Sodium Caseinate and Acetylated Mung Bean Starch for the Encapsulation of Lutein: Enhanced Solubility and Stability of Lutein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Mung Bean Starch Isolating
2.2.2. Preparation of Acetylated Starch
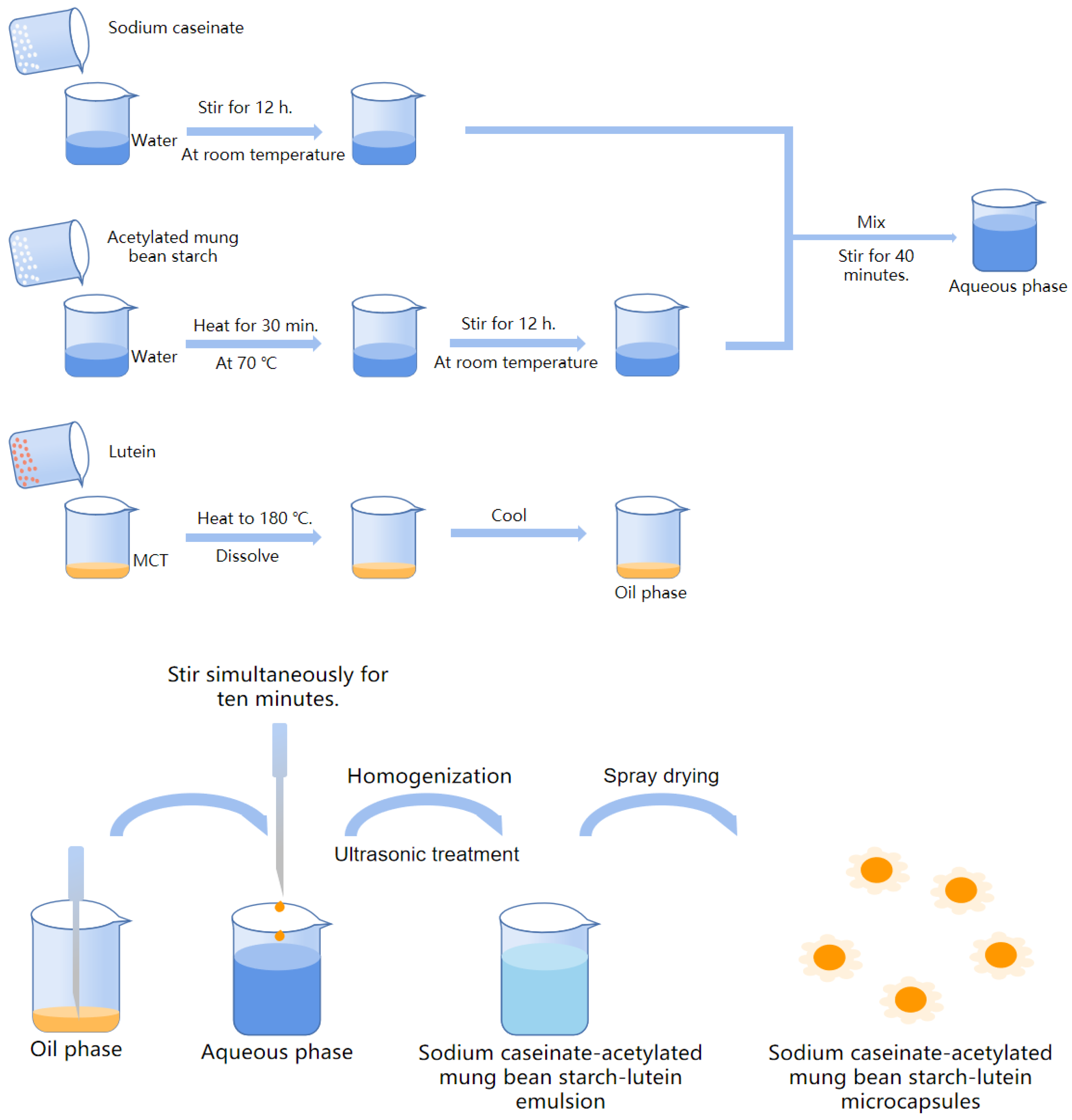
2.2.3. Preparation of Sodium Caseinate-Acetylated Mung Bean Starch–Lutein Emulsion
2.2.4. Preparation of Sodium Caseinate-Acetylated Mung Bean Starch–Lutein Microcapsules
2.3. Characterization of Emulsion
2.3.1. Optical Microscopy Observation for Emulsion
2.3.2. Fluorescence Microscopy Observation for Emulsion
2.3.3. Rheological Property Determination
2.4. Characterization of Microcapsule
2.4.1. Scanning Electron Microscopy (SEM) for Microcapsules
2.4.2. Fourier-Transform Infrared Spectroscopy (FTIR) for Microcapsules
2.4.3. Determination of Yield
2.4.4. Determination of Encapsulation Efficiency
2.4.5. Measurement of Moisture Content
2.4.6. Measurement of Solubility
2.4.7. Measurement of Wettability
2.4.8. Color Characterization
2.4.9. Particle Size Distribution
2.4.10. Differential Scanning Calorimetry (DSC) Analysis
2.5. Stability Tests
2.5.1. Influence of Temperature on the Retention Rate of Microcapsules
2.5.2. Influence of Light on the Retention Rate of Microcapsules
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Emulsion
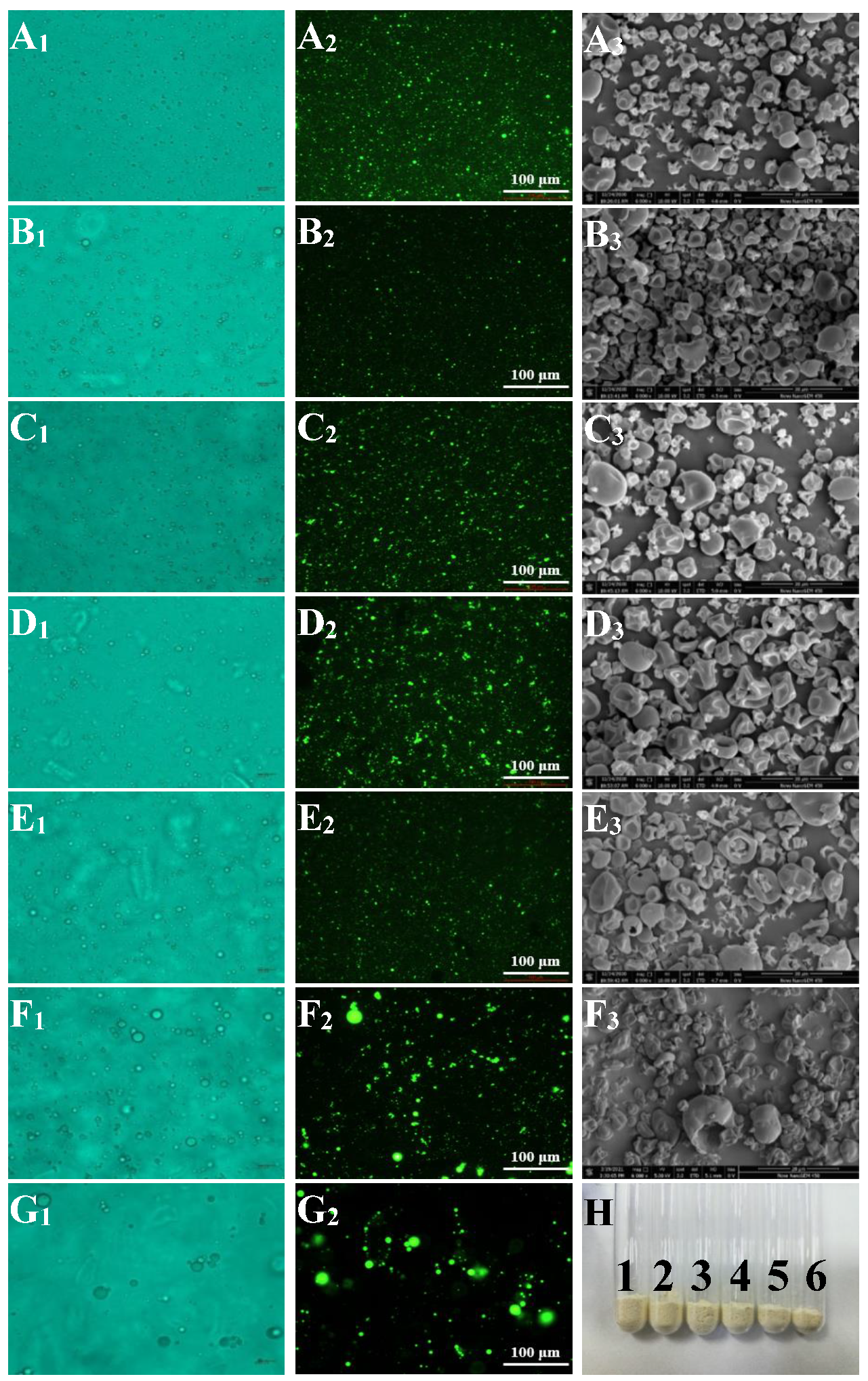
3.1.1. Optical Microscopy Observation for Emulsion
3.1.2. Fluorescence Microscopy Observation for Emulsion
3.1.3. Rheological Properties
3.2. Characterization of Microcapsules
3.2.1. Scanning Electron Microscopy (SEM) Images for Microcapsules
3.2.2. Fourier-Transform Infrared Spectroscopy (FTIR) for Microcapsules
3.2.3. The Yield, Encapsulation Efficiency, Moisture Content, Solubility and Wettability of Microcapsules
