Influence of Emulsifiers and Dairy Ingredients on Manufacturing, Microstructure, and Physical Properties of Butter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Dairy Cream
2.1.2. Additives
2.2. Methods
2.2.1. Preparation of Cream Samples
2.2.2. Butter-Making
2.3. Analyses
2.3.1. Fat and Moisture Content
2.3.2. Microstructure
2.3.3. Thermal Property
2.3.4. Texture Analysis
2.3.5. Rheology
2.3.6. Tribology
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Butter-Making: Churning Time and Moisture Content
3.2. Microstructure
3.3. Physical Properties
3.3.1. Thermal Properties
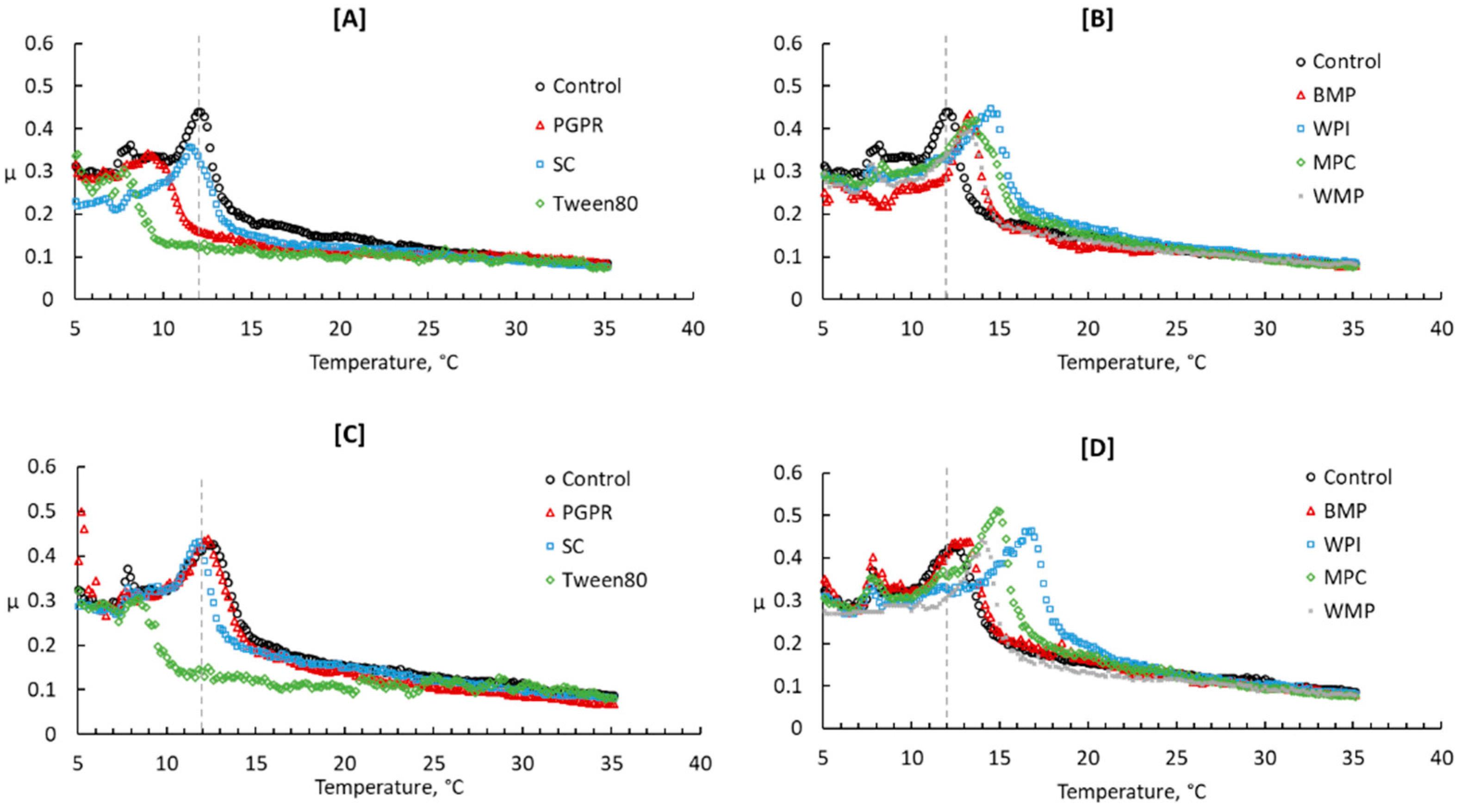
3.3.2. Texture and Rheology
3.3.3. Tribology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Panchal, B.; Bhandari, B. Butter and Dairy Fat Spreads. In Dairy Fat Products and Functionality; Springer: Cham, Switzerland, 2020; pp. 509–532. [Google Scholar]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 1998. [Google Scholar]
- Keogh, M.K. Chemistry and Technology of Butter and Milk Fat Spreads. In Advanced Dairy Chemistry Volume 2 Lipids; Springer: New York, NY, USA, 2007; pp. 333–363. [Google Scholar]
- Bornaz, S.; Fanni, J.; Parmentier, M. Heat treatment of cream: A model of the butter texture response in relation with triglyceride composition. J. Am. Oil Chem. Soc. 1995, 72, 163–169. [Google Scholar] [CrossRef]
- Dolby, R.M. 539. The effect of temperature treatment of cream before churning on the consistency of butter. J. Dairy Res. 1954, 21, 67. [Google Scholar] [CrossRef]
- Rønholt, S.; Kirkensgaard, J.J.K.; Pedersen, T.B.; Mortensen, K.; Knudsen, J.C. Polymorphism, microstructure and rheology of butter. Effects of cream heat treatment. Food Chem. 2012, 135, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Rønholt, S.; Madsen, A.S.; Kirkensgaard, J.J.K.; Mortensen, K.; Knudsen, J.C. Effect of churning temperature on water content, rheology, microstructure and stability of butter during four weeks of storage. Food Struct. 2014, 2, 14–26. [Google Scholar] [CrossRef]
- Wright, A.; Scanlon, M.; Hartel, R.; Marangoni, A. Rheological Properties of Milkfat and Butter. J. Food Sci. 2008, 66, 1056–1071. [Google Scholar] [CrossRef]
- Kaylegian, K.E.; Lindsay, R.C. Performance of Selected Milk Fat Fractions in Cold-Spreadable Butter. J. Dairy Sci. 1992, 75, 3307–3317. [Google Scholar] [CrossRef]
- Shukla, A.; Bhaskar, A.R.; Rizvi, S.S.H.; Mulvaney, S.J. Physicochemical and Rheological Properties of Butter Made from Supercritically Fractionated Milk Fat. J. Dairy Sci. 1994, 77, 45–54. [Google Scholar] [CrossRef]
