergized with green solvents (edible oils). Ind. Crops Prod. 2022, 175, 114257. [Google Scholar] [CrossRef]
- Alzorqi, I.; Sudheer, S.; Lu, T.J.; Manickam, S. Ultrasonically extracted β-D-glucan from artificially cultivated mushroom, characteristic properties and antioxidant activity. Ultrason. Sonochem. 2017, 35, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, C.; Umada, A.; Noma, S.; Demura, M.; Hayashi, N. Extraction of pectin from Satsuma mandarin orange peels by combining pressurized carbon dioxide and deionized water: A green chemistry method. Food Bioprocess Technol. 2021, 14, 1341–1348. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Matias, A.A.; Paiva, A. Recovery of antioxidant protein hydrolysates from shellfish waste streams using subcritical water extraction. Food Bioprod. Process. 2021, 130, 154–163. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, W.; Zhang, A.; Liu, C. Green approach to produce xylo-oligosaccharides and glucose by mechanical-hydrothermal pretreatment. Bioresour. Technol. 2022, 344, 126298. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, H.; Fu, S. Improvement of xylo-oligosaccharides dissolution from Caragana korshinskii through liquid hot water pretreatment with tiny choline chloride. Ind. Crops Prod. 2022, 176, 114418. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Wojciechowska, M.; Marszałek, K.; Skąpska, S. Extraction of galactolipids from waste by-products: The feasibility of green chemistry methods. Appl. Sci. 2021, 11, 12088. [Google Scholar] [CrossRef]
- Yu, M.; Xia, Y.; Xie, W.; Li, Y.; Yu, X.; Zheng, J.; Zhang, Y. Enzymatic extraction of pectic oligosaccharides from finger citron (Citrus medica L. var. sarcodactylis Swingle) pomace with antioxidant potential. Food Funct. 2021, 12, 9855–9865. [Google Scholar] [CrossRef]
- Sabater, C.; Sabater, V.; Olano, A.; Montilla, A.; Corzo, N. Ultrasound-assisted extraction of pectin from artichoke by-products. An artificial neural network approach to pectin characterisation. Food Hydrocoll. 2020, 98, 105238. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Bao, Y.; Ahmed, Z.; Huang, J.Y. Effect of high voltage atmospheric cold plasma on extraction of fenugreek galactomannan and its physicochemical properties. Food Res. Int. 2020, 138, 109776. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane technologies for lactic acid separation from fermentation broths derived from renewable resources. Membranes 2018, 8, 94. [Google Scholar] [CrossRef]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly(3-hydroxybutyrate) synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Fornereto Soldan, A.C.; Arvelos, S.; Watanabe, É.O.; Hori, C.E. Supercritical fluid extraction of oleoresin from Capsicum annuum industrial waste. J. Clean. Prod. 2021, 297, 126593. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef]
- Rico, X.; Nuutinen, E.M.; Gullón, B.; Pihlajaniemi, V.; Yáñez, R. Application of an eco-friendly sodium acetate/urea deep eutectic solvent in the valorization of melon by-products. Food Bioprod. Process. 2021, 130, 216–228. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Khodaiyan, F.; Parastouei, K. Co-optimization of pectin and polyphenols extraction from black mulberry pomace using an eco-friendly technique: Simultaneous recovery and characterization of products. Int. J. Biol. Macromol. 2020, 164, 1025–1036. [Google Scholar] [CrossRef]
- Huerta, R.R.; Saldaña, M.D.A. Pressurized fluid treatment of barley and canola straws to obtain carbohydrates and phenolics. J. Supercrit. Fluids 2018, 141, 12–20. [Google Scholar] [CrossRef]
- Esteban-Lustres, R.; Torres, M.D.; Pazos, A.; Enjamio, C.; Piñeiro, B.; Domínguez, H. Preliminary evaluation of pressurized hot water extraction for the solubilization of valuable components from hospital kitchen wastes. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Barrios, C.; Fernández-Delgado, M.; López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A techno-economic perspective on a microwave extraction process for efficient protein recovery from agri-food wastes. Ind. Crops Prod. 2022, 186, 115166. [Google Scholar] [CrossRef]
