Relationships between Instrumental and Sensory Quality Indices of Shine Muscat Grapes with Different Harvesting Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Experimental Design and Treatments
2.2. Total Soluble Solids Content and Titratable Acidity
2.3. Polymeric Tannin Content
2.4. Texture Analysis
2.5. Volatile Free Aroma Compounds
2.5.1. Free Aroma Extraction and Quantification
2.5.2. Odor Activity Value
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Total Soluble Solids Content and Titratable Acidity
3.2. Polymeric Tannin Content and Textural Properties
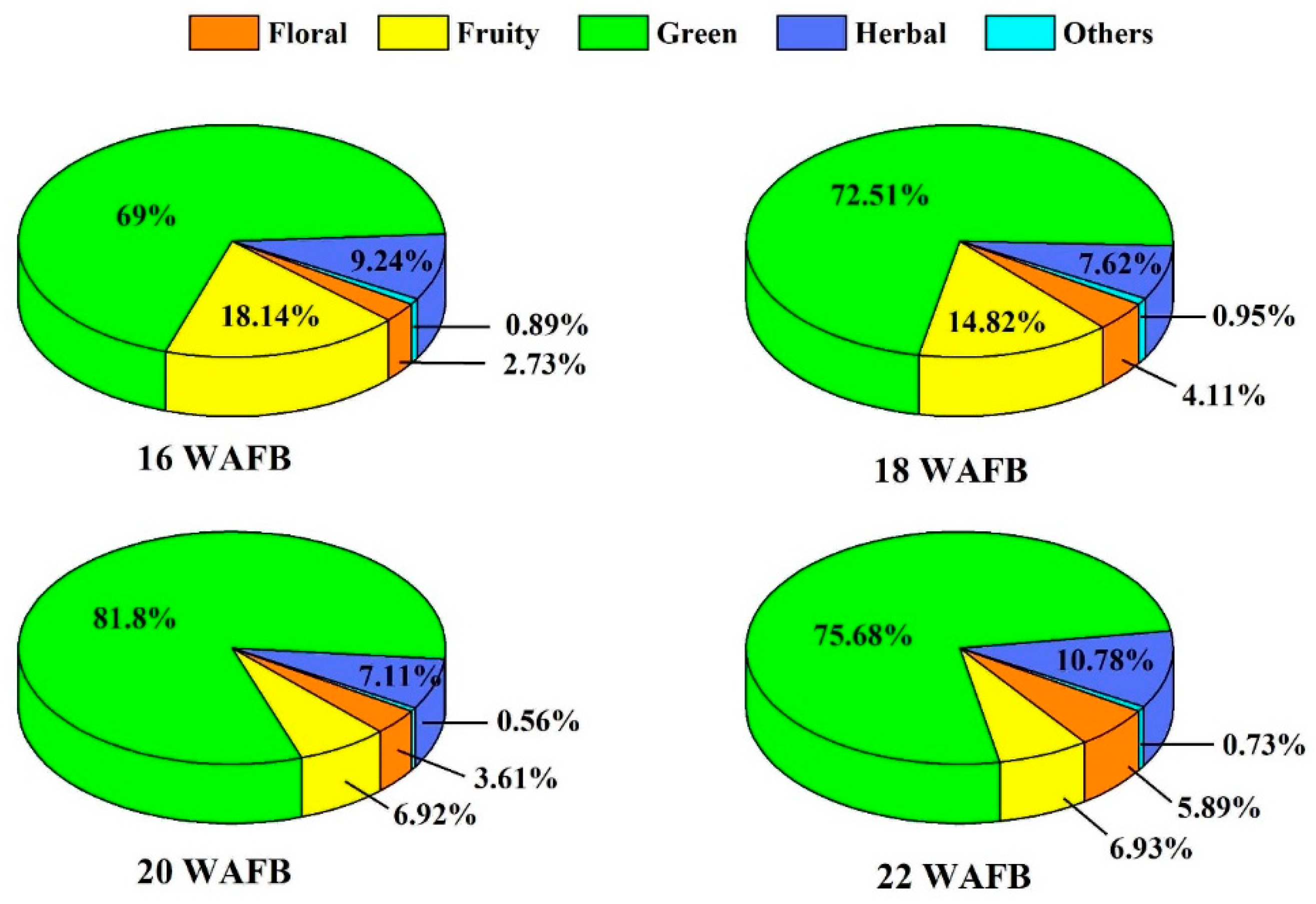
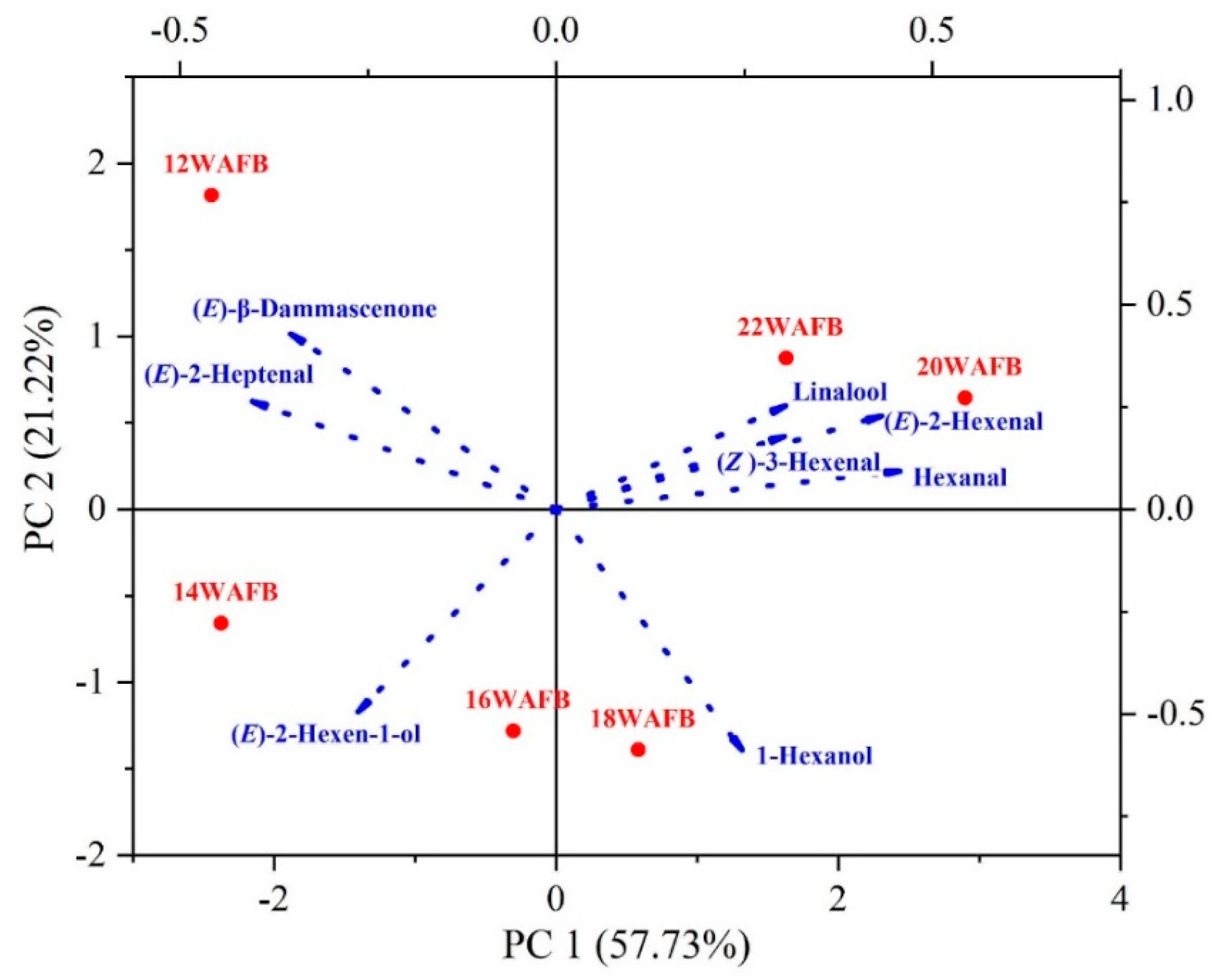
3.3. Free Volatile Aroma Compounds
3.4. Active Odorants
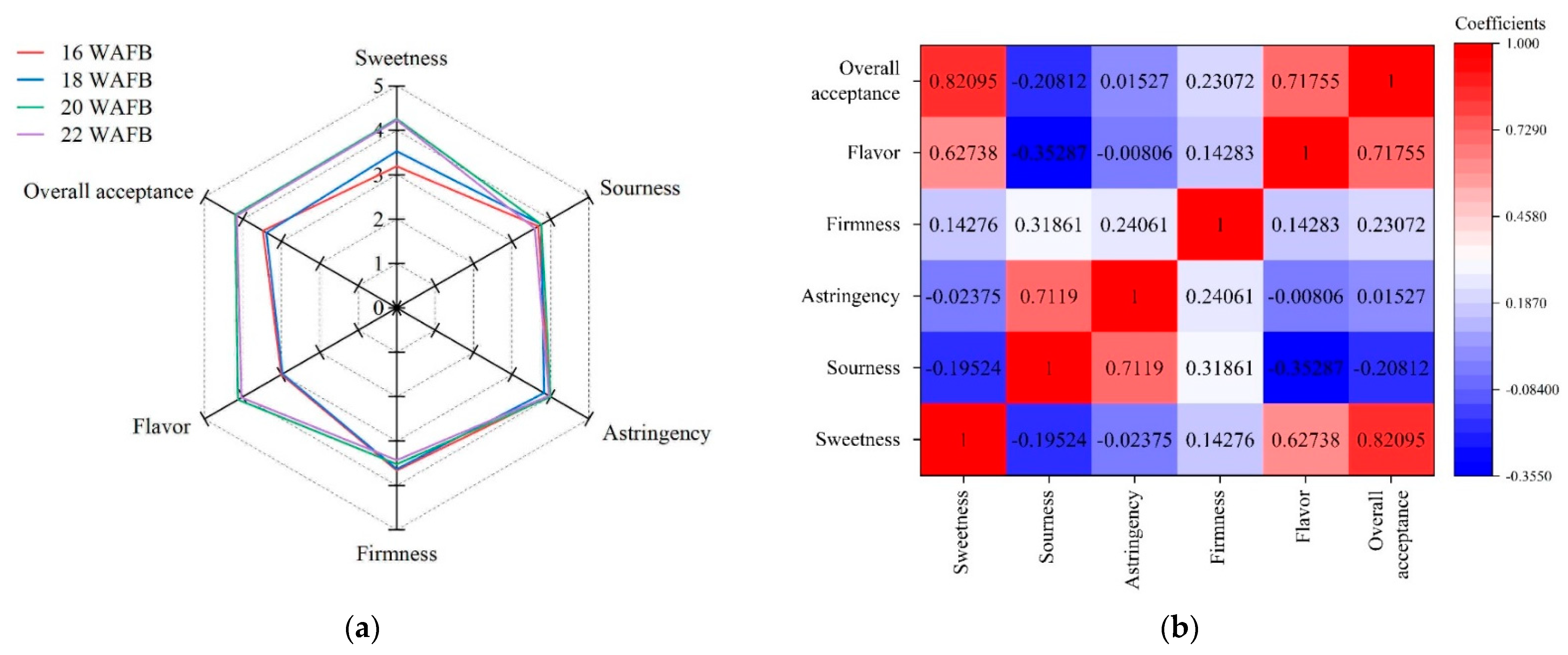
3.5. Sensory Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, K.O.; Im, D.; Park, S.J.; Lee, D.H.; Kim, S.J.; Hur, Y.Y. Effects of berry thinning on the physicochemical, aromatic, and sensory properties of Shine Muscat grapes. Horticulturae 2021, 7, 487. [Google Scholar] [CrossRef]
- Jayasena, V.; Cameron, I. °Brix/acid ratio as a predictor of consumer acceptability of Crimson Seedless table grapes. J. Food Qual. 2008, 31, 736–750. [Google Scholar] [CrossRef]
- Pothen, Z.; Nuske, S. Automated assessment and mapping of grape quality through image-based color analysis. IFAC-PapersOnLine 2016, 49, 72–78. [Google Scholar] [CrossRef]
- Melo, M.; Schultz, H.; Volschenk, C.; Hunter, J.J. Berry size variation of Vitis vinifera L. cv. Syrah: Morphological dimensions, berry composition and wine quality. S. Afr. J. Enol. Vitic. 2015, 36, 1–10. [Google Scholar] [CrossRef]
- Sasaki, T.; Ando, S.; Miyazawa, T.; Yamauchi, D.; Take, H.; Yamazaki, Y.; Enomoto, T. Characterisation of ‘Ruby Roman’ table grapes (Vitis Labruscana Bailey) by sensory evaluation and analysis of aroma and taste compounds. Food Sci. Technol. Int. 2020, 26, 423–434. [Google Scholar] [CrossRef]
- Giacosa, S.; Zeppa, G.; Baiano, A.; Torchio, F.; Segade, S.; Gerbi, V.; Rolle, L. Assessment of sensory firmness and crunchiness of tablegrapes by acoustic and mechanical properties. Aust. J. Grape Wine Res. 2015, 21, 213–225. [Google Scholar] [CrossRef]