- Avramis, C.; Wang, H.; McBride, B.; Wright, T.; Hill, A. Physical and Processing Properties of Milk, Butter, and Cheddar Cheese from Cows Fed Supplemental Fish Meal. J. Dairy Sci. 2003, 86, 2568–2576. [Google Scholar] [CrossRef]
- Banks, W.; Christie, W.W. Feeding Cows for the Production of Butter with Good Spreadability at Refrigeration Temperatures. Outlook Agric. 1990, 19, 43–47. [Google Scholar] [CrossRef]
- Cadden, A.-M.; Urquhart, A.; Jelen, P. Evaluation of Milk and Butter from Commercial Dairy Herds Fed Canola-Based Protected Lipid Feed Supplement. J. Dairy Sci. 1984, 67, 2041–2044. [Google Scholar] [CrossRef]
- Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B. Effects of dissolved carbon dioxide in fat phase of cream on manufacturing and physical properties of butter. J. Food Eng. 2018, 226, 9–21. [Google Scholar] [CrossRef]
- Panchal, B.R.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Effect of fat globule size on the churnability of dairy cream. Food Res. Int. 2017, 99, 229–238. [Google Scholar] [CrossRef]
- Panchal, B.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Influence of fat globule size, emulsifiers, and cream-aging on microstructure and physical properties of butter. Int. Dairy J. 2021, 117, 105003. [Google Scholar] [CrossRef]
- Kapsalis, J.; Kristoffersen, T.; Gould, I.; Betscher, J. Effect of Chemical Additives on the Spreading Quality of Butter. II. Laboratory and Plant Churnings. J. Dairy Sci. 1963, 46, 107–113. [Google Scholar] [CrossRef]
- Panchal, B.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Effect of water content, droplet size, and gelation on fat phase transition and water mobility in water-in-milk fat emulsions. Food Chem. 2020, 333, 127538. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.W.; Bhaggan, K.; Talbot, G.; van Malssen, K.F. Crystallization of fats: Influence of minor components and additives. J. Am. Oil. Chem. Soc. 2011, 88, 1085–1101. [Google Scholar] [CrossRef]
- Walstra, P. Secondary Nucleation in Triglyceride Crystallization. In The Colloid Science of Lipids; Springer: New York, NY, USA, 2008; pp. 4–8. [Google Scholar]
- Di Bari, V.; Sullo, A.; Norton, J.; Norton, I. Material properties of cocoa butter emulsions: Effect of dispersed phase droplet size and compression speed on mechanical response. Colloids Surf. A 2019, 575, 292–298. [Google Scholar] [CrossRef]