- Lin, Z.; Jiao, G.; Zhang, J.; Celli, G.B.; Brooks, M.S.L. Optimization of protein extraction from bamboo shoots and processing wastes using deep eutectic solvents in a biorefinery approach. Biomass Convers. Biorefinery 2021, 11, 2763–2774. [Google Scholar] [CrossRef]
- Hernández Becerra, E.; De Jesús Pérez López, E.; Zartha Sossa, J.W. Recovery of biomolecules from agroindustry by solid-liquid enzyme-assisted extraction: A review. Food Anal. Methods 2021, 14, 1744–1777. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Tsouko, E.; Kachrimanidou, V.; dos Santos, A.F.; do Nascimento Vitorino Lima, M.E.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Koutinas, A.A. Valorization of by-products from palm oil mills for the production of generic fermentation media for microbial oil synthesis. Appl. Biochem. Biotechnol. 2017, 181, 1241–1256. [Google Scholar] [CrossRef]
- Tsakona, S.; Papadaki, A.; Kopsahelis, N.; Kachrimanidou, V.; Papanikolaou, S.; Koutinas, A. Development of a circular oriented bioprocess for microbial oil production using diversified mixed confectionery side-streams. Foods 2019, 8, 300. [Google Scholar] [CrossRef]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-assisted supercritical fluid extraction: An integral approach to extract bioactive compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Sharif, T.; Bhatti, H.N.; Bull, I.D.; Bilal, M. Recovery of high-value bioactive phytochemicals from agro-waste of mango (Mangifera indica L.) using enzyme-assisted ultrasound pretreated extraction. Biomass Convers. Biorefinery 2021, 1–9. [Google Scholar] [CrossRef]
- Granato, D.; Fidelis, M.; Haapakoski, M.; dos Santos Lima, A.; Viil, J.; Hellström, J.; Rätsep, R.; Kaldmäe, H.; Bleive, U.; Azevedo, L.; et al. Enzyme-assisted extraction of anthocyanins and other phenolic compounds from blackcurrant (Ribes nigrum L.) press cake: From processing to bioactivities. Food Chem. 2022, 391, 133240. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Filipigh, A.; Beltrán, S.; Riaño, P. Enzymatic hydrolysis of the industrial solid residue of red seaweed after agar extraction: Extracts characterization and modelling. Food Bioprod. Process. 2021, 126, 356–366. [Google Scholar] [CrossRef]
- Zhang, C.; Song, X.; Cui, W.; Yang, Q. Antioxidant and anti-ageing effects of enzymatic polysaccharide from Pleurotus eryngii residue. Int. J. Biol. Macromol. 2021, 173, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Biel-Nielsen, T.L.; Li, K.; Sørensen, S.O.; Sejberg, J.J.P.; Meyer, A.S.; Holck, J. Utilization of industrial citrus pectin side streams for enzymatic production of human milk oligosaccharides. Carbohydr. Res. 2022, 519, 108627. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Mehlmann, K.; Venus, J. Recent advances in d-lactic acid production from renewable resources: Case studies on agro-industrial waste streams. Food Technol. Biotechnol. 2019, 57, 293–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. [Google Scholar] [CrossRef]
- Carpinelli Macedo, J.V.; de Barros Ranke, F.F.; Escaramboni, B.; Campioni, T.S.; Fernández Núñez, E.G.; de Oliva Neto, P. Cost-effective lactic acid production by fermentation of agro-industrial residues. Biocatal. Agric. Biotechnol. 2020, 27, 101706. [Google Scholar] [CrossRef]
- Alexandri, M.; Blanco-Catalá, J.; Schneider, R.; Turon, X.; Venus, J. High L(+)-lactic acid productivity in continuous fermentations using bakery waste and lucerne green juice as renewable substrates. Bioresour. Technol. 2020, 316, 123949. [Google Scholar] [CrossRef]