- FAO. Standards for Table Grapes (Codex Stan 255-2007). Available online: https://www.fao.org/fao-who-codexalimentarius/search/en/?cx=018170620143701104933%3Aqq82jsfba7w&q=Standards+for+Table+Grapes+&cof=FORID%3A9 (accessed on 18 July 2022).
- Vallone, M.; Alleri, M.; Bono, F.; Catania, P. Quality evaluation of grapes for mechanical harvest using vis NIR spectroscopy. Agric. Eng. Int. CIGR J. 2019, 21, 140–149. [Google Scholar]
- Basile, T.; Perniola, R.; Cardone, M.F.; Marsico, A.D.; Antonacci, D. Analytical and sensory data correlation to understand consumers’ grape preference. BIO Web Conf. 2019, 15, 01017. [Google Scholar] [CrossRef]
- Ha, S.Y.; Hwang, Y.S.; Yang, Y.J.; Park, Y.M. Correlation between instrumental quality attributes and consumers’ sensory evaluation in refrigerated-stored ’Campbell Early’ and ‘Kyoho’ grape. Korean J. Hortic. Sci. Technol. 2007, 25, 125–132. [Google Scholar]
- Choi, K.O.; Lee, D.H.; Park, S.J.; Im, D.; Hur, Y.Y. Correlations between phenolic composition and perceived astringency of wines. Appl. Sci. 2020, 10, 8020. [Google Scholar] [CrossRef]
- Woo, A.H.; Lindsay, R.C. Method for the routine quantitative gas chromatographic analysis of major free fatty acids in butter and cream. J. Dairy Sci. 1980, 63, 1058–1064. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, R.; Hao, L.; Chen, W.; Cheng, S. Profiling of volatile compounds and associated gene expression and enzyme activity during fruit development in two cucumber cultivars. PLoS ONE 2015, 10, e0119444. [Google Scholar] [CrossRef]
- Han, D.; Mi, S.; Zhang, C.H.; Li, J.; Song, H.L.; Fauconnier, M.L.; Tyteca, E. Characterization and discrimination of Chinese marinated pork hocks by volatile compound profiling using solid phase microextraction gas chromatography-mass spectrometry/olfactometry, electronic nose and chemometrics. Molecules 2019, 24, 1385. [Google Scholar] [CrossRef]
