Occurrence of Toxic Metals and Metalloids in Muscle and Liver of Italian Heavy Pigs and Potential Health Risk Associated with Dietary Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Moisture Analysis
2.3. Quantification of TMMs
Validation of the Analytical Method
2.4. Data Processing
2.5. Exposure and Health Risk Assessment of TMMs
2.5.1. Retrieval of Toxicological Reference Values and Reference Points
2.5.2. Calculation of Dietary Intakes of TMMs
2.5.3. Health Risk Estimation
3. Results and Discussion
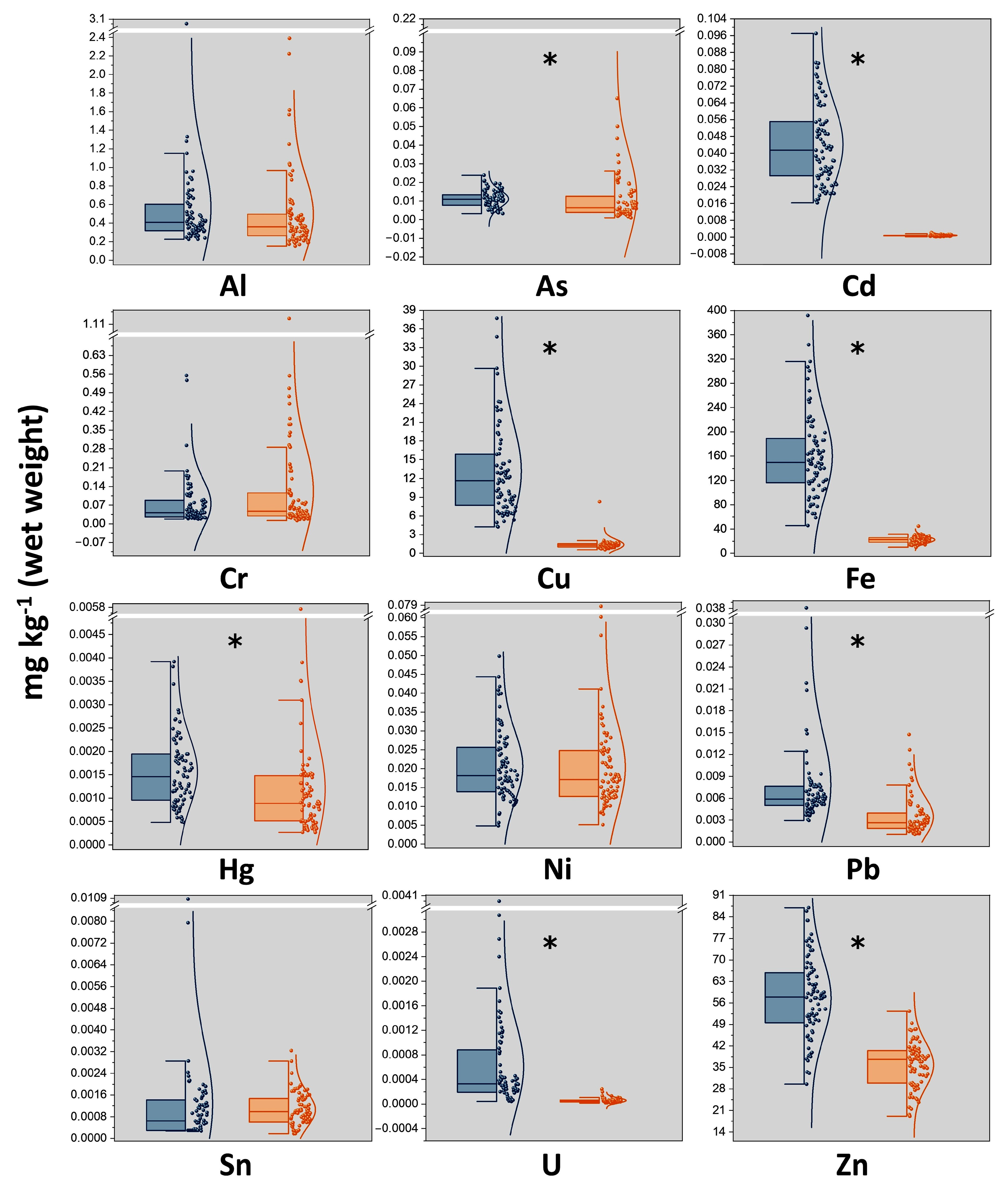
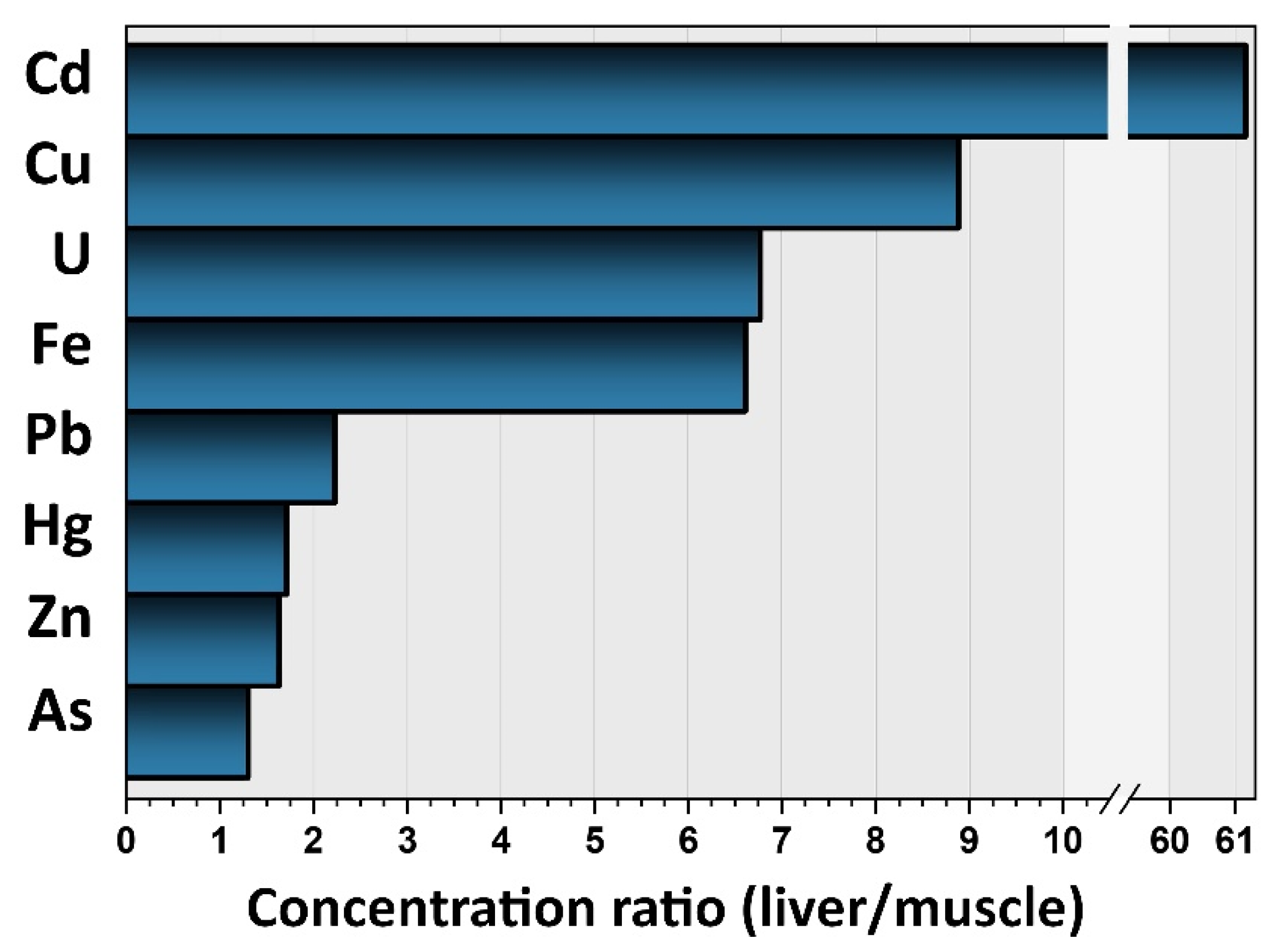
3.1. Contamination Levels of TMMs in Pig Muscle and Liver
3.2. Evaluation of Human Dietary Exposure to TMMs
3.3. Risk Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- ATSDR The Agency for Toxic Substances and Disease Registry. Substance Priority List 2019. Available online: https://www.atsdr.cdc.gov/spl/index.html#2019spl (accessed on 25 April 2022).