- Alexandri, M.; Neu, A.K.; Schneider, R.; López-Gómez, J.P.; Venus, J. Evaluation of various Bacillus coagulans isolates for the production of high purity L-lactic acid using defatted rice bran hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 1321–1329. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Mandl, M.; Venus, J. Production and purification of L-lactic acid in lab and pilot scales using sweet sorghum juice. Fermentation 2019, 5, 36. [Google Scholar] [CrossRef]
- Bevilaqua, D.B.; Montipó, S.; Pedroso, G.B.; Martins, A.F. Sustainable succinic acid production from rice husks. Sustain. Chem. Pharm. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, C.; Li, J.; Hou, J.; Lin, C.S.K.; Qi, Q. Engineering of unconventional yeast Yarrowia lipolytica for efficient succinic acid production from glycerol at low pH. Metab. Eng. 2017, 42, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Dessie, W.; Zhang, W.; Xin, F.; Dong, W.; Zhang, M.; Ma, J.; Jiang, M. Succinic acid production from fruit and vegetable wastes hydrolyzed by on-site enzyme mixtures through solid state fermentation. Bioresour. Technol. 2018, 247, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Papapostolou, H.; Alexandri, M.; Kopsahelis, N.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Koutinas, A.A. Fumaric acid production using renewable resources from biodiesel and cane sugar production processes. Environ. Sci. Pollut. Res. 2018, 25, 35960–35970. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Enhanced fumaric acid production from brewery wastewater by immobilization technique. J. Chem. Technol. Biotechnol. 2015, 90, 1473–1479. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, R.; Chen, D.; Chen, J.; He, W.; Huang, L.; Lin, C.; Chen, H.; Chen, Y.; Zhu, J.; et al. Lipid and carotenoid production by the Rhodosporidium toruloides mutant in cane molasses. Bioresour. Technol. 2021, 326, 124816. [Google Scholar] [CrossRef]
- Liu, Z.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Lipid and carotenoid production from wheat straw hydrolysates by different oleaginous yeasts. J. Clean. Prod. 2020, 249, 119308. [Google Scholar] [CrossRef]
- Rodrigues, T.V.D.; Amore, T.D.; Teixeira, E.C.; de Medeiros Burkert, J.F. Carotenoid production by Rhodotorula mucilaginosa in batch and fed-Batch fermentation using agroindustrial byproducts. Food Technol. Biotechnol. 2019, 57, 388–398. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S. Growth and bioactive peptides production potential of Lactobacillus plantarum strain C2 in soy milk: A LC-MS/MS based revelation for peptides biofunctionality. LWT—Food Sci. Technol. 2017, 86, 293–301. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, F.; Zhang, S.; Zhang, W.; Yang, Q.; Dong, W.; Jiang, M.; Ma, J.; Xin, F. Bio-based succinic acid: An overview of strain development, substrate utilization, and downstream purification. Biofuels Bioprod. Biorefin. 2020, 14, 965–985. [Google Scholar] [CrossRef]
- Li, C.; Ong, K.L.; Cui, Z.; Sang, Z.; Li, X.; Patria, R.D.; Qi, Q.; Fickers, P.; Yan, J.; Lin, C.S.K. Promising advancement in fermentative succinic acid production by yeast hosts. J. Hazard. Mater. 2021, 401, 123414. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Papapostolou, H.; Ladakis, D.; Koutinas, A.; Venus, J. Restructuring the conventional sugar beet industry into a novel biorefinery: Fractionation and bioconversion of sugar beet pulp into succinic acid and value-added coproducts. ACS Sustain. Chem. Eng. 2019, 7, 6569–6579. [Google Scholar] [CrossRef]
- Alexandri, M.; Papapostolou, H.; Komaitis, M.; Stragier, L.; Verstraete, W.; Danezis, G.P.; Georgiou, C.A.; Papanikolaou, S.; Koutinas, A.A. Evaluation of an integrated biorefinery based on fractionation of spent sulphite liquor for the production of an antioxidant-rich extract, lignosulphonates and succinic acid. Bioresour. Technol. 2016, 214, 504–513. [Google Scholar] [CrossRef]