- Han, Z.X.; Rana, M.M.; Liu, G.F.; Gao, M.J.; Li, D.X.; Wu, F.G.; Li, X.B.; Wan, X.C.; Wei, S. Data on green tea flavor determinantes as affected by cultivars and manufacturing processes. Data Brief 2017, 10, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Tortorice, M.; Ryo, S.; Li, X.; Waterman, K.; Hagen, A.; Yin, Y. Sensory lexicons and formation pathways of fff-aromas in dairy ingredients: A review. Molecules 2020, 25, 569. [Google Scholar] [CrossRef]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, W. The volatile constituents in the peel and pulp of a green Thai mango, Khieo Sawoei cultivar (Mangifera indica L.). Food Sci. Technol. Int. 2001, 7, 72–77. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef]
- Choi, K.O.; Lee, D.H.; Park, S.J.; Im, D.; Hur, Y.Y.; Kim, S.J. Changes in biochemical and volatile flavor compounds of Shine Muscat at different ripening stages. Appl. Sci. 2020, 10, 5661. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Ren, J.; Qi, J.; Zhang, G.; Leng, P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010, 10, 257–267. [Google Scholar] [CrossRef]
- Daniels, A.J.; Poblete-Echeverria, C.; Opara, U.L.; Nieuwoudt, H.H. Measuring internal maturity parameters contactless on intact table grape bunches using NIR spectroscopy. Front. Plant Sci. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Munoz-Robredo, P.; Robledo, P.; Manriquez, D.; Molina, R.; Defilippi, B.G. Characterization of sugars and organic acids in commercial varieties of table grapes. Chil. J. Agric. Res. 2011, 71, 452–458. [Google Scholar] [CrossRef]
- Rolle, L.; Río Segade, S.; Torchio, F.; Giacosa, S.; Cagnasso, E.; Marengo, F.; Gerbi, V. Influence of grape density and harvest date on changes in phenolic composition, phenol extractability indices, and instrumental texture properties during ripening. J. Agric. Food Chem. 2011, 59, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Crespan, M.; Migliaro, D.; Vezzulli, S.; Zenoni, S.; Tornielli, G.B.; Giacosa, S.; Paissoni, M.; Segade, S.; Rolle, L. A Major QTL is associated with berry grape texture characteristics. OENO One 2021, 55, 183–206. [Google Scholar] [CrossRef]
- Lanaridis, P.; Salaha, M.-J.; Tzourou, I.; Tsoutsouras, E.; Karagiannis, S. Volatile compounds in grapes and wines from two muscat varieties cultivated in greek islands. OENO One 2002, 36, 39–47. [Google Scholar] [CrossRef]