- Barone, G.; Storelli, A.; Quaglia, N.C.; Garofalo, R.; Meleleo, D.; Busco, A.; Storelli, M.M. Trace Metals in Pork Meat Products Marketed in Italy: Occurrence and Health Risk Characterization. Biol. Trace Elem. Res. 2021, 199, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Bilandžić, N.; Sedak, M.; Čalopek, B.; Đokić, M.; Varenina, I.; Kolanović, B.S.; Luburić, Đ.B.; Varga, I.; Hruškar, M. Dietary exposure of the adult Croatian population to meat, liver, and meat products from the Croatian market: Health risk assessment. J. Food Compos. Anal. 2021, 95, 103672. [Google Scholar] [CrossRef]
- Halagarda, M.; Wójciak, K.M. Health and safety aspects of traditional European meat products. A review. Meat Sci. 2022, 184, 108623. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to cadmium as undesirable substance in animal feed. EFSA J. 2004, 72, 1–24. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to lead as undesirable substance in animal feed. EFSA J. 2004, 71, 1–20. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to arsenic as undesirable substance in animal feed. EFSA J. 2005, 180, 1–35. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Yang, S. Effect of organic and conventional rearing system on the mineral content of pork. Meat Sci. 2016, 118, 103–107. [Google Scholar] [CrossRef]
- European Commission. EU Agricultural Outlook for Markets, Income and Environment 2020–2030; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-25645-8. [Google Scholar]
- Tuyet-Hanh, T.T.; Sinh, D.X.; Phuc, P.D.; Ngan, T.T.; Van Tuat, C.; Grace, D.; Unger, F.; Nguyen-Viet, H. Exposure assessment of chemical hazards in pork meat, liver, and kidney, and health impact implication in Hung Yen and Nghe An provinces, Vietnam. Int. J. Public Health 2017, 62, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Shang, K.; Jia, W.; Zhang, C.H.; Liu, J.Q.; Huang, D.Q. Composition of chemical elements in the edible viscera of Tibetan pigs and its correlation with environment and feed. Food Res. Int. 2020, 129, 108832. [Google Scholar] [CrossRef] [PubMed]
- Gonzàlez-Weller, D.; Karlsson, L.; Caballero, A.; Hernández, F.; Gutiérrez, A.; González-Iglesias, T.; Marino, M.; Hardisson, A. Lead and cadmium in meat and meat products consumed by the population in Tenerife Island, Spain. Food Addit. Contam. 2006, 23, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; Djinovic-stojanovic, J.; Jankovic, S.; Stanisic, N.; Radovic, C.; Pezo, L.; Lausevic, M. Mineral composition and toxic element levels of muscle, liver, and kidney of intensive (Swedish Landrace) and extensive (Mangulica) pigs from Serbia. Food Addit. Contam. Part A 2017, 34, 962–971. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, M.; Miranda, M.; Castillo, C.; Hernández, J.; García-Vaquero, M.; Benedito, J.L. Toxic and essential metals in liver, kidney, and muscle of pigs at slaughter in Galicia, north-west Spain. Food Addit. Contam. 