- Filippi, K.; Papapostolou, H.; Alexandri, M.; Vlysidis, A.; Myrtsi, E.D.; Ladakis, D.; Pateraki, C.; Haroutounian, S.A.; Koutinas, A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value-added fractions. Bioresour. Technol. 2022, 343, 125989. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xue, Q.; Sun, M.; Liu, J.; Hung Wong, M.; Wang, C.; Chen, S. Co-production of polysaccharides, ginsenosides and succinic acid from panax ginseng residue: A typical industrial herbal waste. Bioresour. Technol. 2021, 331, 125073. [Google Scholar] [CrossRef] [PubMed]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Valverde, J.M.; Lipan, L.; Carbonell-Barrachina, Á.A.; Sendra, E. Introduction. In The Age of Clean Label Foods; Galanakis, C.M., Ed.; Springer: Chania, Greece, 2022; pp. 1–37. [Google Scholar]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting synergies of sourdough and antifungal organic acids to delay fungal spoilage of bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef]
- El Dessouky Abdel-Aziz, M.; Darwish, M.S.; Mohamed, A.H.; El-Khateeb, A.Y.; Hamed, S.E. Potential activity of aqueous fig leaves extract, olive leaves extract and their mixture as natural preservatives to extend the shelf life of pasteurized buffalo milk. Foods 2020, 9, 615. [Google Scholar] [CrossRef]
- Tsouko, E.; Alexandri, M.; Fernandes, K.V.; Freire, D.M.G.; Mallouchos, A.; Koutinas, A.A. Extraction of phenolic compounds from palm oil processing residues and their application as antioxidants. Food Technol. Biotechnol. 2019, 57, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pando, G.; Ekonomou, S.I.; Stratakos, A.C.; Pintado, T. Clean label alternatives in meat products. Foods 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Tamkutė, L.; Gil, B.M.; Carballido, J.R.; Pukalskienė, M.; Venskutonis, P.R. Effect of cranberry pomace extracts isolated by pressurized ethanol and water on the inhibition of food pathogenic/spoilage bacteria and the quality of pork products. Food Res. Int. 2019, 120, 38–51. [Google Scholar] [CrossRef]
- Lau, A.T.Y.; Arvaj, L.; Strange, P.; Goodwin, M.; Barbut, S.; Balamurugan, S. Effect of cranberry pomace on the physicochemical properties and inactivation of Salmonella during the manufacture of dry fermented sausages. Curr. Res. Food Sci. 2021, 4, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Sobral, M.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I.M. Incorporation of avocado peel extract to reduce cooking-induced hazards in beef and soy burgers: A clean label ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef] [PubMed]
- Bassijeh, A.; Ansari, S.; Hosseini, S.M.H. Astaxanthin encapsulation in multilayer emulsions stabilized by complex coacervates of whey protein isolate and Persian gum and its use as a natural colorant in a model beverage. Food Res. Int. 2020, 137, 109689. [Google Scholar] [CrossRef]
- Šeregelj, V.; Pezo, L.; Šovljanski, O.; Lević, S.; Nedović, V.; Markov, S.; Tomić, A.; Čanadanović-Brunet, J.; Vulić, J.; Šaponjac, V.T.; et al. New concept of fortified yogurt formulation with encapsulated carrot waste extract. LWT—Food Sci. Technol. 2021, 138, 110732. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N.; Singh, A.K. Stability assessment of emulsion of carotenoids extracted from carrot bio-waste in flaxseed oil and its application in food model system. Food Biosci. 2022, 47, 101631. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Mirab, B.; Ahmadi Gavlighi, H.; Amini Sarteshnizi, R.; Azizi, M.H.; Udenigwe, C.C. Production of low glycemic potential sponge cake by pomegranate peel extract (PPE) as natural enriched polyphenol extract: Textural, color and consumer acceptability. LWT—Food Sci. Technol. 2020, 134, 109973. [Google Scholar] [CrossRef]
- Morina, A.; Shehaj, A. Physico-chemical parameters and antioxidant activity of baked product fortified with fruit waste powder. J. Biol. Stud. 2022, 5, 221–228. [Google Scholar]