- Rafanan, R.; Rousseau, D. Dispersed droplets as active fillers in fat-crystal network-stabilized water-in-oil emulsions. Food Res. Int. 2017, 99, 355–362. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Viscoelastic Properties of Protein-Stabilized Emulsions: Effect of Protein−Surfactant Interactions. J. Agric. Food Chem. 1998, 46, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Chen, J. Heat-set whey protein emulsion gels: Role of active and inactive filler particles. J. Dispers. Sci. Technol. 1999, 20, 197–213. [Google Scholar] [CrossRef]
- Corredig, M. Dairy-Derived Ingredients: Food and Nutraceutical Uses; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Sharma, A.; Jana, A.H.; Chavan, R.S. Functionality of Milk Powders and Milk-Based Powders for End Use Applications—A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 518–528. [Google Scholar] [CrossRef]
- Alimentarius, C. Codex standard for butter, codex standard 279–1971. In Milk and Milk Products; World Health Organization: Rome, Italy, 2011; pp. 36–37. [Google Scholar]
- Bureau of Indian Standards (BIS). IS-1224 Determination of Fat by Gerber Method: Part II Milk Products; Bureau of Indian Standards: Delhi, India, 1977. [Google Scholar]
- AOAC International. Official methods of analysis of AOAC International. In Moisture in Butter, Method No. 920.116; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Truong, T.; Bansal, N.; Sharma, R.; Palmer, M.; Bhandari, B. Effects of emulsion droplet sizes on the crystallisation of milk fat. Food Chem. 2014, 145, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Rohm, H. Rheological behaviour of butter at large deformations. J. Texture Stud. 1993, 24, 139–155. [Google Scholar] [CrossRef]
- Awad, T.S.; Rogers, M.A.; Marangoni, A.G. Scaling Behavior of the Elastic Modulus in Colloidal Networks of Fat Crystals. J. Phys. Chem. B 2004, 108, 171–179. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Schröder, A.; Berton-Carabin, C.; Venema, P.; Cornacchia, L. Interfacial properties of whey protein and whey protein hydrolysates and their influence on O/W emulsion stability. Food Hydrocoll. 2017, 73, 129–140. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Morr, C.V.; Ha, E.Y.W. Structural and Functional Properties of Caseinate and Whey Protein Isolate as Affected by Temperature and pH. J. Food Sci. 1992, 57, 1210–1229. [Google Scholar] [CrossRef]
- Graham, D.E.; Phillips, M. Proteins at liquid interfaces: III. Molecular structures of adsorbed films. J. Colloid Interface Sci. 1979, 70, 427–439. [Google Scholar] [CrossRef]
