Changes in Gel Structure and Chemical Interactions of Hypophthalmichthys molitrix Surimi Gels: Effect of Setting Process and Different Starch Addition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Low Field Nuclear Magnetic Resonance (LF-NMR)
2.4. Scanning Electron Microscope (SEM)
2.5. Light Microscopy (LM)
2.6. Determination of Non-Covalent Bonds
2.7. Determination of Total Sulfhydryl Groups
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
2.9. Texture Profile Analysis (TPA)
2.10. Determination of Whiteness
2.11. Determination of Water Holding Capacity (WHC)
2.12. Statistical Analysis
3. Results and Discussion
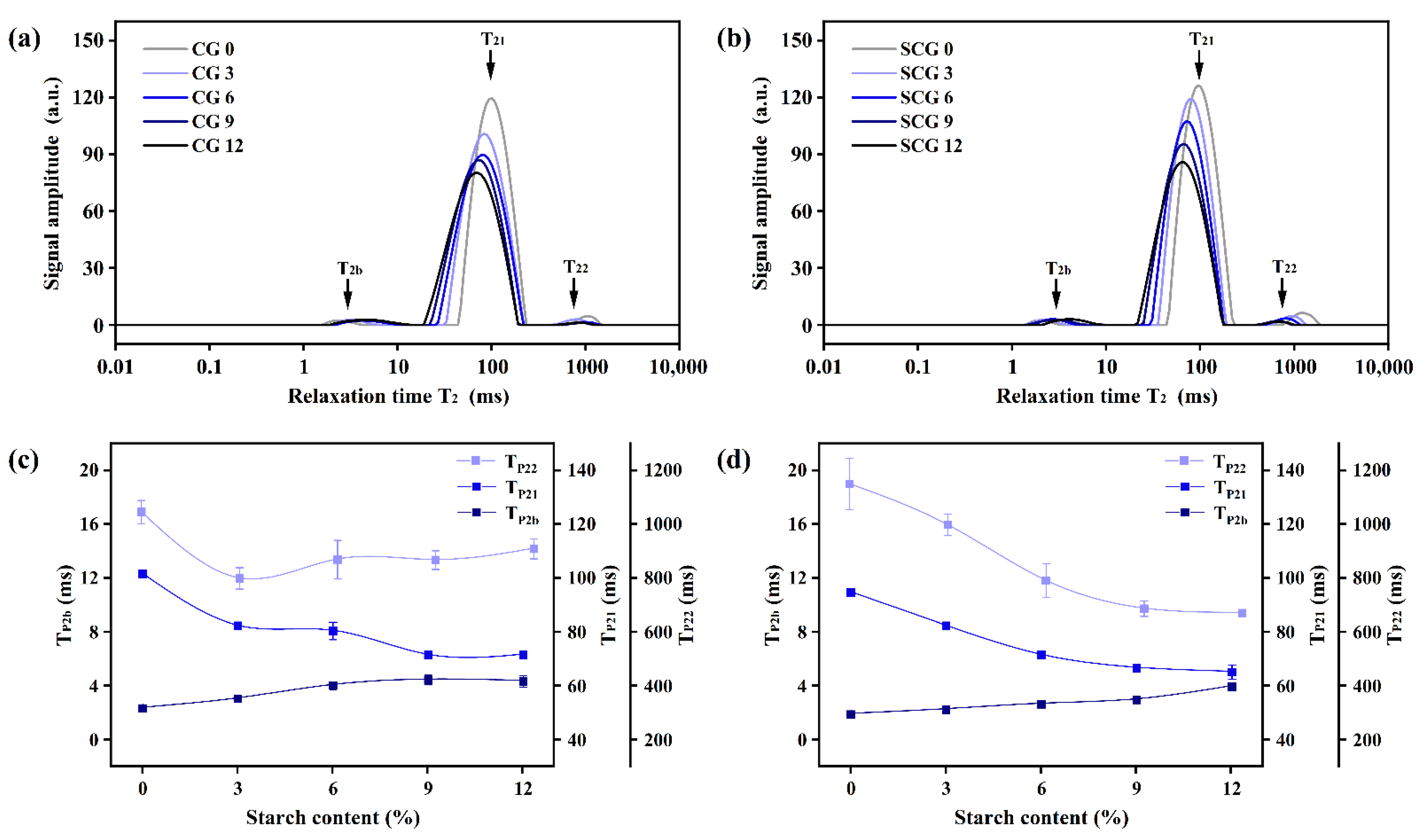
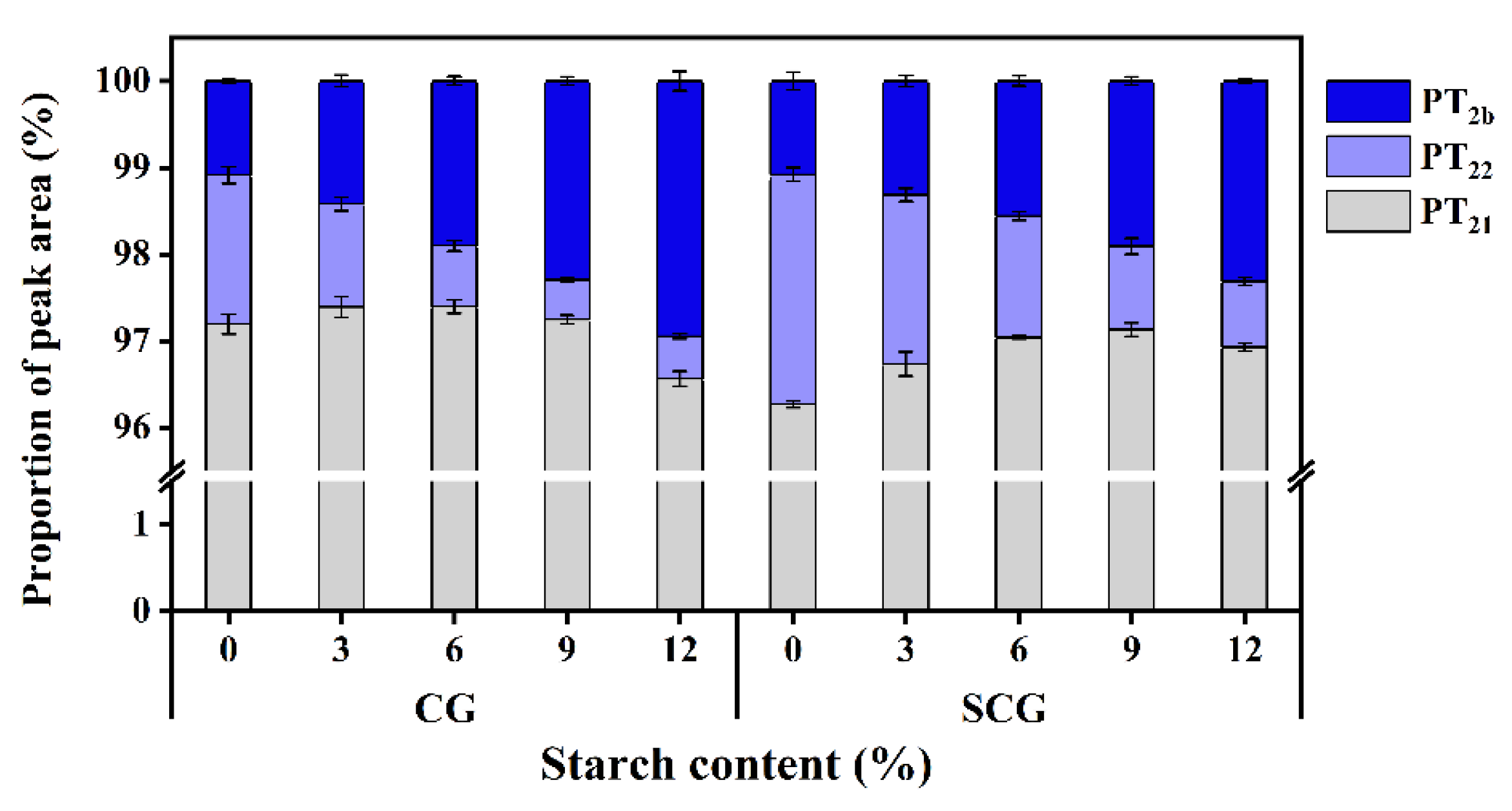
3.1. Changes of Relaxation Time and Water Distribution of Starch–Surimi Gels with Non-Setting or Setting Effect
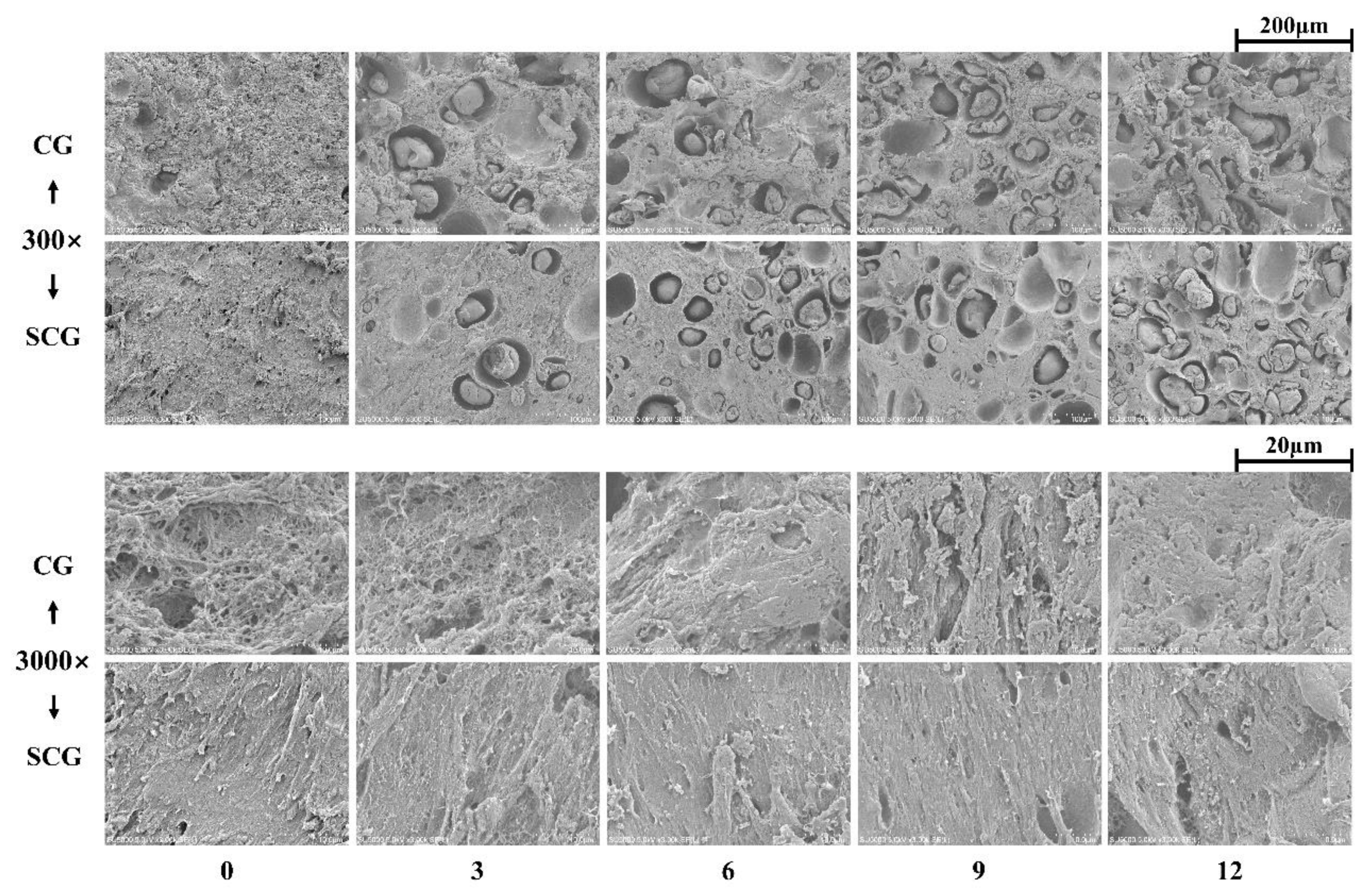
3.2. Changes in Tissue Histology of Starch–Surimi Gels with Non-Setting or Setting Effect
3.2.1. Light Micrograph
3.2.2. Scanning Electron Micrograph
3.3. Changes in Chemical Interactions of Starch–Surimi Gels with Non-Setting or Setting Effect
3.3.1. Non-Covalent Bonds
3.3.2. Total Sulfhydryl Groups
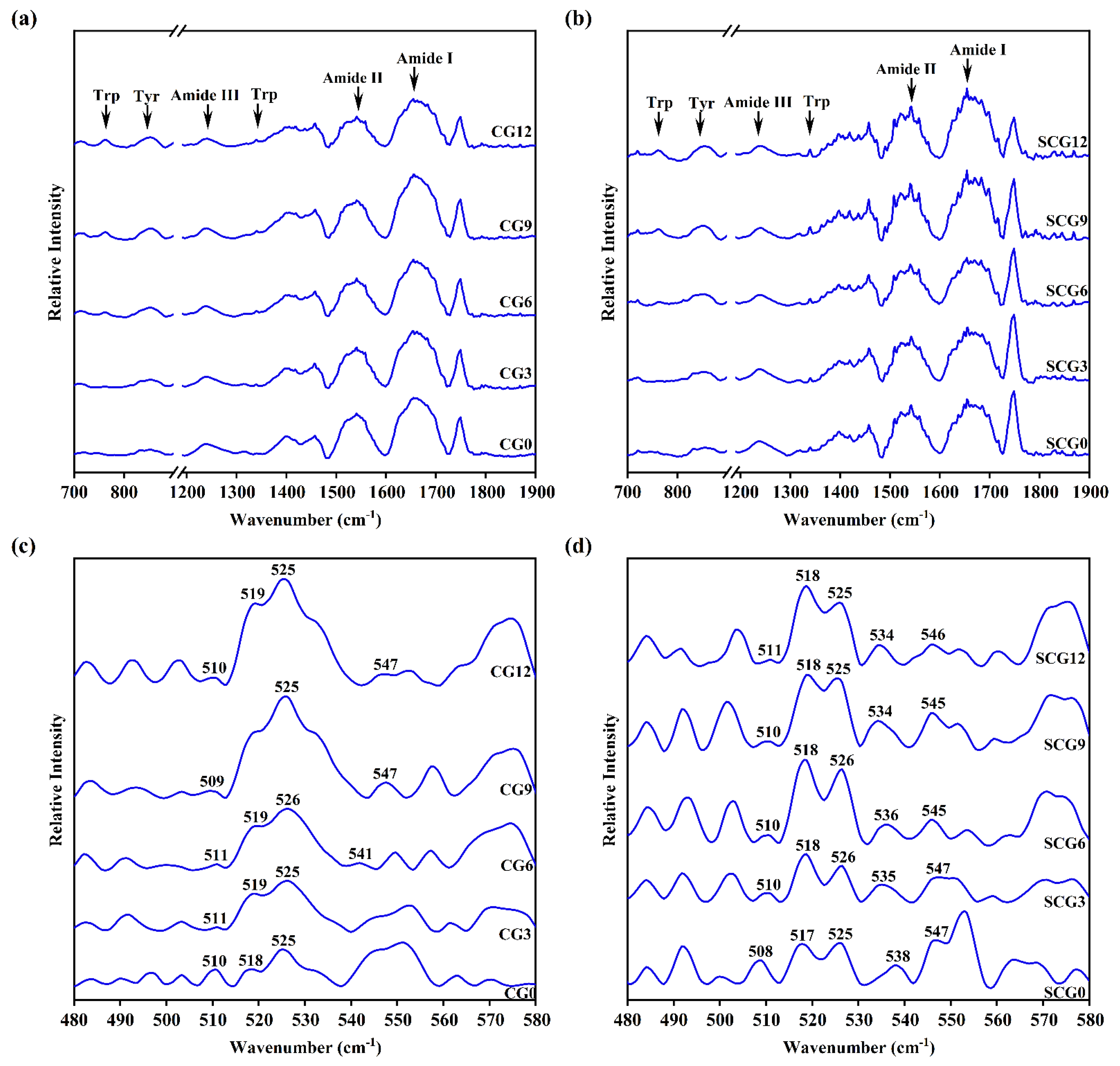
3.4. FT-IR Spectroscopy Analysis of Starch–Surimi Gels with Non-Setting or Setting Effect
3.4.1. Amide Bands of Protein
3.4.2. Tryptophan (Trp) Residue Bands and Tyrosine (Tyr) Doublet Bands
3.4.3. S-S Stretching
3.5. Changes in Gel Properties of Starch–Surimi Gels with Non-Setting or Setting Effect
3.5.1. Texture Profile Analysis
3.5.2. Whiteness
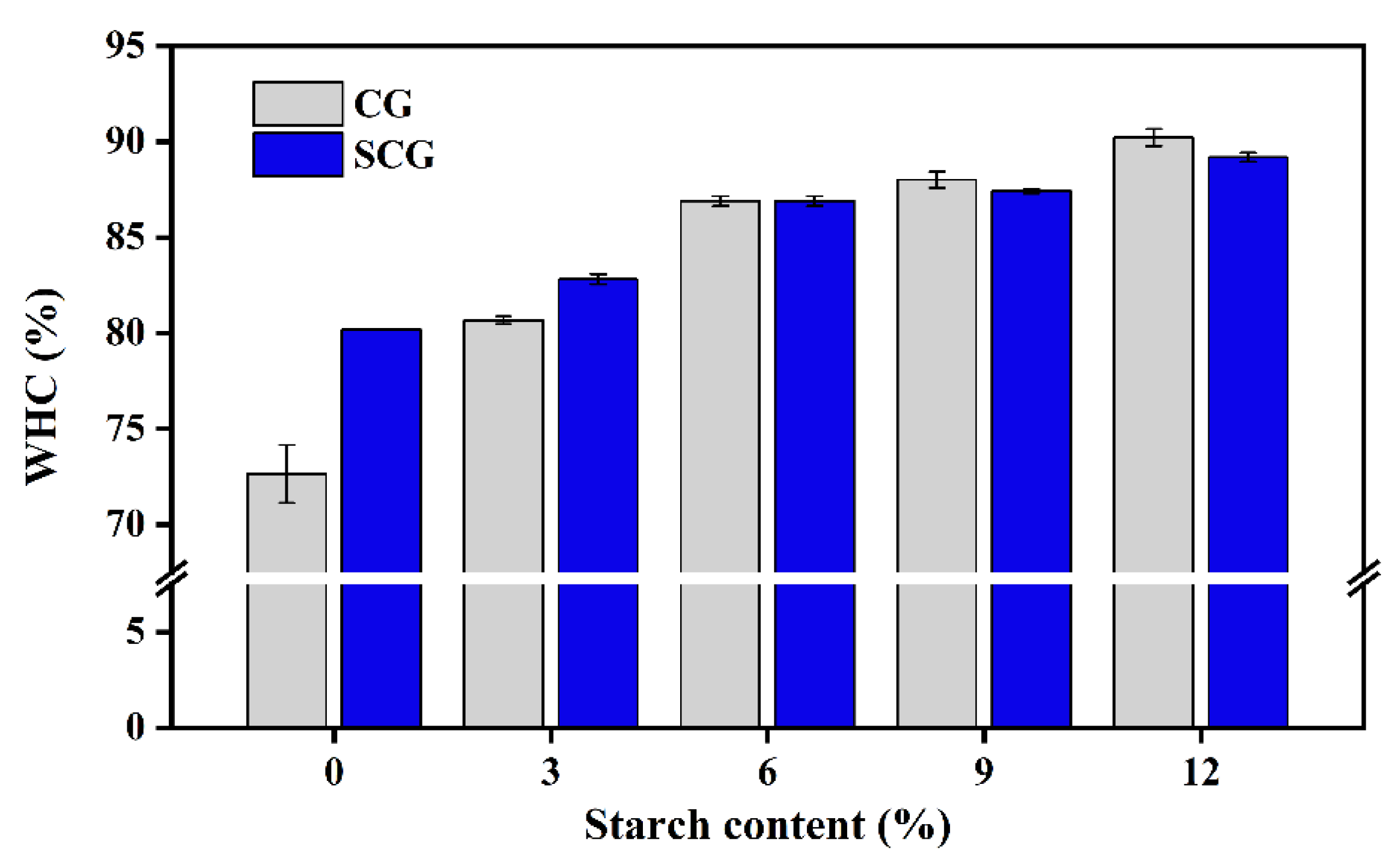
3.5.3. Water Holding Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, S.-W.; Wei, W.; Wang, Y.; Gan, J.-H.; Lu, Y.; Tao, N.-P.; Wang, X.-C.; Liu, Y.; Xu, C.-H. Integrated recognition and quantitative detection of starch in surimi by infrared spectroscopy and spectroscopic imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lin, T.M.; Yongsawatdigul, J. New developments in manufacturing of Surimi and Surimi seafood. Food Rev. Int. 1997, 13, 577–610. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S. Gelling characteristics of surimi from yellow stripe trevally (Selaroides leptolepis). Int. Aquat. Res. 2012, 4, 5. [Google Scholar] [CrossRef]