- Ayati, S.; Eun, J.B.; Atoub, N.; Mirzapour-Kouhdasht, A. Functional yogurt fortified with fish collagen-derived bioactive peptides: Antioxidant capacity, ACE and DPP-IV inhibitory. J. Food Process. Preserv. 2022, 46, e16208. [Google Scholar] [CrossRef]
- Gan, J.; Peng, G.; Liu, S.; Hu, X.; Wang, X.; Guo, S.; Xie, J.; Chen, Y.; Yu, Q. Comparison of structural, functional and in vitro digestion properties of bread incorporated with grapefruit peel soluble dietary fibers prepared by three microwave-assisted modifications. Food Funct. 2020, 11, 6458–6466. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Li, C.; Zhao, F.; Cao, J.; Zhang, X.; Shen, X. Effects of co-fermented collagen peptide-jackfruit juice on the immune response and gut microbiota in immunosuppressed mice. Food Chem. 2021, 365, 130487. [Google Scholar] [CrossRef]
- Hesam, F.; Tarzi, B.G.; Honarvar, M.; Jahadi, M. Valorization of sugarcane bagasse to high value-added xylooligosaccharides and evaluation of their prebiotic function in a synbiotic pomegranate juice. Biomass Convers. Biorefinery 2020, 1–13. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Lappa, I.K.; Andriotis, H.; Eriotou, E.; Mandala, I.; Kopsahelis, N. Tuning the physical and functional properties of whey protein edible films: Effect of pH and inclusion of antioxidants from spent coffee grounds. Sustain. Chem. Pharm. 2022, 27, 100700. [Google Scholar] [CrossRef]
- Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Gonou-Zagou, Z.; Kopsahelis, N. Trametes versicolor as a natural source of bioactive compounds for the production of whey protein films with functional properties: A holistic approach to valorize cheese whey. Waste Biomass Valorization 2022, 13, 3989–3998. [Google Scholar] [CrossRef]
- Çavdaroğlu, E.; Farris, S.; Yemenicioğlu, A. Development of pectin–eugenol emulsion coatings for inhibition of Listeria on webbed-rind melons: A comparative study with fig and citrus pectins. Int. J. Food Sci. Technol. 2020, 55, 1448–1457. [Google Scholar] [CrossRef]
- Linn, K.S.; Kasemsiri, P.; Jetsrisuparb, K.; Iamamornphan, W.; Chindaprasirt, P.; Knijnenburg, J.T.N. Development of biodegradable films with antioxidant activity using pectin extracted from Cissampelos pareira leaves. J. Polym. Environ. 2022, 30, 2087–2098. [Google Scholar] [CrossRef]
- Wen, L.; Liang, Y.; Lin, Z.; Xie, D.; Zheng, Z.; Xu, C.; Lin, B. Design of multifunctional food packaging films based on carboxymethyl chitosan/polyvinyl alcohol crosslinked network by using citric acid as crosslinker. Polymer 2021, 230, 124048. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.; Turner, M.S.; Fletcher, M.T.; Sultanbawa, Y. The impact of commercial prebiotics on the growth, survival and nisin production by Lactococcus lactis 537 in milk. LWT—Food Sci. Technol. 2021, 137, 110356. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Pateiro, M.; Domínguez, R.; Penna, A.L.B.; Lorenzo, J.M.; Silva Barretto, A.C. Impact of fructooligosaccharides and probiotic strains on the quality parameters of low-fat Spanish Salchichón. Meat Sci. 2020, 159, 107936. [Google Scholar] [CrossRef] [PubMed]

| Method | Principle | Advantages | Disadvantages | Target Compounds | Reference |
|---|---|---|---|---|---|
| Subcritical water extraction (SWE) | Use of water as extraction solvent in liquid state under high pressure at a temperature between 100 and 374 °C | Extraction of less-polar compounds with non-toxic solvent (water) | At temperatures above 240 °C, most of bioactive compounds appeared to be unstable and can be destroyed | Polysaccharides, phenolics | [60,61] |
| Sub- and supercritical fluid extraction (SFE) | Use of gases in their sub- or supercritical fluid state | Using CO2: non-toxic, inexpensive, non-flammable, modest critical point, easily recovered, suitable for heat-sensitive compounds, no product degradation due to absence of air and light, low energy consumption | Using CO2: requires a co-solvent (e.g., EOH, water) for the efficient extraction of polar compounds, high capital cost, requires technical expertise | Polyphenols, carotenoids, lipids, essential oils | [62,63,64] |
