Engineering Concanavalin B to Release Bioactive Peptides against Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bioactive Peptides Selection
2.3. In Silico Design
2.4. Expression and Purification
2.4.1. Extraction of Genomic DNA from Canavalia Ensiformis
2.4.2. Primers Design and Extraction of Concanavalin B Gen
2.4.3. Expression and Purification of Recombinant Concanavalin (CNVR) and Modified Concanavalin (CNV44)
2.5. Simulated Gastrointestinal Digestion
2.6. In Vitro Activities
2.6.1. DPPH
2.6.2. ABTS
2.6.3. Fe++ Chelation
2.6.4. Inhibition of Angiotensin Converting Enzyme
2.6.5. Inhibition of DPPIV
2.6.6. Inhibition of HMGR
2.7. Statistical Analysis
3. Results
3.1. Scaffold Selection and Sequence Modification
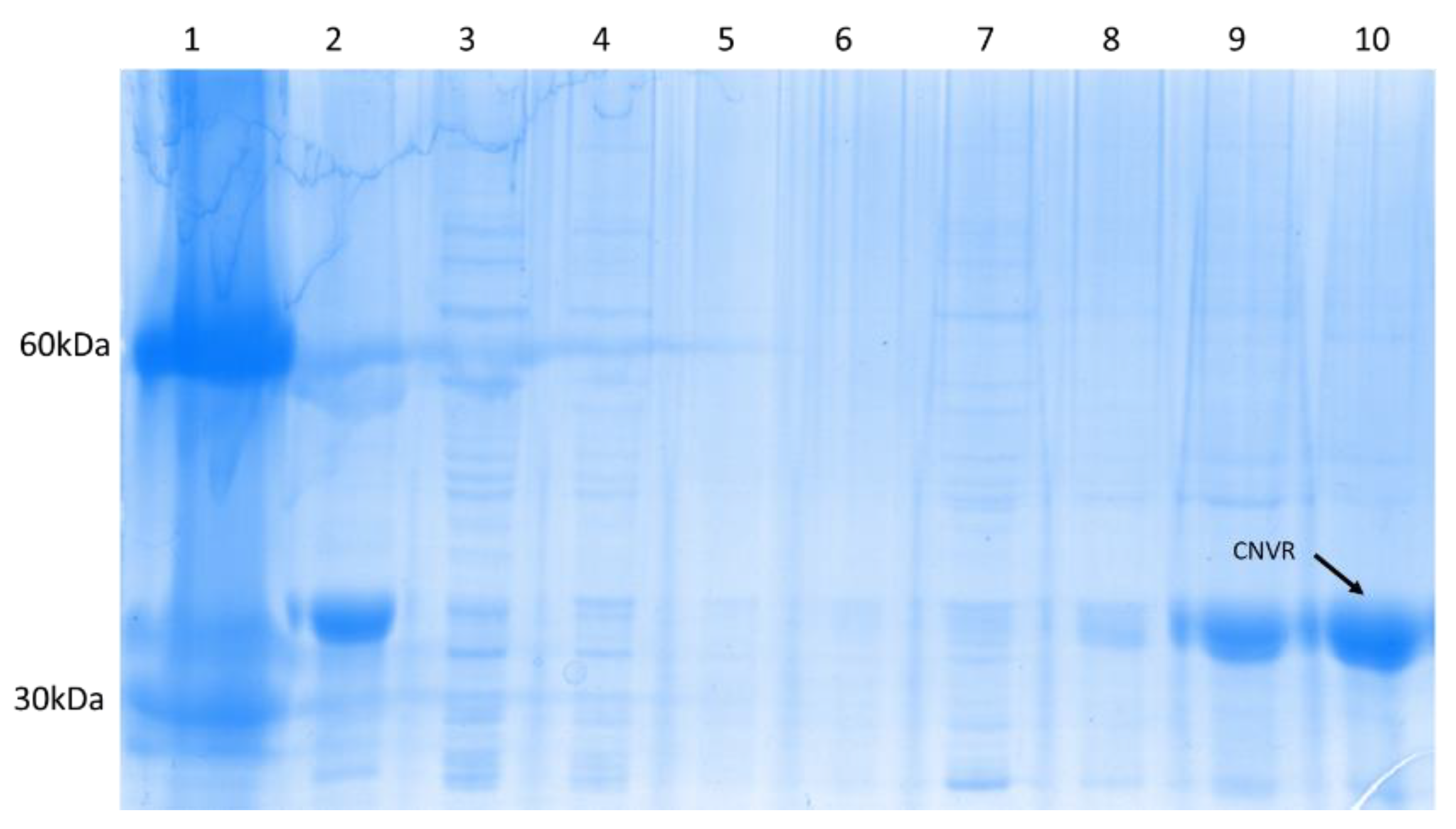
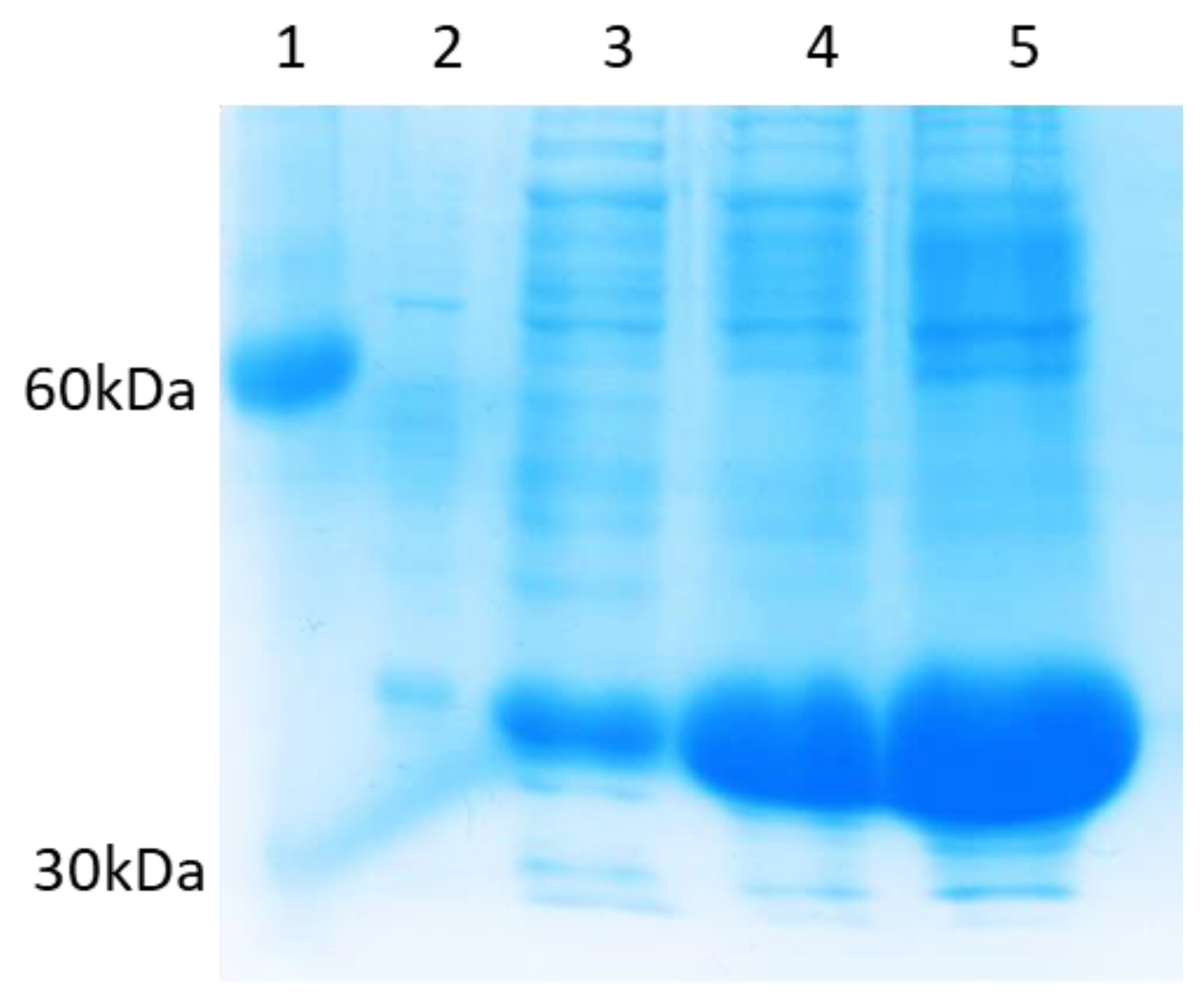
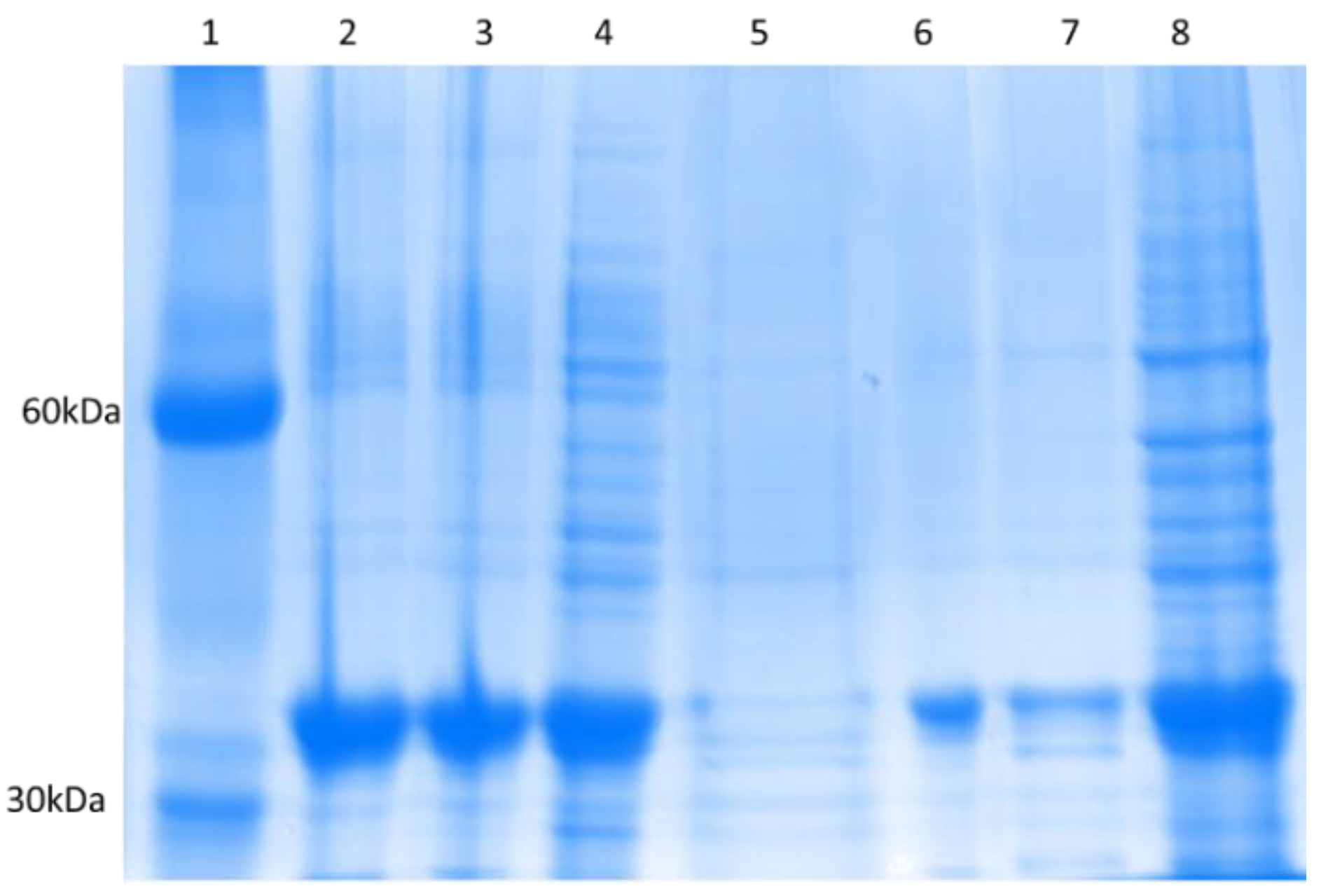
3.2. Expression and Purification of CNVR and CNV44
3.2.1. CNVR
3.2.2. CNV44
3.3. In Vitro Activity
3.3.1. Antioxidant Activity
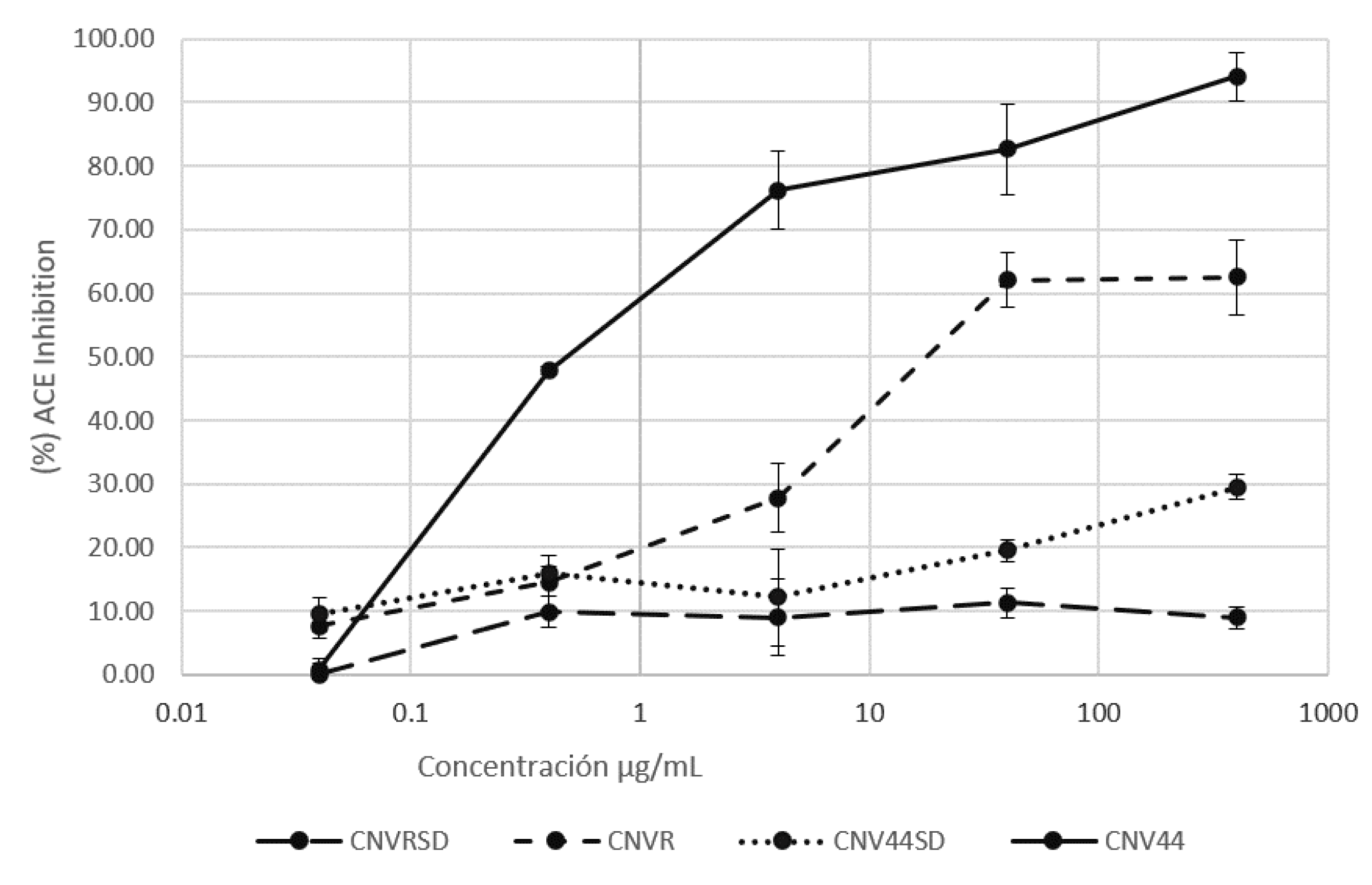
3.3.2. ACEI Activity
3.3.3. DPPIV Inhibition Activity
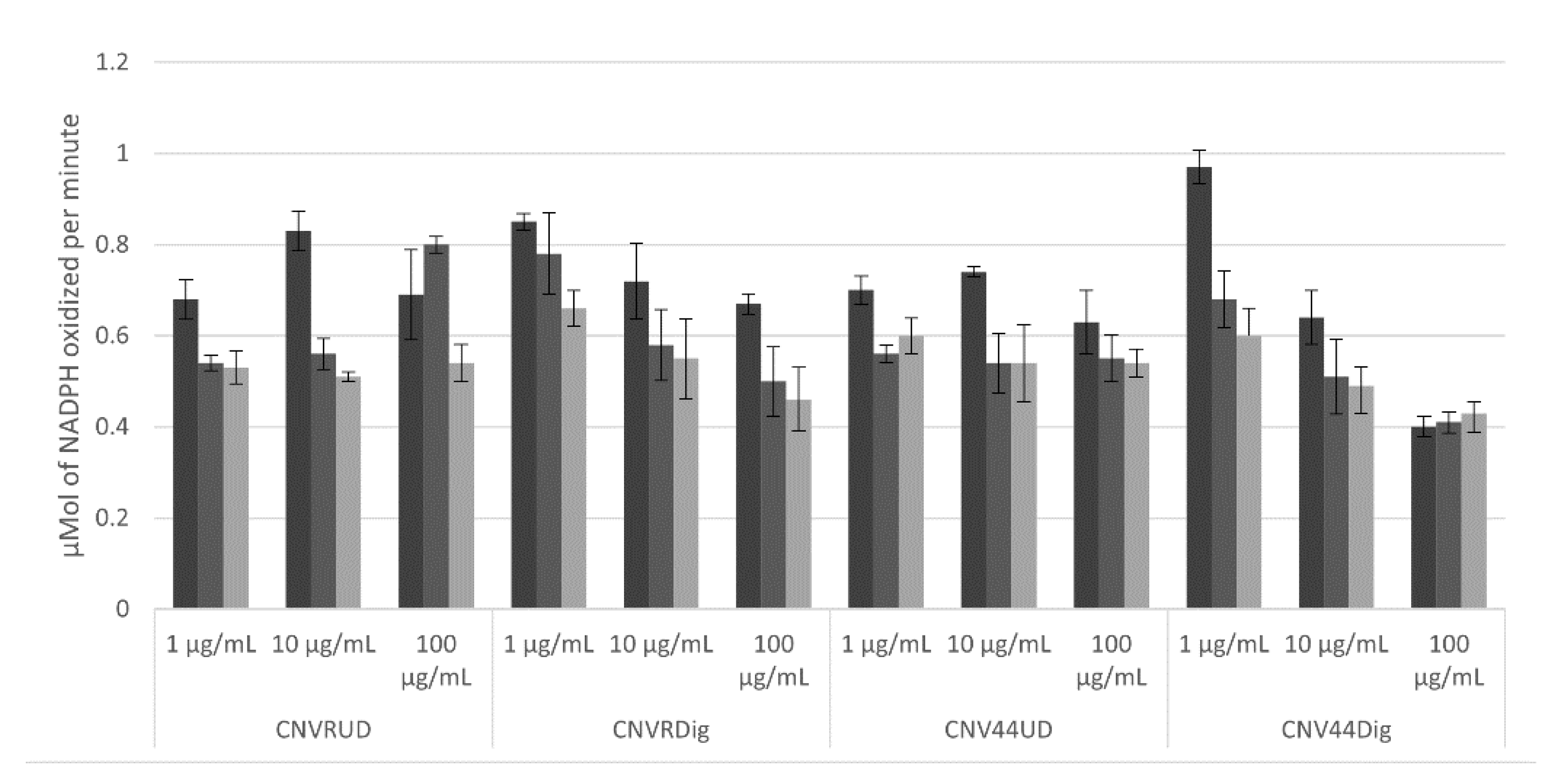
3.3.4. HMGCoA Reductase Activity
4. Discussion
4.1. Scaffold Selection and Sequence Modification
4.2. Expression and Purification
4.3. In Vitro Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKeown, R.E. The epidemiologic transition: Changing patterns of mortality and population dynamics. Am. J. Lifestyle Med. 2009, 3 (Suppl. 1), 19S–26S. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Barba, F.J.; Kovacevic, D.; Lorenzo, J.M.; Cruz, A.; Putnik, P. Functional Foods: Product development, Technological trends, Efficacy testing and Saefty. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekaran, A.; Kalaivani, M. Designer foods and their benefits: A review. J. Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A review on bioactive peptides: Physiological functions, bioavailability and safety. Int. J. Pept. Res. Ther. 2019, 26, 139–150. [Google Scholar] [CrossRef]
- Takenaka, Y.; Utsumi, S.; Yoshikawa, M. Introduction of enterostatin (VPDPR) and a related sequence into soybean proglycininA1aB1B subunit by site-directed mutagenesis. Biosci. Biotechnol. Biochem. 2000, 64, 2731–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shengqi, R.; Xu, Z.; Su, Y.; Li, J.; Sung, J.; Yang, Y. Cloning, soluble expression and production of recombinant antihypertensive peptide multimer (AHPM-2) in Escherichia coli for bioactivity identification. Protein Pept. Lett. 2011, 18, 699–706. [Google Scholar] [CrossRef]
- Maldonado-Torres, D.A.; Fernández-Velasco, D.A.; Morales-Olán, G.; Rosas-Cardenas, F.F.; Luna-Suárez, S. Modification of vegetables proteins to release bioactive peptides able to treat metabolic syndrome—In silico assessment. Appl. Sci. 2020, 10, 2604. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef] [Green Version]
- Schlesier, B.; Nong, V.; Hortsmann, C.; Henning, M. Sequence analysis of Concanavalin B from Canavalia ensiformis reveals homology to chitinases. J. Plant Physiol. 1996, 147, 665–674. [Google Scholar] [CrossRef]
- Espinosa-Hernández, E.; Morales-Camacho, J.I.; Fernández-Velasco, D.A.; Benítez-Cardoza, C.G.; Rosas-Cárdenas, F.F.; Luna-Suárez, S. The insertion of bioactive peptides at the C-terminal end of an 11S globulin changes the structural stability and improves the antihypertensive activity. Electron. J. Biotechnol. 