- Garcia, E.; Chacon, J.; Martinez, J.; Izquierdo, P. Changes in volatile compounds during ripening in grapes of Airen, Macabeo and Chardonnay white varieties grown in La Mancha Region (Spain). Food Sci. Technol. Int. 2003, 9, 33–41. [Google Scholar] [CrossRef]
- Wilson, B.; Strauss, C.R.; Williams, P.J. Changes in free and glycosidically bound monoterpenes in developing muscat grapes. J. Agric. Food Chem. 1984, 32, 919–924. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
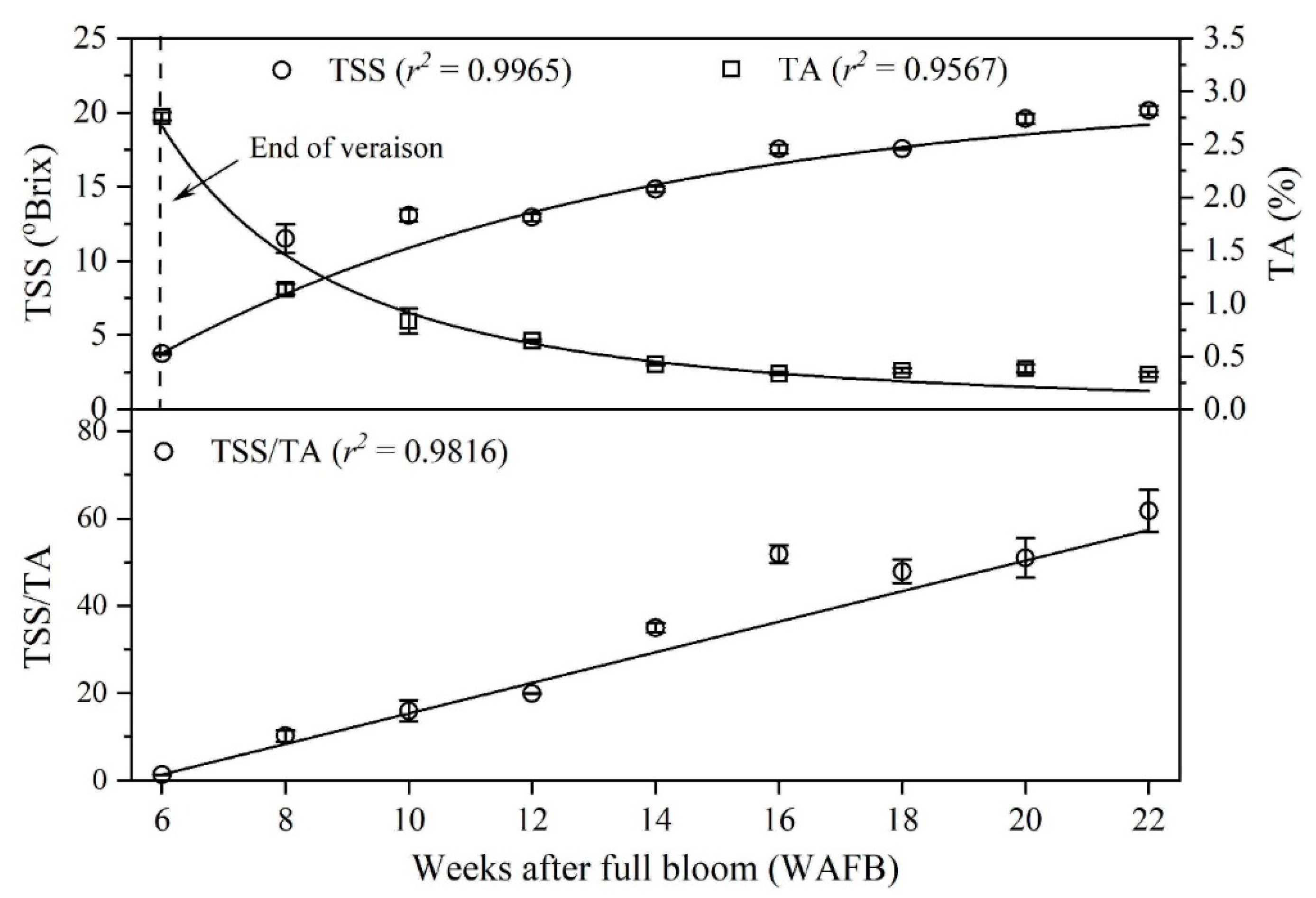
| 1 WAFB | 2 TSS (°Bx) | 3 TA (%) | 4 PTC (mgTAE/gDW) | Textural Properties | |
|---|---|---|---|---|---|
| Skin Rupture Strength (N) | Flesh Firmness (N) | ||||
| 16 | 17.5 ± 0.3 b | 0.34 ± 0.01 a | 9.1 ± 0.3 b | 10.99 ± 1.26 a | 2.48 ± 0.32 a |
| 18 | 17.6 ± 0.1 b | 0.37 ± 0.02 a | 10.2 ± 0.5 a | 6.8 ± 1.11 b | 1.73 ± 0.14 b |
| 20 | 19.6 ± 0.3 a | 0.39 ± 0.04 a | 10.0 ± 0.4 a | 5.99 ± 0.83 b | 1.82 ± 0.25 b |
| 22 | 20.1 ± 0.3 a | 0.33 ± 0.02 a | 10.1 ± 0.4 a | 6.14 ± 0.39 b | 1.99 ± 0.12 b |
| Group | Aroma Compounds | 1 RI | Weeks After Full Bloom | |||||
|---|---|---|---|---|---|---|---|---|
| 12 | 14 | 16 | 18 | 20 | 22 | |||
| Alcohol | 2-Ethyl-1-hexanol | 1510 | 4.0(0.3) c | 4.4(0.2) bc | 4.7(0.3) b | 5.4(0.1) a | 5.4(0.3) a | 4.6(0.2) bc |
| 1-Heptanol | 1472 | 0.3(0.03) b | 0.5(0.1) a | 0.4(0.1) ab | - | - | - | |
| 1-Hexanol | 1373 | 168.6(65.2) d | 366.9(18.8) c | 724.3(18.4) b | 938.7(56.1) a | 653.8(98.6) b | 403.1(39.5) c | |
| (E)-2-Hexen-1-ol | 1426 | 294.7(79.6) cd | 893.6(41.0) a | 623.1(84.0) b | 439.3(22.8) c | 279.1(48.7) d | 279.5(19.9) d | |
| (E)-3-Hexen-1-ol | 1382 | - | 0.9(0.03) a | - | 0.3(0.02) b | 0.8(0.1) a | 0.5(0.1) b | |
| (Z)-3-Hexen-1-ol | 1400 | 33.1(1.4) c | 42.9(0.3) b | 54(2.5) a | 45.1(3.1) b | 29.1(0.6) c | 17.3(1.0) d | |
| 1-Nonanol | 1679 | 0.2(0.01) a | 0.1(0.01) b | 0.1(0.01) b | 0.1(0.003) c | 0.1(0.002) c | 0.1(0.01) c | |
| (E)-2-Hepten-1-ol | 1529 | - | 0.1(0.01) | - | - | - | - | |
| 1-Octanol | 1577 | 0.5(0.03) a | 0.6(0.1) a | - | - | 0.3(0.08) b | 0.5(0.1) a | |
| 1-Octen-3-ol | 1472 | 0.1(0.02) a | 0.2(0.04) a | 0.2(0.02) a | 0.2(0.04) a | 0.1(0.03) a | 0.1(0.03) a | |
| (E)-2-Penten-1-ol | 1335 | - | 0.5(0.04) c | 0.3(0.03) c | 0.4(0.03) c | 1.2(0.3) b | 2.2(0.3) a | |