| Pulsed electric fields (PEF) | Cell membrane rupture due to electrical pulses (0.1–100 kV/cm) with a duration of 100–1000 μs | Extracts of higher purity, selectivity, low energy consumption, short processing time, suitable for temperature-sensitive compounds | Costly equipment maintenance, control parameters optimization | Polyphenols, proteins, polysaccharides | [50,60,63,65] |
| Microwave assisted extraction (MAE) | Application of microwave energy at frequencies of 300 MHz–300 GHz causing dipole rotation or ionic conduction induced by microwave energy | Low solvent requirements or solvent-free extraction, easy set-up, low economic and environmental impacts | Higher effectiveness for polar compounds; non-selective, unsuitable for heat-sensitive compounds, non-homogenous heat distribution, high energy consumption | Polyphenols, pectins, polysaccharides, bioactive peptides | [63,65] |
| Ultrasound assisted extraction (UAE) | Acoustic cavitation generated by ultrasonic waves (20 kHz–10 MHz) | Low solvent requirements, easy set-up, low economic and environmental impacts | No selectivity; long extraction times depending on target compound; uncontrollable energy transfer | Phenolic compounds, polysaccharides, proteins, lipids, pigments | [66] |
| Pressurized liquid extraction (PLE) | Application of high pressure (3–20 MPa) and temperature (50–200 °C); solvent temperature above boiling point | Rapid extraction time/rate, solvent volume reduction, high recovery yields, use of safe solvents (e.g., water), simple equipment | High cost of equipment, unsuitable for temperature-sensitive compounds | Flavonoids (especially anthocyanins), sugars, carotenoids | [4,65,67] |
| Pulsed ohmic heating extraction (POHE) | Ohming heating in conjunction to electric field (E < 100 V/cm) (conversion of electrical energy into heat energy) | Fast extraction | Requires process design to achieve homogenous sample heating; Quality and safety procedures for commercial use | Polyphenols, bioactive peptides | [4] |
| High hydrostatic pressure extraction (HHPE) | Application of high pressure (300–1000 MPa) at room temperature for 3–5 min | Faster extraction times, higher recovery yields and efficiencies, low energy consumption, applicable for polar and non-polar compounds, suitable of heat-labile compounds | Expensive equipment, maintenance, well-trained workforce due to high pressures employed | Carotenoids, pectins, phenolic compounds | [4,50] |
| Cold plasma assisted extraction | Application of cool ionized gas-containing ions, electrons, reactive neutral radicals and ultraviolet photons that rupture plant cell walls | Non-thermal process suitable for thermos-labile compounds, operation at atmospheric pressures, inexpensive equipment, low energy consumption | Available data only in lab-scale experiments, requires process optimization and study on possible alterations in the structure of the compounds | Phenolic compounds, protein, polysaccharides | [4,67] |
| Ionic liquids | Organic salts that are composed of organic cations and organic/inorganic anions—used as green solvents or used alone or in combination with other techniques | Low volatility, wide range of polarity | Expensive, possibly toxic, flammable, low stability | Phenolic compounds, carotenoids, oligosaccharides | [68] |
| Natural deep eutectic solvents | Used as green solvents or used alone or in combination with other techniques | Low volatility, wide range of polarity, low cost, easy preparation, stability, “tenability” | Short life, pending evaluation of potential toxicity | Polar and non-polar compounds | [68] |
| Raw Material | Method | Results | Reference |
|---|---|---|---|