3.2.4. Color Characterization
3.2.5. Particle Size Distribution
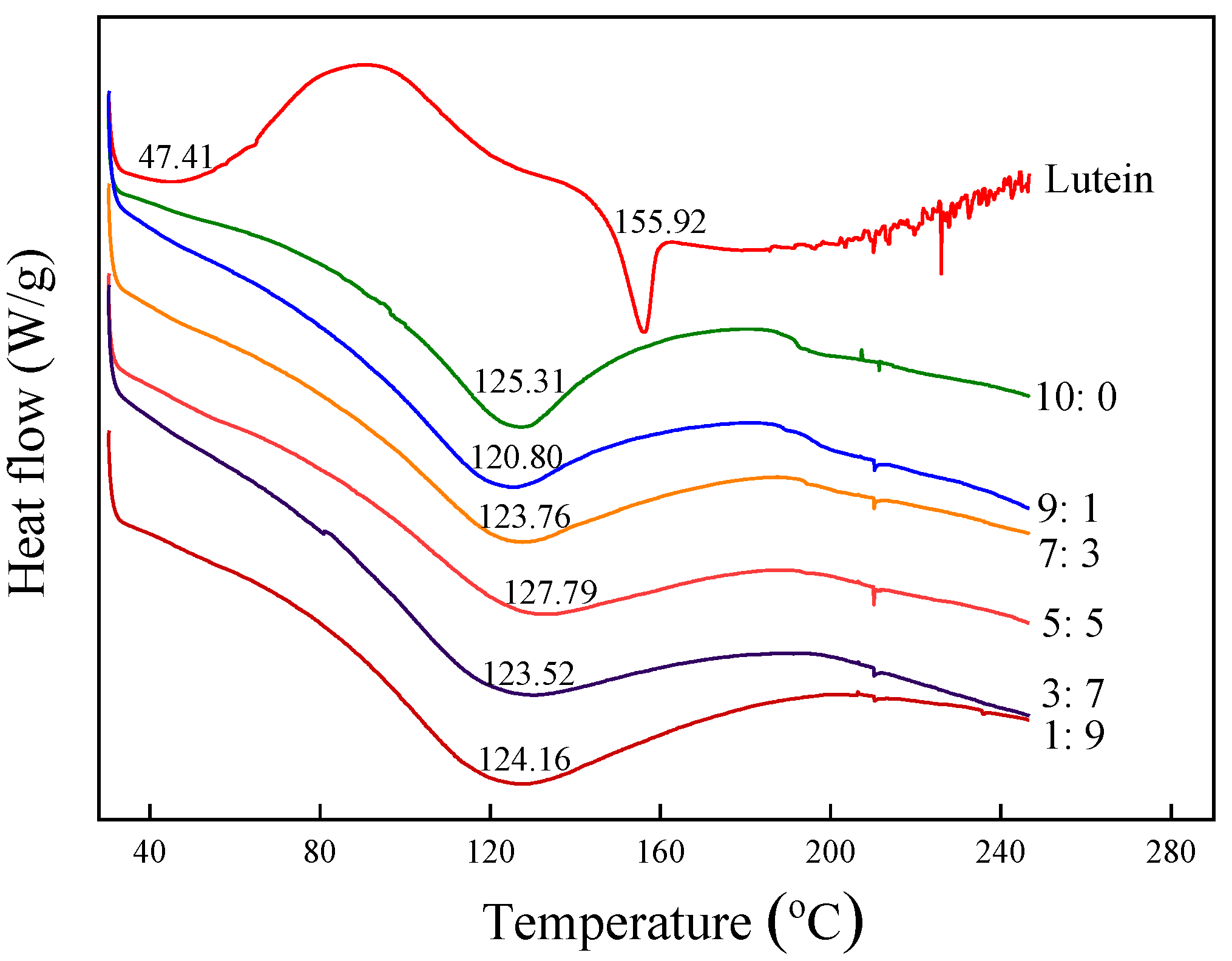
3.2.6. Thermal Characteristics Analysis
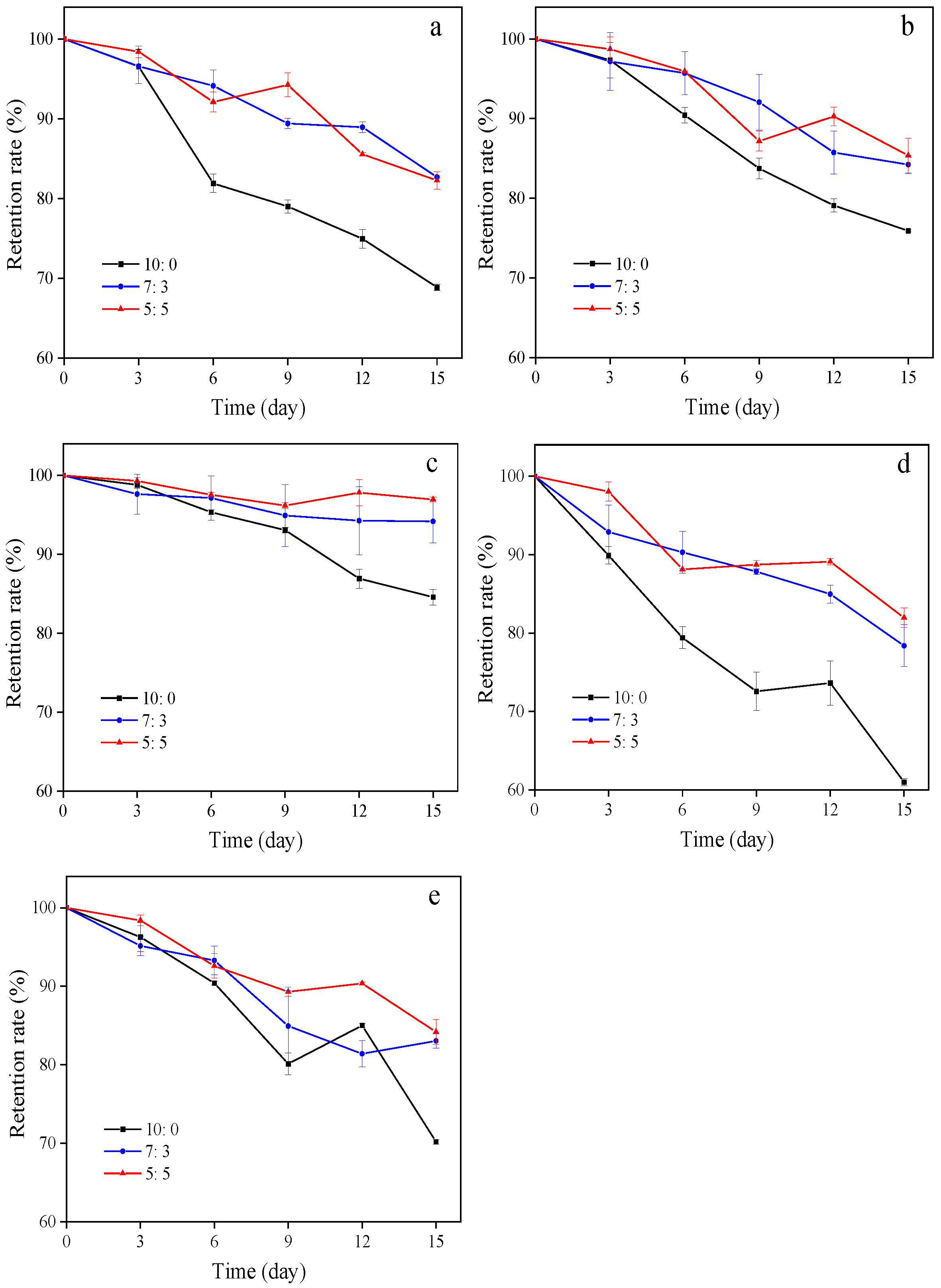
3.2.7. Stability Tests
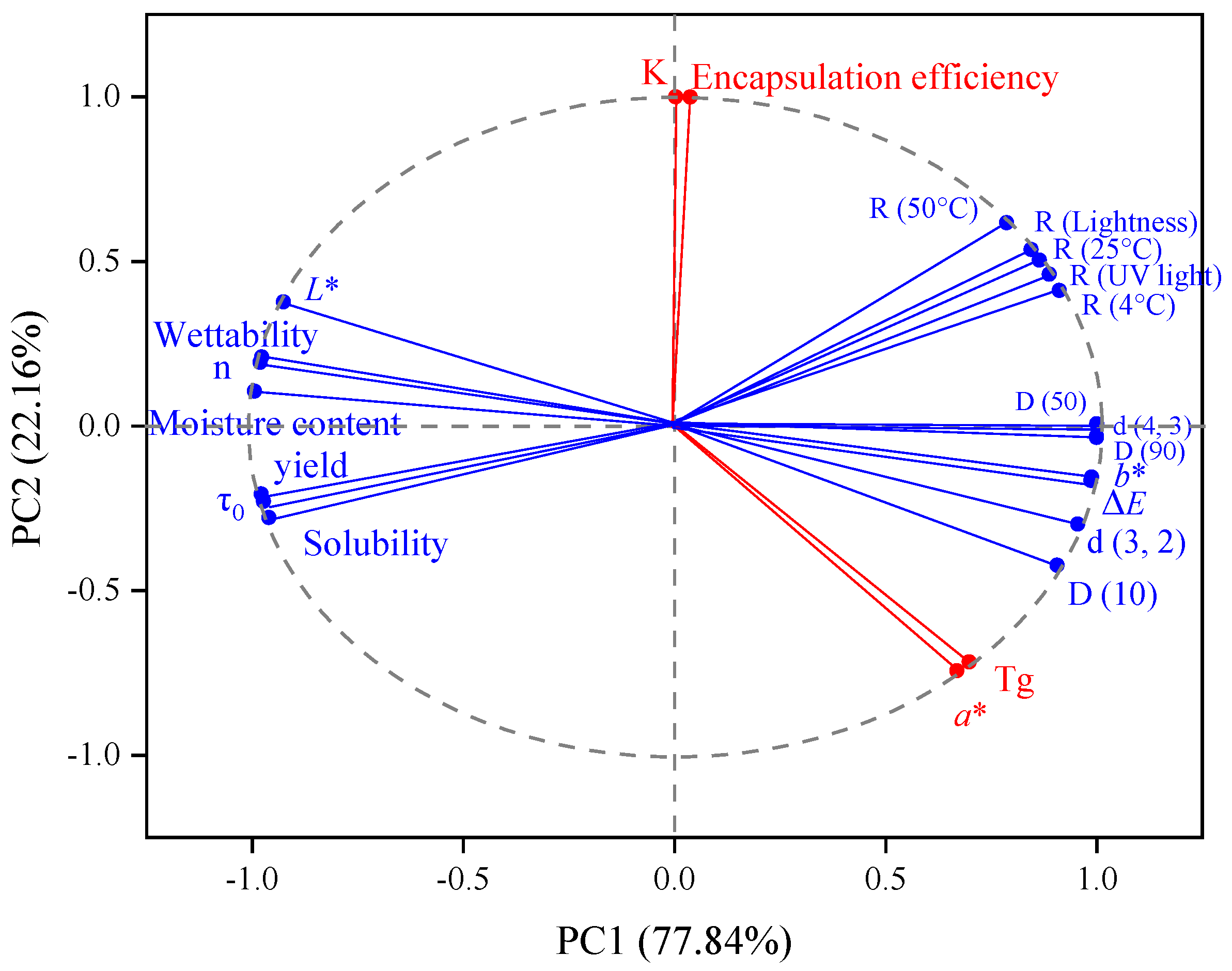
3.2.8. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and zeaxanthin and their potential roles in disease prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar] [CrossRef]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T.J.M. Lutein: More than just a filter for blue light. Prog. Retinal Eye Res. 2012, 31, 303–315. [Google Scholar]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.M. Advances in spray-drying encapsulation of food bioactive ingredients: From microcapsules to nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Peng, Y.Y.; Wen, J.Y.; Quek, S.Y. A Comparison of Microfluidic-Jet Spray Drying, Two-Fluid Nozzle Spray Drying, and Freeze-Drying for Co-Encapsulating β-Carotene, Lutein, Zeaxanthin, and Fish Oil. Foods 2021, 10, 1522. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, T.; Wang, X.; Prakash, S.; Zhao, Y.N.; Han, J.; Wang, Z.P. Influences of different carbohydrates as wall material on powder characteristics, encapsulation efficiency, stability and degradation kinetics of microencapsulated lutein by spray drying. Int. J. Food Sci. Technol. 2020, 55, 2872–2882. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, F.G.; Duan, X.; Xiao, C.X.; Liu, X.B. Physicochemical properties of lutein-loaded microcapsules and their uptake via Caco-2 monolayers. Molecules 2018, 23, 1805. [Google Scholar] [CrossRef] [Green Version]