| Subtotal | 501.5 | 1310.7 | 1407.1 | 1429.5 | 969.9 | 707.9 | ||
| Aldehyde | Benzaldehyde | 1525 | 2.9(0.1) b | 2.1(0.5) cd | 2.5(0.2) bc | 4.0(0.03) a | 1.8(0.2) d | 1.6(0.2) d |
| Hexanal | 1101 | 773.6(79.2) c | 781.8(72.0) c | 1069.2(70.6) b | 1104.5(124.1) b | 1643.6(94.1) a | 1414.7(86.5) a | |
| (E)-2-Heptenal | 1329 | 0.8(0.1) | 0.8(0.01) | - | - | - | - | |
| (E)-2-Hexenal | 1215 | 1067(158.2) ab | 922.9(121.6) b | 1054.7(236.3) ab | 1127.1(182.3) ab | 1411.5(103.6) a | 710.2(55.5) b | |
| (Z)-3-Hexenal | 1154 | 4.3(0.4) e | 3.8(0.001) de | 4.6(0.2) cd | 5.2(0.4) c | 8.5(0.1) a | 6.6(0.3) b | |
| Nonanal | 1404 | 0.3(0.01) ab | 0.3(0.1) ab | 0.4(0.1) a | 0.3(0.1) ab | 0.2(0.01) bc | 0.1(0.004) c | |
| (E)-2-Octenal | 1435 | - | - | - | 0.2(0.02) | - | - | |
| Subtotal | 1848.9 | 1711.7 | 2131.4 | 2241.3 | 3065.6 | 2133.2 | ||
| Ester | Methyl salicylate | 1761 | 0.7(0.1) b | 1.4(0.2) a | 0.4(0.1) c | 0.3(0.04) cd | 0.03(0.002) d | 0.03(0.01) d |
| Ethyl octanoate | 1446 | 0.2(0.1) | - | - | - | - | - | |
| Subtotal | 0.9 | 1.4 | 0.4 | 0.3 | 0.03 | 0.03 | ||
| Ketone | (E)-β-Damascenone | 1817 | 0.68(0.07) a | 0.21(0.01) b | 0.15(0.002) b | 0.02(0.003) c | 0.02(0.001) c | 0.04(0.004) c |
| 2-Heptanone | 1195 | 0.9(0.1) b | 1.2(0.1) a | 0.5(0.1) c | 0.2(0.1) d | 0.3(0.1) d | 0.2(0.04) d | |
| sulcatone | 1351 | 2.8(0.6) b | 3.6(1.0) ab | 3.3(0.8) ab | 3.9(0.4) ab | 4.6(0.5) a | 3.1(0.1) ab | |
| 2-Octanone | 1296 | 4.1(0.5) a | 3.3(0.3) b | 1.7(0.003) c | 0.5(0.1) d | 0.4(0.1) d | 0.4(0.04) d | |
| Subtotal | 9.0 | 8.9 | 5.7 | 4.6 | 5.5 | 3.9 | ||
| Terpenoid | (Z)-Citral | 1685 | - | - | 0.2(0.03) a | 0.1(0.01) b | - | 0.1(0.01) b |
| (R)-(+) -β-Citronellol | 1785 | 1.4(0.1) b | 2.2(0.2) a | 2.2(0.03) a | 1.5(0.1) b | 0.2(0.1) d | 0.6(0.2) c | |
| Epoxylinalool | 1750 | - | - | - | 1.8(0.4) b | 2.0(0.2) b | 3.0(0.6) a | |
| Geraniol | 1863 | 8.9(1.7) d | 14.6(0.5) c | 24.8(2.7) b | 29.7(2.0) a | 7.3(0.6) d | 10.0(0.8) d | |
| Hotrienol | 1632 | 2.1(0.1) bc | 1.9(0.1) bc | 2.4(0.3) ab | 2.6(0.04) a | 1.6(0.2) c | 2.6(0.3) a | |
| D-Limonene | 1204 | 0.1(0.01) c | 0.1(0.02) c | 0.1(0.01) c | 0.2(0.02) b | 0.2(0.04) b | 0.3(0.1) a | |