| Citrus depressa Hayata peels from ripe and urnripe fruits | UAE with 50% EOH at solid-to-solvent ratio 1:100, ambient temperature and 50 min (unripe) or 40 min (ripe) | Unripe fruit peels: Rutin: 6.265 mg/g; Nobiletin: 12.511 mg/g; Tangeretin: 7.016 mg/g. Ripe fruit peels: Rutin: 3.033 mg/g; Nobiletin: 7.439 mg/g; Tangeretin: 3.569 mg/g. | [70]) |
| Dried onion skins | PEF (as pretreatment step) at 2.5 kV/cm for 15 s and SWE at 145 °C for 15 min | 19.25 ± 0.77 mg total quercetin per g (33.22% improved extraction yield in comparison to samples without PEF treatment) | [71]) |
| Green asparagus roots | ILs: 0.5% 1-butyl-3-methylimidazolium chloride, at 1:10 S:L ratio, for 4 min; PEF: 1.6 kV/cm, 200 Hz, 20 μs pulse width | TFC with ILs: 122 mg RE/mL; TFC after PEF: 1.52–1.74 mg RE/mL | [72]) |
| Pomegranate peel waste | SFE using CO2 and EOH as co-solvent at 20 MPa and 40 °C; UAE with EOH | SFE:11,561.84 ± 490.69 μg/g total polyphenols; UAE: 6940.74 ± 264.32 μg/g total polyphenols | [73] |
| Waste mango peels | UA and NADES (lactic acid:glycerol 5:1), 1:30 solid-to-liquid ratio, 30 min, 30 °C | TPC: 69.85 mg GAE/g; TFC: 16.5 mg QE/g | [74] |
| Turmeric residues | DES (choline chloride: propylene glycol, at 1:2 ratio) with 20% water, 1:40 solid-to-liquid ratio, 50 °C, 60 min | Curcumin extraction yield: 54.2 mg/g | [75] |
| Pomegranate peel waste | NADES composed of choline chloride: lactic acid (35:100), 1:20 waste-to-solvent ratio, 45 °C, 25 min extraction time | TPC: 4.14 mg GAE/mL | [76] |
| Onion skin | NADES composed of glycolic acid and L-proline with 30% w/w water, 50 mg solids with 1.5 g NADES, 30 min extraction time, 50 °C temperature | Quercetin: 18.56 ± 0.25 μg/mL | [77] |
| Uvaia residue | UAE with 40% amplitude, water as solvent, and 40 °C for 2.5 min followed by concentration of the extract with reverse osmosis | TPC: 332.22 mg GAE/100 g; TFC: 1300.18 mg/100 g | [78] |
| Cranberry pomace | PLE with ethanol 30–100%, at 40–160 °C, 50 or 200 bar | Max anthocyanins extraction (6.02–8.42 mg Cy3GE d.w.) with 100% EOH; Max TPC (84.96 ± 7.82 mg GAE d.w.) with 30% EOH at 140 °C, and 50 bar | [69]) |
| Acerola and umbu residues | UAE (40 kHZ, 40 °C, 30 min) or simple shaking (120 rpm, 40 °C, 30 min) with 80% acetone | TPC acerola: 444.05 mg GAE/100 g; TPC umbu: 404.36 mg GAE/100 g | [79] |
| Custard apple leaves | PEF at 6 kV/cm with 70% EOH | TPC: 235.55 mg GAE/g dry extract | [80] |
| Mango seed kernels | CO2-SFE with 15% EOH, 11 MPa at 60 °C | TPC: 57.3 mg GAE/g extract; TFC: 13.6 mg QE/g extract | [81] |
| Chestnut shells | MW (2.45 GHz) and NADES (choline chloride: oxalic acid, 1:1), 0.5:5 waste-to-solvent ratio, 85 °C, 60 min extraction time | TPC: 295.2 ± 3.2 mg GAE/g dry waste | [82] |
| Camelia sinensis branches | MAE at 140 °C, 1:15 solid-to-liquid ratio | TPC: ~55 mg GAE/g | [83] |
| Rosemary leaves | PLE at 183 °C, 130 bar for 3 min | Rosmarinic acid: 10 ± 1 mg/g; carnosic acid: 21 ± 1 mg/g | [84] |
| Olive pomace | UAE (10 min) with β-cyclodextrin aqueous solutions, under stirring for 21 h, at 60 °C | TPC ~3 mg GAE/g | [85] |
| Ripe mango peels | UAE (436.45 W, 19.6 min and 59.8 mL/g liquid-to-solid) with NADES composed of lactic acid/sodium acetate/water (3:1:4) | TPC: 56.17 mg GAE/g dry waste | [86] |
| Saffron petals | OHAE at 225 V for 45 min, with 5% petals and 0.3 g NaCl | TPC: 928 mg/100 g; TFC: 238 mg/100 g | [87] |
| Vitis vinifera cv Tannat grape pomace | UAE at 100 W, at 30 °C, 50 min extraction time | TPC: 21.6 ± 3.8 mg GAE/g | [88] |
| Sea bream and sea bass residues | PEF at 7000 V and 10 Hz, 20 μs pulse width and 100 number of pulses, with dH2O at 1:1 liquid-to-solid ratio | Antioxidants with DPPH activity ranging from 33.8–71.8% depending on the residue | [89] |
| Grape pomace | High voltage atmospheric cold plasma treatment at 60 kV for 15 min, followed by extraction with 50% EOH at solvent at 1:25 solid-to-liquid ratio for 2 h, under stirring at 150 rpm | TPC enhanced by 22.8% and DPPH by 34.7% | [90] |