- Dickinson, E. Adsorbed protein layers at fluid interfaces: Interactions, structure and surface rheology. Colloids Surf. B 1999, 15, 161–176. [Google Scholar] [CrossRef]
- Benjamins, J.; Cagna, A.; Lucassen-Reynders, E. Viscoelastic properties of triacylglycerol/water interfaces covered by proteins. Colloids Surf. A 1996, 114, 245–254. [Google Scholar] [CrossRef]
- Marinova, K.; Basheva, E.S.; Nenova, B.; Temelska, M.; Mirarefi, A.Y.; Campbell, B.; Ivanov, I.B. Physico-chemical factors controlling the foamability and foam stability of milk proteins: Sodium caseinate and whey protein concentrates. Food Hydrocoll. 2009, 23, 1864–1876. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Conformations and structures of milk proteins adsorbed to oil-water interfaces. Food Res. Int. 1996, 29, 541–547. [Google Scholar] [CrossRef]
- Courthaudon, J.-L.; Dickinson, E.; Dalgleish, D.G. Competitive adsorption of β-casein and nonionic surfactants in oil-in-water emulsions. J. Colloid Interface Sci. 1991, 145, 390–395. [Google Scholar] [CrossRef]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.B.; Larsen, F.H.; Berg, F.W.J.V.D.; Knudsen, J.C.; Andersen, M.L. Competitive Displacement of Sodium Caseinate by Low-Molecular-Weight Emulsifiers and the Effects on Emulsion Texture and Rheology. Langmuir 2014, 30, 8687–8696. [Google Scholar] [CrossRef] [PubMed]
- Bastida-Rodríguez, J. The Food Additive Polyglycerol Polyricinoleate (E-476): Structure, Applications, and Production Methods. ISRN Chem. Eng. 2013, 2013, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Buldo, P.; Andersen, U.; Wiking, L. Microstructure and Material Properties of Milk Fat Systems During Temperature Fluctuations. Food Biophys. 2013, 8, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Buldo, P.; Kirkensgaard, J.J.; Wiking, L. Crystallization mechanisms in cream during ripening and initial butter churning. J. Dairy Sci. 2013, 96, 6782–6791. [Google Scholar] [CrossRef]
- Rashevskaya, T.; Gulyi, I.; Ukrainets, A.; Nishchenko, M.; Likhtorovich, S.; Buzaneva, E. Identification of moisture nanoparticles in the butter sub-microstructure. Mater. Sci. Eng. C 2002, 19, 33–35. [Google Scholar] [CrossRef]
- Tomaszewska-Gras, J. Melting and crystallization DSC profiles of milk fat depending on selected factors. J. Therm. Anal. Calorim. 2013, 113, 199–208. [Google Scholar] [CrossRef] [Green Version]