2019, 37, 18–24. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vilacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Wild) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of peptide size on antioxidant properties of African Yam Bean Seed (Sphenostylis stenocarpa) protein hydrolysate fraction. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Pretggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Adjimani, J.P.; Asare, P. Antioxidant and free scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Liu, H.; Shi, Y.; Le, G. Direct spectrophotometric measurement of angiotensin-I converting enzyme inhibitory activity for screening bioactive peptides. J. Pharm. Biomed. Anal. 2005, 37, 219–224. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int. Dairy J. 2012, 25, 97–102. [Google Scholar] [CrossRef]
- Lin, S.H.; Chang, D.K.; Chou, M.J.; Huang, K.J.; Shihuam, D. Peptide inhibitors of human HMG-CoA reductase as potential hypocholesterolemia agents. Biochem. Biophys. Res. Commun. 2014, 459, 105–109. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Xiong, Y.L. Chromatographic separation and tandem MS identification of active peptides in potato protein hydrolysate that inhibit autooxidation of soybean oil-in-water emulsions. J. Agric. Food Chem. 2010, 58, 8825–8832. [Google Scholar] [CrossRef]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake less. Biosci. Biotech. Biochem. 1994, 58, 1767–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang-Bo, T.; Jia, C.; Shu-Ling, L.; Min, B. A quantitative structure-activity relationship (QSAR) study of peptide drugs based on a new descriptor of amino acids. J. Serb. Chem. Soc. 2015, 80, 343–353. [Google Scholar] [CrossRef]