| Linalool | 1571 | 37.9(4.7) d | 37.1(3.3) d | 40.3(4.7) cd | 64.2(8.4) c | 90.4(3.6) b | 189.1(19.1) a | |
| (E)-Linalool oxide | 1451 | - | - | - | 0.04(0.002) b | 0.1(0.02) a | 0.1(0.01) a | |
| β-Myrcene | 1181 | - | 0.04(0.01) c | 0.1(0.003) b | 0.1(0.003) b | 0.1(0.01) b | 0.2(0.02) a | |
| Nerol | 1814 | 1.5(0.2) d | 3.5(0.6) c | 4.6(0.4) b | 6.3(0.7) a | 1.6(0.1) d | 2.1(0.3) d | |
| Nerol oxide | 1481 | 1.7(0.1) a | 1.8(0.1) a | 0.6(0.1) c | 1.2(0.2) b | - | - | |
| (E)-β-Ocimene | 1266 | 0.1(0.05) d | 0.2(0.03) cd | 0.2(0.02) c | 0.3(0.04) bc | 0.4(0.1) b | 0.6(0.02) a | |
| (Z)-β-Ocimene | 1251 | 0.2(0.03) e | 0.3(0.01) de | 0.3(0.01) cd | 0.2(0.01) bc | 0.3(0.04) b | 0.6(0.01) a | |
| α-Phellandrene | 1168 | - | 0.01(0.001) b | 0.01(0.003) b | 0.01(0.002) b | 0.01(0.002) b | 0.04(0.01) a | |
| β-Phellandrene | 1210 | - | 0.02(0.01) b | 0.02(0.001) b | 0.03(0.001) b | 0.03(0.01) ab | 0.05(0.01) a | |
| β-Pinene | 1177 | 0.2(0.01) e | 0.4(0.02) d | 0.5(0.01) c | 0.7(0.02) b | 0.6(0.03) b | 0.8(0.09) a | |
| (Z)-Rose oxide | 1358 | 0.01(0.002) e | 0.01(0.002) de | 0.02(0.002) c | 0.01(0.001) cd | 0.03(0.002) b | 0.04(0.001) a | |
| Terpinen-4-ol | 1606 | - | - | - | - | 0.04(0.01) | 0.1(0.01) | |
| γ-Terpinene | 1242 | - | 0.04(0.01) c | 0.1(0.01) a | 0.05(0.01) ab | - | - | |
| α-Terpineol | 1706 | 1.0(0.2) d | 10.8(0.2) c | 11.5(1.1) c | 16.4(0.2) b | 16.8(0.7) b | 26.2(3.9) a | |
| Terpinolene | 1278 | - | 0.04(0.02) c | 0.1(0.004) c | 0.1(0.01) b | 0.2(0.01) a | 0.2(0.04) a | |
| Subtotal | 55.1 | 73.1 | 88.1 | 125.6 | 121.9 | 236.7 | ||
| Total | 2415 | 3106 | 3634 | 3802 | 4163 | 3082 | ||
| Aroma Compounds | Odor Type | Odor Threshold (ug/L) | Odor Activity Value (OAV) | |||||
|---|---|---|---|---|---|---|---|---|
| 12 1 WAFB | 14 WAFB | 16 WAFB | 18 WAFB | 20 WAFB | 22 WAFB | |||
| 2-Ethyl-1-hexanol | Citrus | 270 | 0.015 | 0.016 | 0.017 | 0.02 | 0.02 | 0.017 |
| 1-Heptanol | Green | 425 | 0.001 | 0.001 | 0.001 | - | - | - |
| 1-Hexanol | Herbal | 500 | 0.337 | 0.734 | 1.449 | 1.877 | 1.308 | 0.806 |
| (E)-2-Hexen-1-ol | Fruity | 100 | 2.947 | 8.936 | 6.231 | 4.393 | 2.791 | 2.795 |
| (E)-3-Hexen-1-ol | Green | 1000 | - | 0.001 | - | 0.0003 | 0.001 | 0.001 |