- Katerinopoulou, K.; Giannakas, A.; Grigoriadi, K.; Barkoula, N.M.; Ladavos, A. Preparation and characterization of acetylated corn starch–(PVOH)/clay nanocomposite films. Carbohydr. Polym. 2014, 102, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.; Cuomo, F.; Nostro, P.L.; Ceglie, A. Effects of solvent and alkaline earth metals on the heat-induced precipitation process of sodium caseinate. Food Chem. 2013, 136, 266–272. [Google Scholar] [CrossRef]
- Farshchi, A.; Ettelaie, R.; Holmes, M. Influence of pH value and locust bean gum concentration on the stability of sodium caseinate-stabilized emulsions. Food Hydrocoll. 2013, 32, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Dalgleish, D.G. Conformations and structures of milk proteins adsorbed to oil-water interfaces. Food Res. Int. 1996, 29, 541–547. [Google Scholar] [CrossRef]
- Perugini, L.; Cinelli, G.; Cofelice, M.; Ceglie, A.; Lopez, F.; Cuomo, F. Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloids Surf. B 2018, 168, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Perugini, L.; Marconi, E.; Messia, M.C.; Lopez, F. Enhanced Curcumin Bioavailability through Nonionic Surfactant/Caseinate Mixed Nanoemulsions. J. Food Sci. 2019, 84, 2584–2591. [Google Scholar] [CrossRef]
- Galvez, F.C.F.; Resurreccion, A.V.A. The effects of decortication and method of extraction on the physical and chemical properties of starch from mungbean (Vigna radiata (L.) Wilczec). J. Food Process. Preserv. 1993, 17, 93–107. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Torres, A.V.; Salgado-Delgado, R.; Bello-Pérez, L.A. Influence of the oxidation and acetylation of banana starch on the mechanical and water barrier properties of modified starch and modified starch/chitosan blend films. J. Appl. Polym. Sci. 2010, 115, 991–998. [Google Scholar] [CrossRef]