- Ganapathy, V.; Gupta, N.; Martindale, R.G. Protein digestion and Absorption. In Physiology of the Gastrointestinal Tract; Jhonson, L.R., Ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 1667–1692. [Google Scholar]
- Iwaniak, A.; Mogut, D. Metabolic syndrome-preventing peptides derived from milk proteins and their presence in cheeses: A review. Appl. Sci. 2020, 10, 2772. [Google Scholar] [CrossRef]
- Wang, R.; Xie, N.; Li, B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: A review. J. Food Biochem. 2018, E12571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, R.; DeLozier, G.; Robinson, L.; McPherson, A. Bochemical and X-Ray diffraction analysis of Concanavalin B crystals from Jack Bean. Plant Physiol. 1984, 76, 175–183. [Google Scholar] [CrossRef]
- Oh, K.S.; Park, Y.S.; Sung, H.C. Expression and purification on an ACE-inhibitory peptide multimer from synthetic DNA in Escherichia coli. J. Microbiol. Biotechnol. 2002, 12, 56–64. [Google Scholar] [CrossRef]
- Fida, H.; Kumada, Y.; Terashima, M.; Katsudo, T.; Katoh, S. Tandem multimer expression of angiotensin-I converting enzyme inhibitory peptide in Escherichia coli. Biotechnol. J. 2009, 4, 1345–1356. [Google Scholar] [CrossRef]
- Guruprasad, K.; Reddy, B.V.B.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 1990, 4, 155–161. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.; Chung, S.; Koo, S.; Chun, O. Comparison of DPPH/ABTS assay to measure antioxidant capacity in popular antioxidant-rich