2007, 24, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Bilandžic, N.; Maja, Đ.; Sedak, M. Survey of arsenic, cadmium, copper, mercury, and lead in kidney of cattle, horse, sheep and pigs from rural areas in Croatia. Food Addit. Contam. Part B Surveill. 2010, 3, 172–177. [Google Scholar] [CrossRef]
- Song, O.Y.; Islam, M.A.; Son, J.H.; Jeong, J.Y.; Kim, H.E.; Yeon, L.S.; Khan, N.; Jamila, N.; Kim, K.S. Elemental composition of pork meat from conventional and animal welfare farms by inductively coupled plasma-optical emission spectrometry (ICP-OES) and ICP-mass spectrometry (ICP-MS) and their authentication via multivariate chemometric analysis. Meat Sci. 2021, 172, 108344. [Google Scholar] [CrossRef]
- Batista, B.L.; Grotto, D.; Carneiro, M.F.H.; Barbosa, F. Evaluation of the concentration of nonessential and essential elements in chicken, pork, and beef samples produced in Brazil. J. Toxicol. Environ. Health Part A Curr. Issues 2012, 75, 1269–1279. [Google Scholar] [CrossRef]
- Adei, E.; Forson-adaboh, K. Toxic (Pb, Cd, Hg) and essential (Fe, Cu, Zn, Mn) metal content of liver tissue of some domestic and bush animals in Ghana. Food Addit. Contam. Part B 2008, 1, 100–105. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Liu, G.; Zhang, J.; Wang, J.; Yu, Y.; Lu, S. Concentrations and health risk assessment of trace elements in animal-derived food in southern China. Chemosphere 2016, 144, 564–570. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Kenneth Helrich, Ed.; Association of Official Analytical Chemists INC.: Arlington, VA, USA, 1990; Volume 2, ISBN 0935584420. [Google Scholar]
- Varrà, M.O.; Husáková, L.; Patočka, J.; Ghidini, S.; Zanardi, E. Multi-element signature of cuttlefish and its potential for the discrimination of different geographical provenances and traceability. Food Chem. 2021, 356, 129687. [Google Scholar] [CrossRef] [PubMed]
- Husáková, L.; Urbanová, I.; Šídová, T.; Cahová, T.; Faltys, T.; Šrámková, J. Evaluation of ammonium fluoride for quantitative microwave-assisted extraction of silicon and boron from different solid samples. Int. J. Environ. Anal. Chem. 2015, 95, 922–935. [Google Scholar] [CrossRef]
- GEMS/Food-EURO Second Workshop on Reliable Evaluation of Low-Level Contamination of Food 1995. Available online: http://toolbox.foodcomp.info/References/LOD/GEMS-Food-EURO%20%20-%20%20Reliable%20Evaluation%20of%20Low-Level%20Contamination%20of%20Food.pdf (accessed on 27 April 2022).
- EFSA. Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1557. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA J. 2005, 282, 1–31. [Google Scholar] [CrossRef] [Green Version]