- Kuang, P.Q.; Zhang, H.C.; Bajaj, P.R.; Yuan, Q.P.; Tang, J.M.; Chen, S.L.; Sablani, S.S. Physicochemical properties and storage stability of lutein microcapsules prepared with maltodextrins and sucrose by spray drying. J. Food Sci. 2015, 80, E359–E369. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Xie, X.M.; Zhao, Y.P.; Gao, Y.; Cai, C.; Zhang, Q.X.; Ding, Z.; Fan, Z.P.; Zhang, H.Z.; Liu, M.; et al. Effect of plasticizers on manufacturing ritonavir/copovidone solid dispersions via hot-melt extrusion: Preformulation, physicochemical characterization, and pharmacokinetics in rats. Eur. J. Pharm. Sci. 2019, 127, 60–70. [Google Scholar]
- Bejrapha, P.; Min, S.G.; Surassmo, S.; Choi, M.J. Physicothermal properties of freeze-dried fish oil nanocapsules frozen under different conditions. Dry. Technol. 2010, 28, 481–489. [Google Scholar] [CrossRef]
- Tao, T.; Ding, Z.; Hou, D.P.; Prakash, S.; Zhao, Y.N.; Fan, Z.P.; Zhang, D.M.; Wang, Z.P.; Liu, M.; Han, J. Influence of polysaccharide as co-encapsulant on powder characteristics, survival and viability of microencapsulated Lactobacillus paracasei Lpc-37 by spray drying. J. Food Eng. 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, L.; Xing, Y.; Zhao, Y.; Wang, Z.; Han, J. Enhanced oral bioavailability of celecoxib nanocrystalline solid dispersion based on wet media milling technique: Formulation, optimization and in vitro/in vivo evaluation. Pharmaceutics 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers-Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Sharif, H.R.; Williams, P.A.; Sharif, M.K.; Abbas, S.; Majeed, H.; Masamba, K.G.; Safdar, W.; Zhong, F. Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants-A review. Food Hydrocoll. 2018, 76, 2–16. [Google Scholar] [CrossRef]