U.S. foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Muller, L.; Frohlich, K.; Bohm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tang, X.; He, Z.; Dai, Y.; Xiong, Y.; Xie, M.; Chen, J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.; Moraes, J.; Fernandes, A.C.; Borges, A.; Valencia, E.; Fernandes, K.; Lemes, A.; Fernandes, C.; de Figuereido, H.; Rosa da Silva, E.; et al. Bioactive properties of protein hysrolysate of cottonseed byproduct: Antioxidant, antimicrobial, and angiotendin converting enzyme (ACE) inhibitory activities. Waste Biomass Valorization 2020, 12. [Google Scholar] [CrossRef]
- Karamac, M.; Kosinska-Cagnazzo, A.; Kulczyk, A. Use of different proteases to obtain flaxseed protein hydolysates with antioxidant activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.; Pires, C.; Nunes, M.; Batista, I. Effect of in vitro gastrointestinal digestion on the antioxidant activity of protein hydrolysates prepared for Cape hake by-products. Int. J. Food Sci. Techol. 2016, 51, 2528–2536. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of alcalase-hydrolysed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Zou, T.; He, T.; Li, H.; Tang, H.; Xia, E. The structure activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Saidi, S.; Deretani, A.; Belleville, M.; Amar, R. Antioxidant properties of peptide fractions from tuna dark muscle protein by-product hydrolysate produced by membrane fractionation process. Food Res. Int. 2014, 65, 329–336. [Google Scholar] [CrossRef]
- Girgih, A.; He, R.; Malomo, S.; Offengeden, M.; Wu, J.; Aluko, R. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Kaur, A.; Adesgun, K.B.; Sharma, P.; Sharma, D.; Kaur, S. Recently isolated food-derived antihypertensive hydrolysates and peptides: A review. Food Chem. 2021, 346, 128719. [Google Scholar] [CrossRef]