- FAO; WHO. JECFA Evaluations of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/ (accessed on 1 February 2022).
- EFSA. Chemical Hazards Database (OpenFoodTox). Available online: https://www.efsa.europa.eu/en/microstrategy/openfoodtox (accessed on 22 February 2022).
- EFSA. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA J. 2014, 12, 1–261. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main Results: In terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [Green Version]
- EFSA. The EFSA Comprehensive European Food Consumption Database. Available online: https://www.efsa.europa.eu/en/data-report/food-consumption-data (accessed on 2 February 2022).
- European Commission. Regulation (EU) 2017/625 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products. Off. J. Eur. Union 2017, L95, 1–142. [Google Scholar]
- Commission of the European Communities. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Communities 2002, L140, 10–21. [Google Scholar]
- Commission of the European Communities. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- European Commission Commission. Regulation (EC) No 149/2008 of 29 January 2008 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I thereto. Off. J. Eur. Union 2008, L58, 1–566. [Google Scholar]
- Lindén, A.; Olsson, I.M.; Bensryd, I.; Lundh, T.; Skerfving, S.; Oskarsson, A. Monitoring of cadmium in the chain from soil via crops and feed to pig blood and kidney. Ecotoxicol. Environ. Saf. 2003, 55, 213–222. [Google Scholar] [CrossRef]
- Svoboda, M.; Drápal, J.; Haruštiaková, D.; Svobodová, Z. A multiannual survey of cadmium content in pig tissues collected in the Czech Republic during the years 2015–2019. Acta Vet. Brno 2020, 89, 349–355. [Google Scholar] [CrossRef]
- López-Alonso, M. Trace Minerals and Livestock: Not Too Much Not Too Little. ISRN Vet. Sci. 2012, 2012, 704825. [Google Scholar] [CrossRef] [Green Version]
- Han, J.L.; Pan, X.D.; Chen, Q. Distribution and safety assessment of heavy metals in fresh meat from Zhejiang, China. Sci. Rep. 2022, 12, 3241. [Google Scholar] [CrossRef]
- Guang-zhi, R.E.N.; Ming, W.; Zhen-tian, L.I.; Xin-jian, L.I.; Jun-feng, C. Study on the Correlations between Mineral Contents in Musculus Longissimus Dorsi and Meat Quality for Five Breeds of Pigs. Am. J. Anim. Vet. Sci. 2008, 3, 18–22. [Google Scholar] [CrossRef]
- Tomović, V.M.; Petrović, L.S.; Tomović, M.S.; Kevrešan, Ž.S.; Džinić, N.R. Determination of mineral contents of semimembranosus muscle and liver from pure and crossbred pigs in Vojvodina (northern Serbia). Food Chem. 2011, 124, 342–348. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Halagarda, M.; Rohn, S.; Kęska, P.; Latoch, A.; Stadnik, J. Selected nutrients determining the quality of different cuts of organic and conventional pork. Eur. Food Res. Technol. 2021, 247, 1389–1400. [Google Scholar] [CrossRef]