- Weng, W.; Zheng, W. Silver Carp (Hypophthalmichthys molitrix) Surimi Acid-Induced Gel Extract Characteristics: A Comparison with Heat-Induced Gel. Int. J. Food Prop. 2015, 18, 821–832. [Google Scholar] [CrossRef]
- Alipour, H.J.; Rezaei, M.; Shabanpour, B.; Tabarsa, M. Effects of sulfated polysaccharides from green alga Ulva intestinalis on physicochemical properties and microstructure of silver carp surimi. Food Hydrocoll. 2018, 74, 87–96. [Google Scholar] [CrossRef]
- Fang, M.; Xiong, S.; Hu, Y.; Yin, T.; You, J. In vitro pepsin digestion of silver carp (Hypophthalmichthys molitrix) surimi gels after cross-linking by Microbial Transglutaminase (MTGase). Food Hydrocoll. 2019, 95, 152–160. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Wan, J.; Ushio, H.; Watabe, S. Thermal gelation properties of white croaker, walleye pollack and deepsea bonefish surimi after suwari treatment at various temperatures. Fish. Sci. 2013, 79, 715–724. [Google Scholar] [CrossRef]
- Yasui, T.; Ishioroshi, M.; Samejima, K. Effect of Actomyosin on Heat-induced Gelation of Myosin. Agric. Biol. Chem. 1982, 46, 1049–1059. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.-M.; Xie, B.-J.; Xiong, S.-B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Jia, R.; Jiang, Q.; Kanda, M.; Tokiwa, J.; Nakazawa, N.; Osako, K.; Okazaki, E. Effects of heating processes on changes in ice crystal formation, water holding capacity, and physical properties of surimi gels during frozen storage. Food Hydrocoll. 2019, 90, 254–265. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Pan, D.; Bu, G. Gel properties of surimi from silver carp (Hypophthalmichthys molitrix) as affected by heat treatment and soy protein isolate. Food Hydrocoll. 2008, 22, 1513–1519. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Zhou, A.; Xiao, J.; Benjakul, S. Insight into the Effect of Ice Addition on the Gel Properties of Nemipterus virgatus Surimi Gel Combined with Water Migration. Foods 2021, 10, 1815. [Google Scholar] [CrossRef]