| Tomato pomace | High voltage atmospheric cold plasma treatment with nitrogen gas at 60 kV for 15 min, followed by extraction with 50% EOH at solvent at 1:40 solid-to-liquid ratio for 15 min, under stirring at 150 rpm | TPC: 1.033 ± 0.020 mg GAE/g (9.8% improvement) | [91] |
| Compound | Fermentation Substrate | Conditions | Results | References |
|---|---|---|---|---|
| Lactic acid | Poplar hydrolysate; Corn stover hydrolysate | L. brevis ATCC 367 and L. plantarum ATCC 2102, Enzymatic hydrolysis of corn stover | 31.8 g/L from poplar hydrolysate with yield 0.80 g/g and productivity 0.48 g/L/h; 31.2 g/L from corn stover hydrolysate with yield 0.78 g/g and productivity 0.43 g/L/h | [142] |
| Cassava bagasse (CB) | Lactobacillus amylovorus (ATCC-33620) and Lactobacillus acidophilus (ATCC-4356), Enzymatic hydrolysis of CB, Corn Steep Liquor as nutrient and nitrogen source, No pH control | 31.6 g/L productivity, 0.11 g/L/h | [143] | |
| Crust bread waste (CBW) hydrolysate and lucerne green juice | Bacillus coagulans, Enzymatic hydrolysis of CBW, Continuous fermentation | 55 g/L yield, 0.48 g/g productivity, 11.28 g/L/h | [144] | |
| Defatted rice bran (DRB) hydrolysates | Bacillus coagulans A107, Enzymatic hydrolysis of DRB, Batch fermentations | 75.9 g/L yield, 0.90 g/g productivity, 2.7 g/L/h | [145] | |
| Sweet sorghum juice | Bacillus coagulans, Pilot scale fermentation (50 L) | 73 g/L lactic acid yield, 0.70 g/g productivity, 1.47 g/L/h | [146] | |
| Succinic acid | Rice husk hydrolysate | Acid hydrolysis of rice husks at pressurized reactor with 2.2% (v/v) HCl at 174 °C (59 bar) for 46 min | 12.5 g/L succinic acid yield 59.9% | [147] |
| Glycerol | Metabolic engineered Y. lipolytica based on the strain ATCC MYA-2613 no pH control | 110.7 g/L succinic acid yield 0.53 g/g | [148] | |
| Fruit and vegetable wastes hydrolyzed | A. succinogenes NJ113, Enzymatic hydrolysis of fruit and vegetable wastes with Solid State Fermentation with Aspergillus niger and Rhizopus oryzae | 27.03 g/L succinic acid yield, 1.18 g SA/g sugar productivity, 1.28 g/L/h | [149] | |
| Fumaric acid | Very high polarity (VHP) sugar and molasses from sugarcane mills | Rhizopus arrhizus NRRL 2582, 200 mg/L FAN from soybean cake hydrolysate | 40 g/L fumaric acid yield, 0.86 g/g of total consumed sugars | [150] |
| Brewery wastewater | Rhizopus oryzae 1526 immobilized mycelia on muslin cloth | 43.67 g/L fumaric acid productivity, 1.21 g/L/h | [151] | |
| Carotenoids | Cane molasses hydrolysate | Wild-type R. toruloides ACCC 20,341 and mutant R. toruloides M18 | 12.32 mg/L torularhodin and 5.21 mg/L torulene | [152] |
| Wheat straw hydrolysate | R. toruloides NRRL Y-1091, Decolorization of the cellulosic hydrolysate with activated charcoal | 24.58 mg/L carotenoids, yield 0.32 mg/g, productivity 0.26 mg/L/h | [153] | |
| Sugar cane molasses | Rhodotorula mucilaginosa CCT 7688, Corn Steep Liquor as nutrient and nitrogen source, Fed-batch fermentation | 3726 μg/L total carotenoides | [154] | |
| Bioactive peptides | Soy milk | Lactobacillus plantarum C2, Fermentation of soy milk, Analysis with LC-MS/MS | Detection of 17 biofunctional peptides with antioxidant and ACE-inhibitory activities | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandri, M.; Kachrimanidou, V.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products. Foods 2022, 11, 2796. https://doi.org/10.3390/foods11182796
Alexandri M, Kachrimanidou V, Papapostolou H, Papadaki A, Kopsahelis N. Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products. Foods. 2022; 11(18):2796. https://doi.org/10.3390/foods11182796
Chicago/Turabian StyleAlexandri, Maria, Vasiliki Kachrimanidou, Harris Papapostolou, Aikaterini Papadaki, and Nikolaos Kopsahelis. 2022. "Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products" Foods 11, no. 18: 2796. https://doi.org/10.3390/foods11182796