| (Z)-3-Hexen-1-ol | Green | 70 | 0.473 | 0.613 | 0.771 | 0.644 | 0.416 | 0.247 |
| 1-Nonanol | Floral | 50 | 0.004 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| (E)-2-Hepten-1-ol | Fatty | N.A | N.A | N.A | N.A | N.A | N.A | N.A |
| 1-Octanol | Waxy | 110 | 0.005 | 0.005 | - | - | 0.003 | 0.005 |
| 1-Octen-3-ol | Earthy | 1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 |
| (Z)-2-Penten-1-ol | Green | 720 | - | 0.001 | 0.0004 | 0.001 | 0.002 | 0.003 |
| Benzaldehyde | Fruity | 350 | 0.008 | 0.006 | 0.007 | 0.011 | 0.005 | 0.005 |
| Hexanal | Green | 4.5 | 171.911 | 173.733 | 237.6 | 245.444 | 365.244 | 314.378 |
| (E)-2-Heptenal | Green | 0.8 | 1 | 1 | - | - | - | - |
| (E)-2-Hexenal | Green | 17 | 62.765 | 54.288 | 62.041 | 66.3 | 83.029 | 41.776 |
| (Z)-3-Hexenal | Green | 0.25 | 17.2 | 15.2 | 18.4 | 20.8 | 34 | 26.4 |
| Nonanal | Aldehydic | 1 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.1 |
| (E)-2-Octenal | Fatty | 3 | - | - | - | 0.067 | - | - |
| Methyl salicylate | Minty | 40 | 0.018 | 0.035 | 0.01 | 0.008 | 0.001 | 0.001 |
| Ethyl octanoate | Waxy | 194 | 0.001 | - | - | - | - | - |
| (E)-β-Damascenone | Fruity | 0.002 | 340 | 105 | 75 | 10 | 10 | 20 |
| 2-Heptanone | Cheese | 140 | 0.006 | 0.009 | 0.004 | 0.001 | 0.002 | 0.001 |
| sulcatone | 50 | 0.056 | 0.072 | 0.066 | 0.078 | 0.092 | 0.062 | |
| 2-Octanone | Earthy | 50 | 0.082 | 0.066 | 0.034 | 0.01 | 0.008 | 0.008 |
| (Z)-Citral | Citrus | 30 | - | - | 0.007 | 0.003 | - | 0.003 |
| (R)-(+)-β-Citronellol | Floral | 40 | 0.035 | 0.055 | 0.055 | 0.038 | 0.005 | 0.015 |
| Epoxylinalool | Floral | 6 | - | - | - | 0.3 | 0.333 | 0.5 |
| Geraniol | Floral | 40 | 0.223 | 0.365 | 0.62 | 0.743 | 0.183 | 0.25 |
| Hotrienol | Tropical | 110 | 0.019 | 0.017 | 0.022 | 0.024 | 0.015 | 0.024 |
| D-Limonene | Citrus | 10 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 |
| Linalool | Floral | 6 | 6.317 | 6.183 | 6.717 | 10.7 | 15.067 | 31.517 |
| (E)-Linalool oxide | Floral | 6 | - | - | - | 0.007 | 0.017 | 0.017 |
| β-Myrcene | Spicy | 36 | - | 0.001 | 0.003 | 0.003 | 0.003 | 0.006 |
| Nerol | Floral | 300 | 0.005 | 0.012 | 0.015 | 0.021 | 0.005 | 0.007 |