- Watabe, S.; Ikeda, D.; Mashiro, T.; Kagetakubo, Y.; Takahashi, Y.; Uemura, M.; Mizusawa, N.; Koyama, H.; Yasumoto, K.; Jimbo, M.; et al. Suitability of Japanese codling as a raw material for surimi-based products revealed by primary sequence analysis of myosin heavy chain and thermal gel properties. Fish. Sci. 2020, 86, 711–719. [Google Scholar] [CrossRef]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined Use of Rheology, LF-NMR, and MRI for Characterizing the Gel Properties of Hairtail Surimi with Potato Starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Watanabe, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Sweet potato starch with low pasting temperature to improve the gelling quality of surimi gels after freezing. Food Hydrocoll. 2018, 81, 467–473. [Google Scholar] [CrossRef]
- Kong, C.; Ogawa, H.; Iso, N. Temperature Dependency of Compression Properties of Fish-meat Gel as Affected by Added Starch. J. Food Sci. 1999, 64, 1048–1051. [Google Scholar] [CrossRef]
- Mi, H.; Wang, C.; Su, Q.; Li, X.; Yi, S.; Li, J. The effect of modified starches on the gel properties and protein conformation of Nemipterus virgatus surimi. J. Texture Stud. 2019, 50, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Huang, Q.; Hu, T.; Xiong, S.; Zhao, S. Effects and mechanism of modified starches on the gel properties of myofibrillar protein from grass carp. Int. J. Biol. Macromol. 2014, 64, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, W.; Wang, H.; Ye, Q. Effects of a highly resistant rice starch and pre-incubation temperatures on the physicochemical properties of surimi gel from grass carp (Ctenopharyn Odon Idellus). Food Chem. 2014, 145, 212–219. [Google Scholar] [CrossRef]
- Pan, T.; Guo, H.; Li, Y.; Song, J.; Ren, F. The effects of calcium chloride on the gel properties of porcine myosin–κ-carrageenan mixtures. Food Hydrocoll. 2017, 63, 467–477. [Google Scholar] [CrossRef]
- Zhang, H.; She, W.; Li, L.; Wang, W. Effect of temperature rising inhibitor on autogenous shrinkage of cement pastes. Constr. Build. Mater. 2019, 220, 329–339. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Effect of small granules in potato starch and wheat starch on quality changes of direct heated surimi gels after freezing. Food Hydrocoll. 2020, 104, 105732. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- Reichardt, W.; Eckert, B. Zur Bestimmung des Proteingehaltes von Milch, Käse und Fleisch mit Hilfe der Biuret-Reaktion. Food/Nahrung 1991, 35, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp ( Hypophthalmichthys molitrix ) fillets during setting and heating: Effects of different washing solutions. Food Hydrocoll. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T.; Nuthong, P. Effect of Psyllium (Plantago ovata Forks) Husk on Characteristics, Rheological and Textural Properties of Threadfin Bream Surimi Gel. Foods 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017, 34, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, M.; Huang, Q.; Zhao, S.; Xiong, S.; Yin, T.; Zhang, B. Effect of micro- and nano-starch on the gel properties, microstructure and water mobility of myofibrillar protein from grass carp. Food Chem. 2022, 366, 130579. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Effects of emulsified lard and TGase on gel properties of threadfin bream (Nemipterus virgatus) surimi. LWT 2021, 146, 111513. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, C.M.; Hufnagel, L.A. Textural properties and structure of starch-reinforced surimi gels as affected by heat-setting. Food Struct. 1987, 6, 81–89. [Google Scholar]
- Stone, A.; Stanley, D. Mechanisms of fish muscle gelation. Food Res. Int. 1992, 25, 381–388. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Wei, S.; Xia, Q.; Pan, Y.; Ji, H.; Deng, C.; Hao, J.; Liu, S. Insight into the correlations among rheological behaviour, protein molecular structure and 3D printability during the processing of surimi from golden pompano (Trachinotus ovatus). Food Chem. 2022, 371, 131046. [Google Scholar] [CrossRef]
- Jia, D.; Huang, Q.; Xiong, S. Chemical interactions and gel properties of black carp actomyosin affected by MTGase and their relationships. Food Chem. 2016, 196, 1180–1187. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, L.; Ren, Y.; Zhao, Q. Chemical Interactions and Protein Conformation Changes During Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Formation. Int. J. Food Prop. 2014, 17, 1702–1713. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Effects of microwave combined with conduction heating on surimi quality and morphology. J. Food Eng. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Chemical interactions and rheological properties of hairtail (Trichiurus haumela) surimi: Effects of chopping and pressure. Food Biosci. 2020, 38, 100781. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Z.; Pan, Y.; Jiang, P.; Pan, J.; Yu, C.; Dong, X. Effect of κ-carrageenan on quality improvement of 3D printed Hypophthalmichthys molitrix-sea cucumber compound surimi product. LWT 2021, 154, 112279. [Google Scholar] [CrossRef]