- Williams, P.A. Food Emulsions: Principles, Practice, and Techniques. Int. J. Food Sci. Technol. 2001, 36, 223–224. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Mohammed, J.K.; Mahdi, A.A.; Al-Ansi, W.; Zhang, M.; Al-Adeeb, A.; Wei, M.P.; Phyo, H.M.; Yao, W.R. Physicochemical properties, microstructure, and storage stability of Pulicaria jaubertii extract microencapsulated with different protein biopolymers and gum arabic as wall materials. Int. J. Biol. Macromol. 2021, 187, 939–954. [Google Scholar] [CrossRef]
- Xu, L.L.; Wang, J.; Su, Y.J.; Chang, C.H.; Gu, L.P.; Yang, Y.J.; Li, J.H. Utilization of high internal phase emulsion stabilized by egg yolk-modified starch complex for the delivery of lutein. LWT-Food Sci. Technol. 2021, 142, 111024. [Google Scholar] [CrossRef]
- Moukhametov, R.; Srivastava, A.; Akhter, S.; Bautista, J.; Ferroudji, H.; Hadear, H.; Hassan, I.; Rahman, M.A. Effects of salinity on solid particle settling velocity in non-Newtonian Herschel-Bulkley fluids. J. Pet. Explor. Prod. Technol. 2021, 11, 3333–3347. [Google Scholar] [CrossRef]
- Franco, J.M.; Partal, P.; Ruiz-Marquez, D.; Conde, B.; Gallegos, C. Influence of pH and protein thermal treatment on the rheology of pea protein-stabilized oil-in-water emulsions. J. Am. Oil Chem. Soc. 2000, 77, 975–983. [Google Scholar] [CrossRef]
- Batista, A.P.; Raymundo, A.; Sousa, I.; Empis, J. Rheological characterization of coloured oil-in-water food emulsions with lutein and phycocyanin added to the oil and aqueous phases. Food Hydrocoll. 2006, 20, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate-gum Arabic complex coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Qv, X.Y.; Zeng, Z.P.; Jiang, J.G. Preparation of lutein microencapsulation by complex coacervation method and its physicochemical properties and stability. Food Hydrocoll. 2011, 25, 1596–1603. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Jarzębski, M.; Pratap-Singh, A.; Guo, Y.G.; Abd-Manap, Y. Encapsulation of betacyanins from the peel of red dragon fruit (Hylocereus polyrhizus L.) in alginate microbeads. Food Hydrocoll. 2021, 113, 106535. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ye, H.; Zhou, C.H.; Lv, F.X.; Bie, X.M.; Lu, Z.X. Study on the spray-drying encapsulation of lutein in the porous starch and gelatin mixture. Eur. Food Res. Technol. 2012, 234, 157–163. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Morris, G.A.; Sims, I.M.; Robertson, A.J.; Furneaux, R.H. Investigation into the physical and chemical properties of sodium caseinate-maltodextrin glyco-conjugates. Food Hydrocoll. 2004, 18, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Zhang, M.; Devahastin, S. Color/aroma changes of 3D-Printed buckwheat dough with yellow flesh peach as triggered by microwave heating of gelatin-gum Arabic complex coacervates. Food Hydrocoll. 2021, 112, 106358. [Google Scholar] [CrossRef]
- Kippax, P. Appraisal of the laser diffraction particle-sizing. Pharm. Technol. 2005, 3, 88–89. [Google Scholar]
- Polekkad, A.; Franklin, M.E.E.; Pushpadass, H.A.; Battula, S.N.; Rao, S.B.N.; Pal, D.T. Microencapsulation of zinc by spray-drying: Characterisation and fortification. Powder Technol. 2021, 381, 1–16. [Google Scholar] [CrossRef]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef] [Green Version]
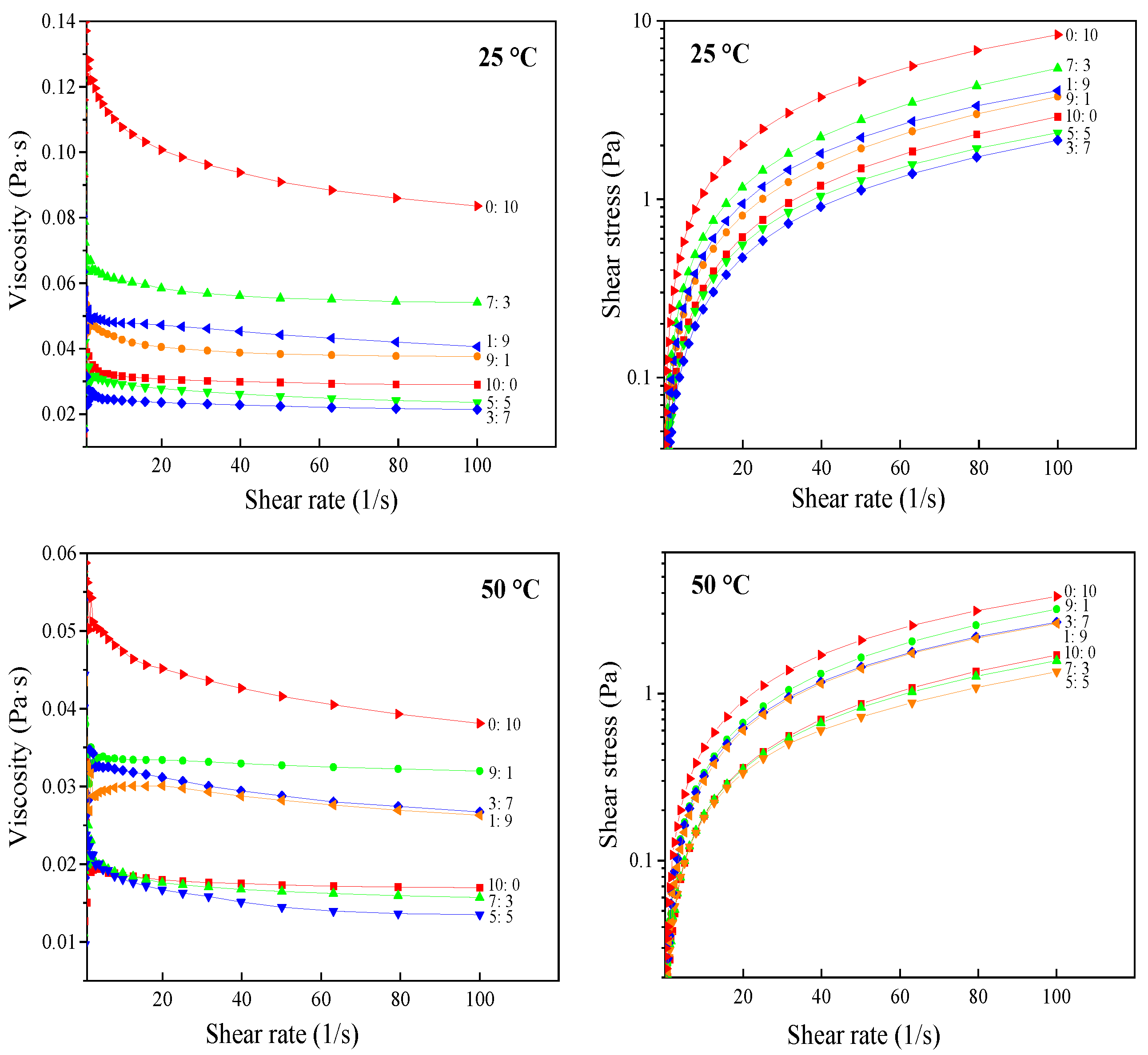

| Temperatures | Sample | τ0 1 (Pa) | K 2 (Pa·s−1) | N 3 | R2,4 |
|---|---|---|---|---|---|
| 25 °C | 10:0 | 0.0055 ± 0.0001 a | 0.0333 ± 0.0000 f | 0.9695 ± 0.0001 a | 1.0000 |
| 9:1 | 0.0029 ± 0.0009 ab | 0.0461 ± 0.0002 d | 0.9546 ± 0.0011 b | 0.9999 | |
| 7:3 | 0.0016 ± 0.0001 abc | 0.0667 ± 0.0000 b | 0.9539 ± 0.0001 b | 1.0000 | |
| 5:5 | −0.0010 ± 0.0004 bc | 0.0376 ± 0.0000 e | 0.8992 ± 0.0003 e | 0.9999 | |