- Pedroche, J.; Yust, M.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Utilization of chickpea protein isolates for production of peptides with angiotensin-Iconverting enzyme (ACE)-inhibitory activity. J. Sci. Food Agric. 2002, 82, 960–965. [Google Scholar] [CrossRef]
- Je, J.Y.; Lee, K.H.; Lee, M.H.; Ahn, C. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009, 49, 1266–1272. [Google Scholar] [CrossRef]
- Magaña, M.D.; Segura-Campos, M.; Dávila-Ortiz, G.; Betancour-Ancona, D.; Chel-Guerrero, L. ACE-I inhibitory properties of hydrolysates from germinated and ungerminated Phaseolus lunatus proteins. Food Sci. Technol. 2015, 35, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Daliri, E.; Odusu, E.K.; Chelliah, R.; Park, M.H.; Kim, J.H.; Oh, D.H. Development of soy protein hydrolysate with an antihypertensive effect. Int. J. Mol. Sci. 2019, 20, 1496. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Xue, Z.; Ma, Q.; Guo, Q.; Xing, L.; Santhanam, R.K.; Zhang, M.; Chen, H. Antioxidant and antihypertensive effects of garlic protein and its hydrolysates and the related mechanism. J. Food Biochem. 2019, 44, e1312. [Google Scholar] [CrossRef]
- Suwannapan, O.; Wachirattanapongmette, K.; Thawornchinsombut, S.; Katekaew, S. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from Thai jasmine rice bran protein hydrolysates. Int. J. Food Sci. Technol. 2020, 55, 2441–2450. [Google Scholar] [CrossRef]
- Jia, C.; Hussain, N.; Ujiroghene, O.; Pang, X.; Zhang, S.; Lu, J.; Liu, L.; Lu, J. Generation and characterization of dipeptidyl peptidase-IV inhibitory peptides from trypsin -hydrolyzed α-lactalbumin-rich whey proteins. Food Chem. 2020, 318, 126333. [Google Scholar] [CrossRef]