- Fevzioglu, M.; Ozturk, O.K.; Hamaker, B.R.; Campanella, O.H. Quantitative approach to study secondary structure of proteins by FT-IR spectroscopy, using a model wheat gluten system. Int. J. Biol. Macromol. 2020, 164, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Jiang, X.; Zhou, H.; Chen, Y.; Zhao, Y.; Yang, H.; Zhou, G. Insight into the mechanism of physicochemical influence by three polysaccharides on myofibrillar protein gelation. Carbohydr. Polym. 2020, 229, 115449. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, M.; Zhou, Y.; Wang, X.; Ma, J.; Xiong, G.; Wang, L.; Wang, S.; Sun, W. Gel properties of grass carp myofibrillar protein modified by low-frequency magnetic field during two-stage water bath heating. Food Hydrocoll. 2020, 107, 105920. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, Q.; Zhao, M.; Yang, B.; Cui, C.; Ren, J. Structural Evaluation of Myofibrillar Proteins during Processing of Cantonese Sausage by Raman Spectroscopy. J. Agric. Food Chem. 2011, 59, 11070–11077. [Google Scholar] [CrossRef]
- Herrero, A.; Carmona, P.; Cofrades, S.; Jimenez-Colmenero, F. Raman spectroscopic determination of structural changes in meat batters upon soy protein addition and heat treatment. Food Res. Int. 2008, 41, 765–772. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Zou, W.; Tang, H.; Yang, F.; Wang, L.; Elfalleh, W. Raman spectroscopy analysis of the effect of electrolysis treatment on the structure of soy protein isolate. J. Food Meas. Charact. 2021, 15, 1294–1300. [Google Scholar] [CrossRef]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Adsorption of S—S Containing Proteins on a Colloidal Silver Surface Studied by Surface-Enhanced Raman Spectroscopy. Appl. Spectrosc. 2004, 58, 1147–1156. [Google Scholar] [CrossRef]
- Pfeuti, G.; Brown, L.S.; Longstaffe, J.G.; Peyronel, F.; Bureau, D.P.; Kiarie, E.G. Predicting the standardized ileal digestibility of crude protein in feather meal fed to broiler chickens using a pH-stat and a FT-Raman method. Anim. Feed. Sci. Technol. 2020, 261, 114340. [Google Scholar] [CrossRef]