- De Man, J.; Wood, F. Hardness of butter II. Influence of setting. J. Dairy Sci. 1959, 42, 56–61. [Google Scholar] [CrossRef]
- Douaire, M.; Di Bari, V.; Norton, J.E.; Sullo, A.; Lillford, P.; Norton, I.T. Fat crystallisation at oil-water interfaces. Adv. Colloid Interface Sci. 2014, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.; Dungan, S.; German, J.; Simoneau, C.; Kinsella, J. Droplet Size and Emulsifier Type Affect Crystallization and Melting of Hydrocarbon-in-Water Emulsions. J. Food Sci. 1993, 58, 1148–1151. [Google Scholar] [CrossRef]
- Tempel, M.V.D. Rheology of plastic fats. Rheol. Acta 1958, 1, 115–118. [Google Scholar] [CrossRef]
- Boodhoo, M.; Humphrey, K.; Narine, S. Relative Hardness of Fat Crystal Networks Using Force Displacement Curves. Int. J. Food Prop. 2009, 12, 129–144. [Google Scholar] [CrossRef]
- Dedinaite, A.; Campbell, B. Interactions between Mica Surfaces across Triglyceride Solution Containing Phospholipid and Polyglycerol Polyricinoleate. Langmuir 2000, 16, 2248–2253. [Google Scholar] [CrossRef]
- Di Bari, V.; Macnaughtan, W.; Norton, J.; Sullo, A.; Norton, I. Crystallisation in water-in-cocoa butter emulsions: Role of the dispersed phase on fat crystallisation and polymorphic transition. Food Struct. 2017, 12, 82–93. [Google Scholar] [CrossRef]
- Zafeiri, I.; Norton, J.E.; Smith, P.; Norton, I.T.; Spyropoulos, F. The role of surface active species in the fabrication and functionality of edible solid lipid particles. J. Colloid Interface Sci. 2017, 500, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.L.; Hartel, R.W. Effect of processing conditions on physical properties of a milk fat model system: Rheology. J. Am. Oil Chem. Soc. 2000, 77, 1189–1196. [Google Scholar] [CrossRef]
- Narine, S.S.; Marangoni, A.G. Fractal nature of fat crystal networks. Phys. Rev. E 1999, 59, 1908. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Bhandari, B.; Prakash, S. Tribological method to measure lubricating properties of dairy products. J. Food Eng. 2016, 168, 27–34. [Google Scholar] [CrossRef]
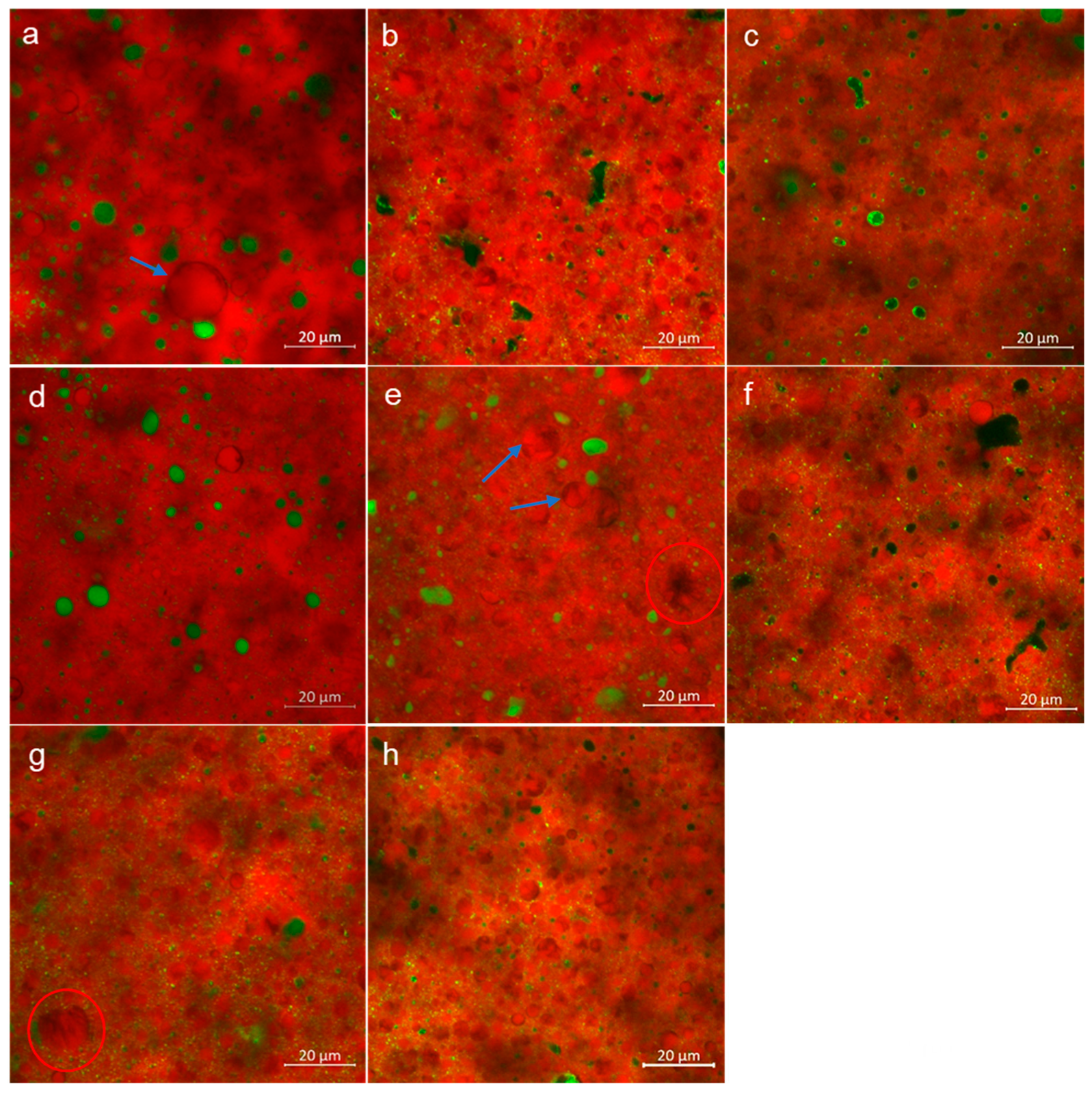

| Sample | Churning Time, Sec | Moisture, % (w/w) |
|---|---|---|
| Control * | 225 ± 21 b | 15.47 ± 2.45 ab |
| PGPR | 218 ± 11 b | 21.50 ± 1.19 a |
| Tween 80 | 160 ± 28 b | 15.35 ± 0.28 b |
| SC | 315 ± 21 a | 16.89 ± 2.47 ab |
| WPI | 225 ± 21 b | 15.17 ± 0.09 b |
| BMP | 195 ± 7 b | 14.91 ± 2.04 b |
| MPC | 212 ± 18 b | 16.45 ± 1.18 ab |
| WMP | 205 ± 7 b | 15.19 ± 0.24 b |
| Sample | Peak Melting Temperature (°C) | SFC, % | ||