- Hsu, K.; Tung, Y.; Huang, S.; Jao, C. Dipeptidyl peptidase-IV inhibitory activity of peptides in porcine skin gelatine hydrolysates. In Bioactive Food Peptides in Health and Disease, 1st ed.; Hernandes-Ledesma, B., Hsie, C., Eds.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Hayes, M. Effect of pre-treatment on the generation of dipeptidyl peptidase-IV and prolyl endopeptidase-inhibitory hydrolysates from bovine lung. Irish J. Agric. Food Res. 2017, 56, 12–24. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E. Production and identification of dipeptidyl peptidase IV (DPPIV) inhibitory peptides from discarded Sardine pilchardus protein. Food Chem. 2020, 328, 127096. [Google Scholar] [CrossRef] [PubMed]
- Harnedy-Rotwell, P.; McLaughlin, C.; O’Keffe, B.; Le Gouic, A.; Allsopp, P.; McSorley, E.; Sharkey, S.; Whooley, J.; McGovern, B.; O´Harte, F.; et al. Identification and characterization of peptides from boarfish (Capros aper) protein hydrolysate displaying In vitro dipeptidyl peptidase-IV (DPP-IV) inhibitory and insulinotropic activity. Food Res. Int. 2020, 131, 108989. [Google Scholar] [CrossRef] [PubMed]
- Nogonierma, A.; FitzGerald, R. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2017, 43, e1245. [Google Scholar] [CrossRef] [Green Version]
- Istvan, E.; Paltnikar, M.; Buchanan, S.; Disenhofer, J. Crystal structure of the catalytic portion of human HMG-CoA reductase: Insights into regulation activity and catalysis. EMBO J. 2000, 19, 819–830. [Google Scholar] [CrossRef]