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Academic Press: Salt Lake City, UT, USA, 2002; pp. 182–186. [Google Scholar]
- Petcharat, T.; Benjakul, S. Effect of gellan incorporation on gel properties of bigeye snapper surimi. Food Hydrocoll. 2018, 77, 746–753. [Google Scholar] [CrossRef]
- Uresti, R.M.; Velazquez, G.; Ramírez, J.A.; Vázquez, M.; Torres, J.A. Effect of high-pressure treatments on mechanical and functional properties of restructured products from arrowtooth flounder (Atheresthes stomias). J. Sci. Food Agric. 2004, 84, 1741–1749. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Pan, Y.; Wang, K.; Prakash, S.; Zhu, B. Investigation of sweet potato starch as a structural enhancer for three-dimensional printing of Scomberomorus niphonius surimi. J. Texture Stud. 2019, 50, 316–324. [Google Scholar] [CrossRef]
- Duflot, M.; Sánchez-Alonso, I.; Duflos, G.; Careche, M. LF 1H NMR T2 relaxation rate as affected by water addition, NaCl and pH in fresh, frozen and cooked minced hake. Food Chem. 2019, 277, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 indicates large granule starch;
indicates large granule starch;  indicates small granule starch. Caption: see Figure 1.
indicates small granule starch. Caption: see Figure 1.
 indicates large granule starch;
indicates large granule starch;  indicates small granule starch. Caption: see Figure 1.
indicates small granule starch. Caption: see Figure 1.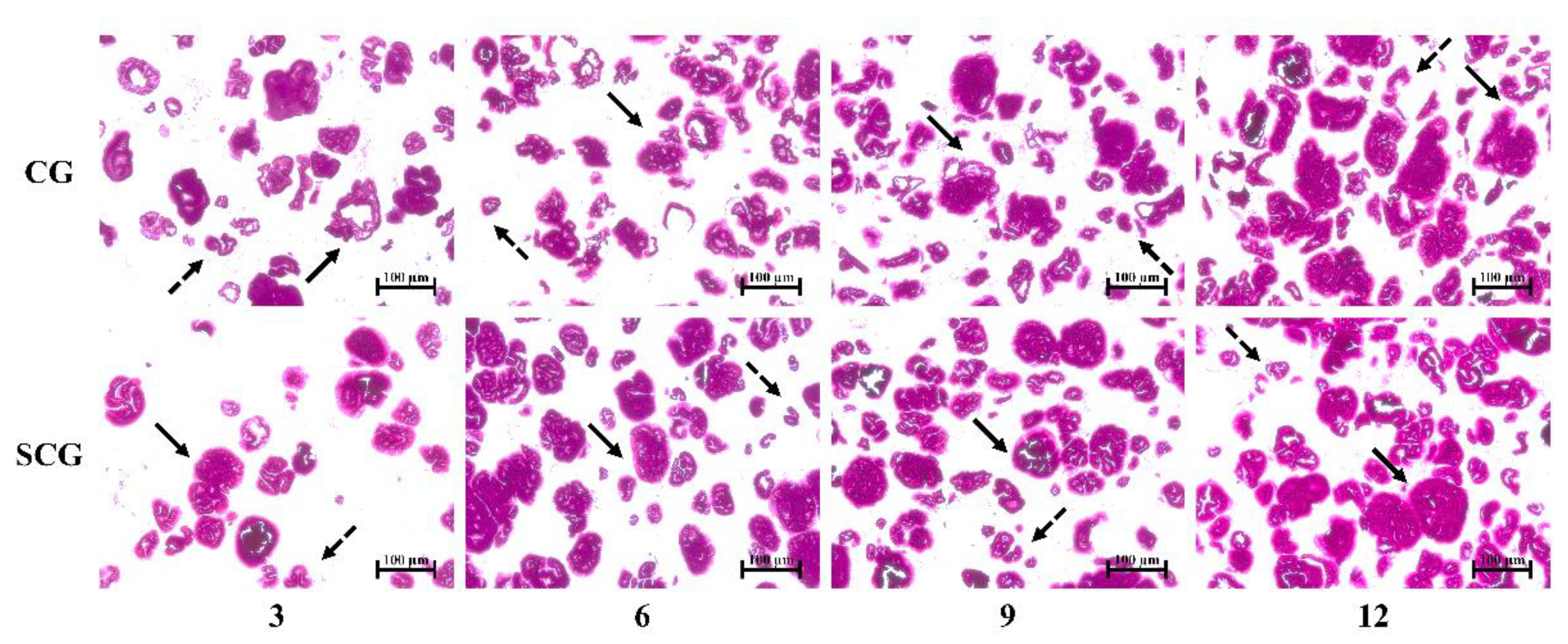
| CPMG | SF | SW | RFD | Tw | RG1 | DRG1 | PRG | P1 | P2 | TE | NECH | NS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | 21 | 200 | 0.08 | 2500 | 10 | 3 | 1 | 20 | 40 | 0.3 | 8000 | 8 |
| Samples | Starch Content (%) | Non-Covalent Bonds (g/L) | Total Sulfhydryl Groups (μmol/g Protein) | |||
|---|---|---|---|---|---|---|
| Non-Specific Associations | Ionic Bonds | Hydrogen Bonds | Hydrophobic Interactions | |||
| CG | 0 | 0.99 ± 0.03 Be | 0.85 ± 0.02 Bd | 0.39 ± 0.02 Bd | 7.71 ± 0.35 Aa | 25.17 ± 0.96 Aa |
| 3 | 1.22 ± 0.01 Bd | 1.33 ± 0.02 Ac | 0.63 ± 0.03 Bc | 7.75 ± 0.27 Aa | 20.07 ± 1.75 Ab | |
| 6 | 1.30 ± 0.02 Bc | 1.90 ± 0.03 Ab | 1.21 ± 0.01 Bb | 5.64 ± 0.10 Ab | 19.44 ± 2.60 Ab | |