- Lopez Alonso, M.; Benedito, J.L.; Miranda, M.; Castillo, C.; Hernandez, J.; Shore, R.F. Toxic and trace elements in liver, kidney and meat from cattle slaughtered in Galicia (NW Spain). Food Addit. Contam. 2000, 17, 447–457. [Google Scholar] [CrossRef]
- Amici, A.; Danieli, P.P.; Russo, C.; Primi, R.; Amici, A.; Danieli, P.P.; Russo, C.; Primi, R.; Amici, A.; Danieli, P.P.; et al. Concentrations of some toxic and trace elements in wild boar (Sus scrofa) organs and tissues in different areas of the Province of Viterbo, Central Italy. Ital. J. Anim. Sci. 2012, 11, e65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhou, G.; Zhang, S.; Yang, Y.; Dev, S.; Su, Q.; Deng, X.; Chen, Q.; Niu, B. Risk assessment of heavy metals contamination in pork. Food Control 2022, 135, 108793. [Google Scholar] [CrossRef]
- Pei, F.; Wang, Y.; Fang, Y.; Li, P.; Yang, W.; Ma, N.; Ma, G.; Hu, Q. Concentrations of heavy metals in muscle and edible offal of pork in Nanjing city of China and related health risks. J. Food Sci. 2020, 85, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Tomović, V.M.; Petrović, L.S.; Tomović, M.S.; Kevrešan, Ž.S.; Jokanović, M.R.; Dzinić, N.R.; Despotović, A.R. Cadmium concentrations in the liver of 10 different pig genetic lines from Vojvodina, Serbia. Food Addit. Contam. Part B 2011, 4, 180–184. [Google Scholar] [CrossRef]
- Chałabis-Mazurek, A.; Valverde Piedra, J.L.; Muszynski, S.; Tomaszewska, E.; Szymanczyk, S.; Kowalik, S.; Arciszewski, M.B.; Zacharko-Siembida, A.; Schwarz, T. The Concentration of Selected Heavy Metals in Muscles, Liver and Kidneys of Pigs Fed Standard Diets and Diets Containing 60% of New Rye Varieties. Animals 2021, 11, 1377. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Mercury as undesirable substance in animal feed—Scientific opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 654, 1–76. [Google Scholar] [CrossRef]
- EFSA. Uranium in foodstuffs, in particular mineral water. Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2009, 1018, 1–59. [Google Scholar]
- Anke, M.; Seeber, O.; Müller, R.; Schäfer, U.; Zerull, J. Uranium transfer in the food chain from soil to plants, animals and man. Geochemistry 2009, 69, 75–90. [Google Scholar] [CrossRef]
- Blunden, S.; Wallace, T. Tin in canned food: A review and understanding of occurrence and effect. Food Chem. Toxicol. 2003, 41, 1651–1662. [Google Scholar] [CrossRef]
- EFSA. Re-evaluation of stannous chloride (E 512) as food additive. EFSA J. 2018, 16, 5295. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] to assess the health risks to consumers associated with exposure to organotins in foodstuffs. EFSA J. 2004, 102, 1–119. [Google Scholar] [CrossRef]