| 3:7 | −0.0020 ± 0.0001 cd | 0.0287 ± 0.0001 g | 0.9369 ± 0.0005 c | 0.9999 | |
| 1:9 | −0.0059 ± 0.0024 de | 0.0625 ± 0.0006 c | 0.9087 ± 0.0019 d | 0.9997 | |
| 0:10 | −0.0083 ± 0.0013 e | 0.1437 ± 0.0005 a | 0.8829 ± 0.0008 f | 1.0000 | |
| 50 °C | 10:0 | 0.0026 ± 0.0007 ab | 0.0196 ± 0.0001 g | 0.9682 ± 0.0014 b | 0.9999 |
| 9:1 | −0.0014 ± 0.0004 c | 0.0363 ± 0.0001 d | 0.9731 ± 0.0004 a | 1.0000 | |
| 7:3 | 0.0010 ± 0.0006 b | 0.0218 ± 0.0001 f | 0.9284 ± 0.0012 c | 0.9999 | |
| 5:5 | 0.0032 ± 0.0003 a | 0.0236 ± 0.0001 e | 0.8762 ± 0.0006 g | 0.9996 | |
| 3:7 | −0.0071 ± 0.0004 de | 0.0418 ± 0.0000 b | 0.9045 ± 0.0003 e | 0.9999 | |
| 1:9 | −0.0086 ± 0.0000 e | 0.0389 ± 0.0000 c | 0.9172 ± 0.0000 d | 0.9998 | |
| 0:10 | −0.0055 ± 0.0003 d | 0.0627 ± 0.0000 a | 0.8935 ± 0.0000 f | 0.9999 |
| Sample | Yield (%) | EE 1 (%) | Moisture (%) | Solubility (%) | Wettability (s) |
|---|---|---|---|---|---|
| SA10:0 | 79.64 ± 0.84 a | 79.84 ± 0.20 b | 1.65 ± 0.01 b | 97.31 ± 0.00 a | 328.65 ± 8.73 a |
| SA9:1 | 70.02 ± 0.29 b | 72.40 ± 0.49 c | 1.40 ± 0.27 bc | 94.98 ± 0.62 ab | 302.93 ± 4.84 b |
| SA7:3 | 68.91 ± 0.25 c | 89.44 ± 0.81 a | 1.30 ± 0.08 bc | 93.92 ± 1.31 ab | 319.50 ± 4.69 ab |
| SA5:5 | 61.13 ± 1.23 d | 81.41 ± 1.11 b | 0.50 ± 0.07 cd | 92.05 ± 2.61 b | 284.23 ± 5.66 c |
| SA3:7 | 36.40 ± 1.39 e | 69.72 ± 2.83 c | 0.07 ± 0.00 d | 78.00 ± 0.02 c | 294.65 ± 4.51 c |
| SA1:9 | 22.49 ± 0.57 f | 71.42 ± 1.32 c | 4.45 ± 0.58 a | 74.59 ± 0.34 c | 299.88 ± 0.14 bc |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| SA10:0 | 94.87 ± 0.03 a | −1.66 ± 0.05 cd | 18.59 ± 0.52 e | 0.60 ± 0.36 e |
| SA9:1 | 93.22 ± 0.13 bc | −1.24 ± 0.16 b | 30.74 ± 0.47 c | 12.74 ± 0.48 c |
| SA7:3 | 94.83 ± 0.14 a | −1.79 ± 0.04 d | 22.17 ± 0.33 d | 4.04 ± 0.33 d |