| 9 | 2.00 ± 0.01 Bb | 1.84 ± 0.03 Ab | 2.19 ± 0.04 Aa | 4.84 ± 0.10 Ac | 18.29 ± 1.34 Bb | |
| 12 | 2.60 ± 0.06 Ba | 2.16 ± 0.06 Aa | 2.21 ± 0.02 Ba | 3.94 ± 0.10 Ad | 12.55 ± 1.10 Bc | |
| SCG | 0 | 1.58 ± 0.05 Ae | 1.03 ± 0.03 Ad | 1.16 ± 0.02 Ae | 2.95 ± 0.09 Be | 25.62 ± 1.91 Aa |
| 3 | 1.97 ± 0.03 Ad | 1.21 ± 0.02 Bc | 1.82 ± 0.01 Ad | 4.06 ± 0.04 Ba | 21.03 ± 2.02 Abc | |
| 6 | 2.43 ± 0.02 Ac | 1.25 ± 0.03 Bc | 2.69 ± 0.04 Aa | 3.47 ± 0.02 Bb | 17.97 ± 1.75 Ac | |
| 9 | 2.96 ± 0.04 Ab | 1.33 ± 0.02 Bb | 2.19 ± 0.01 Ac | 3.41 ± 0.03 Bc | 23.20 ± 3.09 Aab | |
| 12 | 4.07 ± 0.05 Aa | 1.56 ± 0.01 Ba | 2.36 ± 0.03 Ab | 3.17 ± 0.03 Bd | 22.43 ± 1.89 Aab | |
| Samples | Starch Content (%) | α-Helix (%) | Random Coil (%) | β-Sheet (%) | β-Turn (%) |
|---|---|---|---|---|---|
| CG | 0 | 26.16 ± 0.75 Aa | 26.49 ± 0.26 Aa | 25.49 ± 0.18 Aa | 21.85 ± 0.73 Aa |
| 3 | 26.89 ± 1.38 Ba | 25.80 ± 1.13 Aa | 25.20 ± 0.96 Aa | 22.11 ± 2.84 Aa | |
| 6 | 26.47 ± 1.07 Aa | 26.03 ± 0.53 Aa | 25.63 ± 0.61 Aa | 21.88 ± 1.02 Aa | |
| 9 | 27.20 ± 1.01 Ba | 26.00 ± 0.23 Aa | 25.05 ± 0.47 Aa | 21.75 ± 0.35 Aa | |
| 12 | 27.69 ± 1.03 Ba | 25.97 ± 0.21 Aa | 25.34 ± 0.16 Aa | 21.00 ± 1.06 Aa | |
| SCG | 0 | 28.14 ± 2.39 Aa | 25.03 ± 0.75 Ba | 24.47 ± 1.32 Ab | 22.36 ± 2.34 Aa |
| 3 | 28.98 ± 0.55 Aa | 25.25 ± 0.32 Aa | 24.92 ± 0.29 Aab | 20.85 ± 0.26 Aa | |
| 6 | 27.74 ± 1.19 Aa | 24.82 ± 0.31 Ba | 25.89 ± 0.15 Aa | 21.55 ± 0.99 Aa | |
| 9 | 29.12 ± 0.62 Aa | 24.66 ± 0.48 Ba | 25.65 ± 0.47 Aa | 20.56 ± 0.49 Ba | |
| 12 | 29.03 ± 0.38 Aa | 25.17 ± 0.40 Ba | 25.13 ± 0.31 Aab | 20.67 ± 0.52 Aa |
| Samples | Starch Content (%) | Hardness (g) | Springiness | Cohesiveness | Resilience | Chewiness (g) |
|---|---|---|---|---|---|---|
| CG | 0 | 476.41 ± 8.31 Be | 0.94 ± 0.00 Ba | 0.73 ± 0.00 Be | 0.40 ± 0.00 Be | 325.83 ± 4.29 Be |
| 3 | 757.80 ± 20.71 Bd | 0.95 ± 0.01 Aa | 0.73 ± 0.00 Bd | 0.42 ± 0.00 Bd | 528.43 ± 20.04 Bd | |
| 6 | 1097.31 ± 11.53 Bc | 0.96 ± 0.01 Aa | 0.74 ± 0.00 Bc | 0.43 ± 0.00 Bc | 780.30 ± 2.10 Bc | |
| 9 | 1382.00 ± 17.42 Bb | 0.94 ± 0.02 Aa | 0.75 ± 0.00 Bd | 0.44 ± 0.00 Bb | 983.86 ± 13.48 Bb | |
| 12 | 1559.28 ± 17.46 Ba | 0.91 ± 0.01 Ab | 0.76 ± 0.00 Ba | 0.45 ± 0.00 Ba | 1090.18 ± 20.44 Ba | |