- EFSA. Safety of aluminium from dietary intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 6, 1–34. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Cadmium in food. EFSA J. 2009, 980, 1–139. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Dietary Reference Values for copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- Duran, A.; Tuzen, M.; Soylak, M. Trace element concentrations of some pet foods commercially available in Turkey. Food Chem. Toxicol. 2010, 48, 2833–2837. [Google Scholar] [CrossRef]
- Vessecchi, R.; Zafalon, A.; Pedreira, R.S.; Henrique, T.; Vendramini, A.; Rentas, M.F.; Pedrinelli, V.; Bueno, R.; Rodrigues, A.; Risolia, L.W.; et al. Toxic element levels in ingredients and commercial pet foods. Sci. Rep. 2021, 11, 21007. [Google Scholar] [CrossRef]
- Gundert-Remy, U.; Damm, G.; Foth, H.; Freyberger, A.; Gebel, T.; Golka, K.; Röhl, C.; Schupp, T.; Wollin, K.M.; Hengstler, J.G. High exposure to inorganic arsenic by food: The need for risk reduction. Arch. Toxicol. 2015, 89, 2219–2227. [Google Scholar] [CrossRef]
- IARC. Arsenic, Metals, Fibres and Dusts; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100C, ISBN 9789283213208. [Google Scholar]
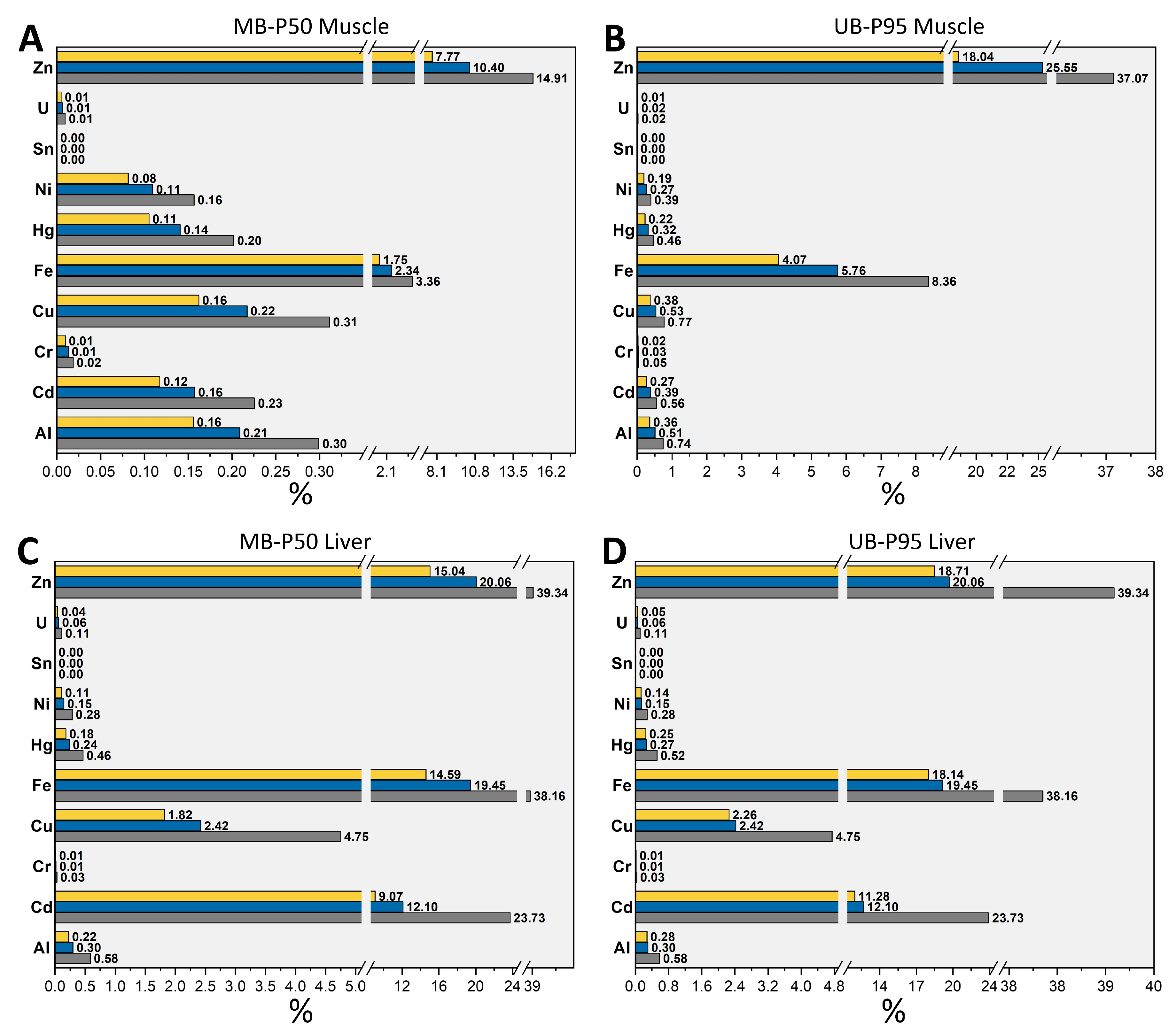

| Exposure by Pig Meat | Exposure by Pig Liver | |||||||
|---|---|---|---|---|---|---|---|---|
| TMM | HBGV/BMDL | Age Group | MB-P50 a | UB-P95 b | MB-P50 a | UB-P95 b | ||
| Al | TWI 1 mg kg bw−1 week−1 | Children | EWI (mg kg bw−1 week−1) | 2.99 × 10−3 | 7.44 × 10−3 | EWI (mg kg bw−1 week−1) | 5.83 × 10−3 | 5.83 × 10−3 |
| Adolescents | 2.09 × 10−3 | 5.13 × 10−3 | 2.97 × 10−3 | 2.97 × 10−3 | ||||
| Adults | 1.56 × 10−3 | 3.62 × 10−3 | 2.23 × 10−3 | 2.77 × 10−3 | ||||
| iAs | BDML01 0.69, 3.7, 7.5 µg kg bw−1 day−1 | Children | EDI (µg kg bw−1 day−1) | 7.63 × 10−3 | 3.98 × 10−2 | EDI (µg kg bw−1 day−1) | 3.27 × 10−2 | 3.27 × 10−2 |
| Adolescents | 5.32 × 10−3 | 2.75 × 10−2 | 1.67 × 10−2 | 1.67 × 10−2 | ||||
| Adults | 3.98 × 10−3 | 1.94 × 10−2 | 1.25 × 10−2 | 1.55 × 10−2 | ||||
| Cd | TWI 2.5 µg kg bw−1 week−1 | Children | EWI (µg kg bw−1 week−1) | 5.64 × 10−3 | 1.40 × 10−2 | EWI (µg kg bw−1 week−1) | 5.93 × 10−1 | 5.93 × 10−1 |
| Adolescents | 3.93 × 10−3 | 9.67 × 10−3 | 3.02 × 10−1 | 3.02 × 10−1 | ||||
| Adults | 2.94 × 10−3 | 6.82 × 10−3 | 2.27 × 10−1 | 2.82 × 10−1 | ||||
| Cr | TDI 0.3 mg kg bw−1 day−1 | Children | EDI (mg kg bw−1 day−1) | 5.62 × 10−5 | 1.40 × 10−4 | EDI (mg kg bw−1 day−1) | 8.48 × 10−5 | 8.48 × 10−5 |
| Adolescents | 3.92 × 10−5 | 9.64 × 10−4 | 4.32 × 10−5 | 4.32 × 10−5 | ||||
| Adults | 2.93 × 10−5 | 6.81 × 10−5 | 3.24 × 10−5 | 4.03 × 10−5 | ||||
| Cu | PMTDI 0.5 mg kg bw−1 day−1 | Children | EDI (mg kg bw−1 day−1) | 1.56 × 10−3 | 3.87 × 10−3 | EDI (mg kg bw−1 day−1) | 2.38 × 10−2 | 2.38 × 10−2 |
| Adolescents | 1.09 × 10−3 | 2.67 × 10−3 | 1.21 × 10−2 | 1.21 × 10−2 | ||||
| Adults | 8.11 × 10−4 | 1.88 × 10−3 | 9.08 × 10−3 | 1.13 × 10−2 | ||||