| SA5:5 | 93.17 ± 0.09 c | −1.48 ± 0.08 bc | 32.43 ± 0.35 b | 14.42 ± 0.36 b |
| SA3:7 | 93.93 ± 0.06 b | −1.71 ± 0.03 cd | 30.81 ± 0.35 c | 12.72 ± 0.35 c |
| SA1:9 | 92.81 ± 0.61 c | −0.75 ± 0.13 a | 36.58 ± 0.37 a | 18.50 ± 0.26 a |
| Sample | d(4,3) (μm) | d(3,2) (μm) | Particle Size Distributions (μm) | ||
|---|---|---|---|---|---|
| D(10) | D(50) | D(90) | |||
| SA10:0 | 1.05 ± 0.01 c | 0.74 ± 0.01 d | 0.40 ± 0.01 c | 0.86 ± 0.00 c | 1.95 ± 0.00 d |
| SA9:1 | 1.17 ± 0.07 c | 0.78 ± 0.01 d | 0.39 ± 0.01 c | 1.08 ± 0.09 c | 2.12 ± 0.12 d |
| SA7:3 | 1.76 ± 0.23 c | 0.88 ± 0.14 d | 0.39 ± 0.07 c | 1.65 ± 0.18 c | 3.28 ± 0.48 cd |
| SA5:5 | 2.96 ± 0.06 c | 1.96 ± 0.77 c | 0.69 ± 0.01 c | 2.81 ± 0.02 c | 5.56 ± 0.36 c |
| SA3:7 | 23.00 ± 1.56 b | 6.37 ± 0.06 b | 4.52 ± 0.35 b | 23.47 ± 2.06 b | 40.40 ± 2.25 b |
| SA1:9 | 35.37 ± 0.12 a | 11.20 ± 0.10 a | 5.89 ± 0.12 a | 34.23 ± 0.31 a | 66.90 ± 0.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, B.; Shen, H.; Ge, X.; Sun, X.; Zhang, Q.; Zhang, X.; Sun, Z.; Li, W. Sodium Caseinate and Acetylated Mung Bean Starch for the Encapsulation of Lutein: Enhanced Solubility and Stability of Lutein. Foods 2022, 11, 65. https://doi.org/10.3390/foods11010065
Lu Y, Zhang B, Shen H, Ge X, Sun X, Zhang Q, Zhang X, Sun Z, Li W. Sodium Caseinate and Acetylated Mung Bean Starch for the Encapsulation of Lutein: Enhanced Solubility and Stability of Lutein. Foods. 2022; 11(1):65. https://doi.org/10.3390/foods11010065
Chicago/Turabian StyleLu, Yifan, Bo Zhang, Huishan Shen, Xiangzhen Ge, Xiangxiang Sun, Qian Zhang, Xiuyun Zhang, Zhuangzhuang Sun, and Wenhao Li. 2022. "Sodium Caseinate and Acetylated Mung Bean Starch for the Encapsulation of Lutein: Enhanced Solubility and Stability of Lutein" Foods 11, no. 1: 65. https://doi.org/10.3390/foods11010065