|---|---|---|---|---|
| Day 1 | Day 28 | Day 1 | Day 28 | |
| Control * | 21.8 ± 0.5 a | 23.3 ± 0.4 a | 42.4 ± 2.4 a | 46.3 ± 1.4 a |
| PGPR | 21.2 ± 0.1 a | 21.9 ± 0.7 ab | 41.3 ± 3.8 a | 46.5 ± 0.3 a |
| Tween 80 | 23.4 ± 0.1 a | 22.9 ± 0.4 ab | 38.9 ± 2.1 a | 44.2 ± 3.5 ab |
| SC | 23.0 ± 0.2 a | 22.7 ± 0.5 ab | 37.4 ± 3.3 a | 38.3 ± 1.7 b |
| WPI | 22.7 ± 0.2 a | 21.9 ± 0.3 ab | 38.2 ± 1.5 a | 46.1 ± 1.7 a |
| BMP | 21.8 ± 0.6 a | 22.1 ± 0.6 ab | 42.3 ± 3.7 a | 45.4 ± 0.5 a |
| MPC | 22.3 ± 0.1 a | 22.2 ± 0.2 ab | 44.7 ± 2.6 a | 47.1 ± 0.7 a |
| WMP | 22.5 ± 1.4 a | 21.6 ± 0.4 b | 45.8 ± 0.4 a | 50.4 ± 2.1 a |
| Sample | Hardness, N | Adhesiveness, N | G’, MPa | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 28 | Day 1 | Day 28 | Day 1 | Day 28 | |
| Control * | 6.67 ± 0.45 bc | 8.44 ± 0.70 ab | 1.92 ± 0.10 bc | 2.25 ± 0.15 ab | 2.62 ± 0.52 c | 3.08 ± 0.08 cd |
| PGPR | 5.17 ± 0.28 d | 6.58 ± 0.27 c | 1.49 ± 0.05 d | 1.84 ± 0.10 b | 1.45 ± 0.23 d | 4.07 ± 0.34 abc |
| Tween 80 | 6.72 ± 0.19 bc | 9.30 ± 1.10 a | 1.97 ± 0.25 bc | 2.07 ± 0.34 ab | 2.18 ± 0.16 cd | 3.47 ± 0.39 bcd |
| SC | 5.88 ± 0.92 cd | 7.75 ± 1.07 bc | 1.64 ± 0.17 cd | 2.50 ± 0.28 a | 1.75 ± 0.12 d | 2.71 ± 0.80 d |
| WPI | 7.86 ± 0.49 a | 8.66 ± 0.46 ab | 2.34 ± 0.19 a | 2.53 ± 0.27 a | 3.96 ± 0.40 a | 4.91 ± 0.48 ab |
| BMP | 7.25 ± 0.23 ab | 8.05 ± 0.54 abc | 2.31 ± 0.15 ab | 2.36 ± 0.16 ab | 2.47 ± 0.74 cd | 3.83 ± 0.53 abc |
| MPC | 6.43 ± 0.13 bc | 8.42 ± 0.32 ab | 1.93 ± 0.06 bc | 2.13 ± 0.10 ab | 2.76 ± 0.75 bc | 3.92 ± 0.31 abc |
| WMP | 8.08 ± 0.74 a | 8.93 ± 0.14 ab | 2.48 ± 0.22 a | 2.50 ± 0.31 a | 3.61 ± 0.57 ab | 4.92 ± 0.33 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchal, B.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Influence of Emulsifiers and Dairy Ingredients on Manufacturing, Microstructure, and Physical Properties of Butter. Foods 2021, 10, 1140. https://doi.org/10.3390/foods10051140
Panchal B, Truong T, Prakash S, Bansal N, Bhandari B. Influence of Emulsifiers and Dairy Ingredients on Manufacturing, Microstructure, and Physical Properties of Butter. Foods. 2021; 10(5):1140. https://doi.org/10.3390/foods10051140
Chicago/Turabian StylePanchal, Bhavesh, Tuyen Truong, Sangeeta Prakash, Nidhi Bansal, and Bhesh Bhandari. 2021. "Influence of Emulsifiers and Dairy Ingredients on Manufacturing, Microstructure, and Physical Properties of Butter" Foods 10, no. 5: 1140. https://doi.org/10.3390/foods10051140