| Nerol oxide | Green | 3000 | 0.001 | 0.001 | 0.0002 | 0.0004 | - | - |
| (E)-β-Ocimene | Floral | 340 | 0.0003 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 |
| (Z)-β-Ocimene | Floral | 34 | 0.006 | 0.009 | 0.009 | 0.006 | 0.009 | 0.018 |
| α-Phellandrene | Terpenic | 8 | - | 0.001 | 0.001 | 0.001 | 0.001 | 0.005 |
| β-Phellandrene | Minty | 8 | - | 0.003 | 0.003 | 0.004 | 0.004 | 0.006 |
| β-Pinene | Herbal | 140 | 0.001 | 0.003 | 0.004 | 0.005 | 0.004 | 0.006 |
| (Z)-Rose oxide | Floral | 0.5 | 0.02 | 0.02 | 0.04 | 0.02 | 0.06 | 0.08 |
| Terpinen-4-ol | Spicy | 130 | - | - | - | - | 0.0003 | 0.001 |
| γ-Terpinene | Terpenic | 1000 | - | 0.00004 | 0.0001 | 0.00005 | - | - |
| α-Terpineol | Terpenic | 330 | 0.003 | 0.033 | 0.035 | 0.05 | 0.051 | 0.079 |
| Terpinolene | Herbal | 200 | - | 0.0002 | 0.001 | 0.001 | 0.001 | 0.001 |
| 1 WAFB | Sweetness | Sourness | Astringency | Firmness | Flavor | Overall Acceptance |
|---|---|---|---|---|---|---|
| 16 | 3.19 ± 0.8 b | 3.68 ± 0.8 a | 3.97 ± 1.1 a | 3.66 ± 1.1 a | 3.00 ± 1.0 b | 3.48 ± 1.0 b |
| 18 | 3.53 ± 1.2 ab | 3.76 ± 1.2 a | 3.83 ± 1.2 a | 3.63 ± 1.1 a | 2.97 ± 1.1 b | 3.39 ± 0.8 b |
| 20 | 4.26 ± 1.1 a | 3.76 ± 0.9 a | 4.00 ± 1.2 a | 3.52 ± 1.0 a | 4.13 ± 1.5 a | 4.20 ± 1.1 a |
| 22 | 4.23 ± 1.1 a | 3.59 ± 1.5 a | 3.96 ± 0.5 a | 3.43 ± 0.7 a | 4.04 ± 1.0 a | 4.16 ± 0.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.-O.; Hur, Y.Y.; Park, S.J.; Lee, D.H.; Kim, S.J.; Im, D. Relationships between Instrumental and Sensory Quality Indices of Shine Muscat Grapes with Different Harvesting Times. Foods 2022, 11, 2482. https://doi.org/10.3390/foods11162482
Choi K-O, Hur YY, Park SJ, Lee DH, Kim SJ, Im D. Relationships between Instrumental and Sensory Quality Indices of Shine Muscat Grapes with Different Harvesting Times. Foods. 2022; 11(16):2482. https://doi.org/10.3390/foods11162482
Chicago/Turabian StyleChoi, Kyeong-Ok, Youn Young Hur, Seo Jun Park, Dong Hoon Lee, Su Jin Kim, and Dongjun Im. 2022. "Relationships between Instrumental and Sensory Quality Indices of Shine Muscat Grapes with Different Harvesting Times" Foods 11, no. 16: 2482. https://doi.org/10.3390/foods11162482