- Holdgate, H.A.; Ward, W.H.J.; McTaggart, F. Molecular mechanism for inhibition of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase by rosuvastatin. Biochem. Soc. Trans. 2003, 31, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Manólio Soares, R.A.; Mendoca, S.; Andrade de Castro, L.I.; Menezes, A.C.; Gomes Areas, J.A. Major peptides from amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]



| Protein Version | Instability Index | FFE (kcal/mol) | Total Contacts |
|---|---|---|---|
| 1CNV | 36.38 | −287.16 | 16,138 |
| 44 | 29.97 | −294.14 | 11,519 |
| 40 | 29.71 | −293.44 | 11,510 |
| 45 | 30.4 | −293.45 | 11,521 |
| 41 | 30.14 | −292.74 | 11,508 |
| 68 | 30.4 | −292.23 | 11,467 |
| Activity | BPs in 1CNV | BPs in CNV44 |
|---|---|---|
| ACEI | IVF, GK, DY, VR, IR | DG, IVF, VY, GK, IW, IY, IR |
| DPPIV inhibitors | TY, NW, DN, VR, IR, SW, TK, PY, NR | VY, IW, DN, EY, VR, IR, TK, QV, NR, IPI |
| Antioxidant | TY, IR, EL | TY, VY 1, IR, DW, EL |
| Lipid lowering | - | DE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Torres, D.A.; Jara-Romero, G.J.; Rosas-Cárdenas, F.d.F.; Fernández-Velasco, D.A.; Luna-Suárez, S. Engineering Concanavalin B to Release Bioactive Peptides against Metabolic Syndrome. Foods 2021, 10, 1554. https://doi.org/10.3390/foods10071554
Maldonado-Torres DA, Jara-Romero GJ, Rosas-Cárdenas FdF, Fernández-Velasco DA, Luna-Suárez S. Engineering Concanavalin B to Release Bioactive Peptides against Metabolic Syndrome. Foods. 2021; 10(7):1554. https://doi.org/10.3390/foods10071554
Chicago/Turabian StyleMaldonado-Torres, Diego Armando, G. Janet Jara-Romero, Flor de Fátima Rosas-Cárdenas, D. Alejandro Fernández-Velasco, and Silvia Luna-Suárez. 2021. "Engineering Concanavalin B to Release Bioactive Peptides against Metabolic Syndrome" Foods 10, no. 7: 1554. https://doi.org/10.3390/foods10071554