| SCG | 0 | 868.25 ± 20.61 Ae | 0.97 ± 0.00 Aa | 0.85 ± 0.01 Aa | 0.59 ± 0.00 Aa | 716.50 ± 18.45 Ae |
| 3 | 1023.91 ± 17.23 Ad | 0.96 ± 0.02 Aab | 0.84 ± 0.00 Ab | 0.57 ± 0.00 Ab | 826.50 ± 30.43 Ad | |
| 6 | 1301.55 ± 27.10 Ac | 0.94 ± 0.00 Abc | 0.82 ± 0.00 Ac | 0.54 ± 0.01 Ac | 1005.24 ± 20.65 Ac | |
| 9 | 1595.79 ± 4.69 Ab | 0.93 ± 0.01 Ac | 0.81 ± 0.00 Ad | 0.52 ± 0.00 Ad | 1200.29 ± 5.83 Ab | |
| 12 | 1874.12 ± 26.36 Aa | 0.92 ± 0.01 Ac | 0.80 ± 0.00 Ae | 0.51 ± 0.00 Ae | 1383.62 ± 26.91 Aa |
| Samples | Starch Content (%) | L * | a * | b * | Whiteness |
|---|---|---|---|---|---|
| CG | 0 | 77.45 ± 0.17 Aa | −0.54 ± 0.12 Aa | 6.06 ± 0.35 Aa | 76.64 ± 0.14 Ba |
| 3 | 75.51 ± 0.31 Ab | −0.60 ± 0.08 Aa | 5.77 ± 0.15 Ab | 74.83 ± 0.28 Bb | |
| 6 | 73.95 ± 0.39 Bc | −0.73 ± 0.05 Ab | 5.63 ± 0.17 Ab | 73.33 ± 0.36 Bc | |
| 9 | 71.65 ± 0.17 Bd | −1.02 ± 0.08 Ac | 4.96 ± 0.18 Ac | 71.20 ± 0.19 Bd | |
| 12 | 71.06 ± 0.28 Be | −1.06 ± 0.05 Ac | 4.67 ± 0.06 Ad | 70.67 ± 0.27 Be | |
| SCG | 0 | 77.46 ± 0.24 Aa | −0.83 ± 0.06 Ba | 5.19 ± 0.16 Ba | 76.86 ± 0.25 Aa |
| 3 | 75.77 ± 0.35 Ab | −0.88 ± 0.04 Bb | 4.92 ± 0.22 Bb | 75.26 ± 0.31 Ab | |
| 6 | 74.87 ± 0.33 Ac | −0.97 ± 0.05 Bc | 4.79 ± 0.14 Bb | 74.40 ± 0.34 Ac | |
| 9 | 73.32 ± 0.24 Ad | −1.07 ± 0.05 Bd | 4.46 ± 0.20 Bc | 72.93 ± 0.21 Ad | |
| 12 | 71.84 ± 0.21 Ae | −1.28 ± 0.04 Be | 4.07 ± 0.06 Bd | 71.51 ± 0.21 Ae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Chen, Q.; Xiao, N.; Du, Y.; Feng, Q.; Shi, W. Changes in Gel Structure and Chemical Interactions of Hypophthalmichthys molitrix Surimi Gels: Effect of Setting Process and Different Starch Addition. Foods 2022, 11, 9. https://doi.org/10.3390/foods11010009
Jiang X, Chen Q, Xiao N, Du Y, Feng Q, Shi W. Changes in Gel Structure and Chemical Interactions of Hypophthalmichthys molitrix Surimi Gels: Effect of Setting Process and Different Starch Addition. Foods. 2022; 11(1):9. https://doi.org/10.3390/foods11010009
Chicago/Turabian StyleJiang, Xin, Qing Chen, Naiyong Xiao, Yufan Du, Qian Feng, and Wenzheng Shi. 2022. "Changes in Gel Structure and Chemical Interactions of Hypophthalmichthys molitrix Surimi Gels: Effect of Setting Process and Different Starch Addition" Foods 11, no. 1: 9. https://doi.org/10.3390/foods11010009