| Fe | PMTDI 0.8 mg kg bw−1 day−1 | Children | EDI (mg kg bw−1 day−1) | 2.69 × 10−2 | 6.69 × 10−2 | EDI (mg kg bw−1 day−1) | 3.05 × 10−1 | 3.05 × 10−1 |
| Adolescents | 1.87 × 10−2 | 4.61 × 10−2 | 1.56 × 10−1 | 1.56 × 10−1 | ||||
| Adults | 1.40 × 10−2 | 3.25 × 10−2 | 1.17 × 10−1 | 1.45 × 10−1 | ||||
| iHg | TWI 4 µg kg bw−1 week−1 | Children | EWI (µg kg bw−1 week−1) | 8.07 × 10−3 | 1.84 × 10−2 | EWI (µg kg bw−1 week−1) | 2.08 × 10−2 | 2.08 × 10−2 |
| Adolescents | 5.63 × 10−3 | 1.27 × 10−2 | 9.48 × 10−3 | 1.06 × 10−2 | ||||
| Adults | 4.21 × 10−3 | 8.95 × 10−3 | 7.11 × 10−3 | 9.90 × 10−3 | ||||
| Ni | TDI 13 µg kg bw−1 day−1 | Children | EDI (µg kg bw−1 day−1) | 2.04 × 10−2 | 5.70 × 10−2 | EDI (µg kg bw−1 day−1) | 3.70 × 10−2 | 3.70 × 10−2 |
| Adolescents | 1.42 × 10−2 | 3.49 × 10−2 | 1.89 × 10−2 | 1.89 × 10−2 | ||||
| Adults | 1.06 × 10−2 | 2.47 × 10−2 | 1.42 × 10−2 | 1.76 × 10−2 | ||||
| Pb | BMDL01/10/01 0.5/0.63/1.5 µg kg bw−1 day−1 | Children | EDI (µg kg bw−1 day−1) | 3.14 × 10−3 | 7.80 × 10−3 | EDI (µg kg bw−1 day−1) | 1.20 × 10−2 | 1.20 × 10−2 |
| Adolescents | 2.19 × 10−3 | 5.38 × 10−3 | 6.14 × 10−3 | 6.14 × 10−3 | ||||
| Adults | 1.63 × 10−3 | 3.80 × 10−3 | 4.60 × 10−3 | 5.72 × 10−3 | ||||
| U | TDI 0.6 µg kg bw−1 day−1 | Children | EDI (µg kg bw−1 day−1) | 5.76 × 10−5 | 1.43 × 10−4 | EDI (µg kg bw−1 day−1) | 6.70 × 10−4 | 6.70 × 10−4 |
| Adolescents | 4.02 × 10−5 | 9.87 × 10−5 | 3.41 × 10−4 | 3.41 × 10−4 | ||||
| Adults | 3.00 × 10−5 | 6.97 × 10−5 | 2.56 × 10−4 | 3.18 × 10−4 | ||||
| Zn | PMTDI 0.3 mg kg bw−1 day−1 | Children | EDI (mg kg bw−1 day−1) | 4.47 × 10−2 | 1.11 × 10−1 | EDI (mg kg bw−1 day−1) | 1.18 × 10−1 | 1.18 × 10−1 |
| Adolescents | 3.12 × 10−2 | 7.67 × 10−2 | 6.02 × 10−2 | 6.02 × 10−2 | ||||
| Adults | 2.33 × 10−2 | 5.41 × 10−2 | 4.51 × 10−2 | 5.61 × 10−2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidini, S.; Varrà, M.O.; Husáková, L.; Alborali, G.L.; Patočka, J.; Ianieri, A.; Zanardi, E. Occurrence of Toxic Metals and Metalloids in Muscle and Liver of Italian Heavy Pigs and Potential Health Risk Associated with Dietary Exposure. Foods 2022, 11, 2530. https://doi.org/10.3390/foods11162530
Ghidini S, Varrà MO, Husáková L, Alborali GL, Patočka J, Ianieri A, Zanardi E. Occurrence of Toxic Metals and Metalloids in Muscle and Liver of Italian Heavy Pigs and Potential Health Risk Associated with Dietary Exposure. Foods. 2022; 11(16):2530. https://doi.org/10.3390/foods11162530
Chicago/Turabian StyleGhidini, Sergio, Maria Olga Varrà, Lenka Husáková, Giovanni Loris Alborali, Jan Patočka, Adriana Ianieri, and Emanuela Zanardi. 2022. "Occurrence of Toxic Metals and Metalloids in Muscle and Liver of Italian Heavy Pigs and Potential Health Risk Associated with Dietary Exposure" Foods 11, no. 16: 2530. https://doi.org/10.3390/foods11162530







