Angiotensin-I Converting Enzyme Inhibition and Antioxidant Activity of Papain-Hydrolyzed Camel Whey Protein and Its Hepato-Renal Protective Effects in Thioacetamide-Induced Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CWP, CWPH, SEC-1, SCE2, and Estimation of Their Activities
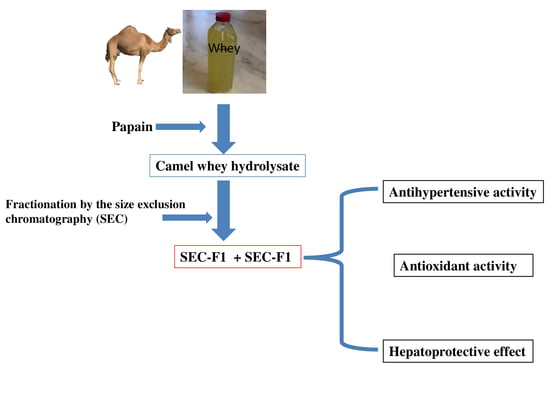
2.1.1. Hydrolysis and Fractionation of Camel Whey Protein (CWP)
2.1.2. Estimation of the ACE Inhibition Activity (ACE-IA)
2.1.3. Estimation of the Antioxidant Activity
2.2. Estimation of the Protective Effect of CWPH on Thioacetamide-Induced Toxicity
2.2.1. Animals and Experimental Design
2.2.2. Blood and Tissue Sampling
2.2.3. Biochemical Assay
Assessment of Liver Function, Kidney Function, and Lipid Profile
Evaluation of the Redox State in the Liver
2.2.4. Histopathological Examination
2.3. Statistical Analysis
3. Results and Discussion
3.1. ACE-Inhibitory Activity (ACE-IA) of CWP, CWPH, and Its (SEC-F1 and SEC-F2) Fractions
3.2. Antioxidant Activity of CWPH and its Fractions
3.3. Effect of CWPH Treatment on Thioacetamide (TAA)-Induced Hepatorenal Toxicity
3.3.1. Hepatic Oxidative-Antioxidative Balance
3.3.2. TAA-Induced Hepatotoxicity and the Effect of CWPH Treatment
3.3.3. Serum Lipid Profile
3.3.4. TAA-Induced Renal Toxicity and the Effect of CWPH Treatment
3.3.5. Histopathological Changes in the Liver
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamrozik, K. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- World Health Organization. Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Hagen, S.; Stark, D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst. Rev. 2011, CD003882. [Google Scholar] [CrossRef] [PubMed]
- Sorbets, E.; Labreuche, J.; Simon, T.; Delorme, L.; Danchin, N.; Amarenco, P.; Goto, S.; Meune, C.; Eagle, K.A.; Bhatt, D.L.; et al. Renin-angiotensin system antagonists and clinical outcomes in stable coronary artery disease without heart failure. Eur. Heart J. 2014, 35, 1760–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Fandiño, R.; Otte, J.; Van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Ding, F.; Qian, B.; Zhao, X.; Shen, S.; Deng, Y.; Wang, D.; Zhang, F.; Sui, Z.; Jing, P. VPPIPP and IPPVPP: Two Hexapeptides Innovated to Exert Antihypertensive Activity. PLoS ONE 2013, 8, e62384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Behare, P.; Rana, R.; Kumar, A.; Kumar, M.; Arora, S.; Morotta, F.; Jain, S.; Yadav, H. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food Funct. 2010, 2, 18–27. [Google Scholar] [CrossRef]
- Ricci, I.; Artacho, R.; Olalla, M. Milk Protein Peptides With Angiotensin I-Converting Enzyme Inhibitory (ACEI) Activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Sakurai, T.; Ochi, D.; Mitsuyama, E.; Yamauchi, K.; Abe, F. Antihypertensive effect of the bovine casein-derived peptide Met-Lys-Pro. Food Chem. 2015, 172, 441–446. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and Modification of the Carcinogenic Response by bha, Bht, and Other Antioxidants. CRC Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef]
- Fu, X. Effect on plant leaf protein on lipotropy peroxidase system of rats. Chin J. Vet. Sci. Technol. 2003, 11, 1–18. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.-R.; Osman, A.; Enan, G.; Abdel-Shafi, S.; El-Nemer, M.; Sitohy, M.; Taha, M.A. Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics 2020, 9, 901. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Saporito, P.; Osman, A.; Mateiu, R.V.; Mojsoska, B.; Jenssen, H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control. 2020, 111, 107056. [Google Scholar] [CrossRef]
- Jrad, Z.; El Hatmi, H.; Adt, I.; Girardet, J.-M.; Cakir-Kiefer, C.; Jardin, J.; Degraeve, P.; Khorchani, T.; Oulahal, N. Effect of digestive enzymes on antimicrobial, radical scavenging and angiotensin I-converting enzyme inhibitory activities of camel colostrum and milk proteins. Dairy Sci. Technol. 2014, 94, 205–224. [Google Scholar] [CrossRef]
- Kappeler, S.; Heuberger, C.; Farah, Z.; Puhan, Z. Expression of the Peptidoglycan Recognition Protein, PGRP, in the Lactating Mammary Gland. J. Dairy Sci. 2004, 87, 2660–2668. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hamid, M.; Goda, H.A.; De Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- Espejo-Carpio, F.J.; De Gobba, C.; Guadix, A.; Guadix, E.M.; Otte, J. Angiotensin I-converting enzyme inhibitory activity of enzymatic hydrolysates of goat milk protein fractions. Int. Dairy J. 2013, 32, 175–183. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Osman, A.; Abd-Elaziz, S.; Salama, A.; Eita, A.A.; Sitohy, M. Health protective actions of phycocyanin obtained from an egyptian isolate of spirulina platensis on albino rats. Eur. Asia. J. BioSci. 2019, 13, 105–112. [Google Scholar]
- Otte, J.; Shalaby, S.M.; Zakora, M.; Pripp, A.H.; El-Shabrawy, S.A. Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: Effect of substrate, enzyme and time of hydrolysis. Int. Dairy J. 2007, 17, 488–503. [Google Scholar] [CrossRef]
- A Alhaj, O.; A Metwalli, A.; A Ismail, E.; Ali, H.S.; Al-Khalifa, A.S.; Kanekanian, A.D. Angiotensin converting enzyme-inhibitory activity and antimicrobial effect of fermented camel milk (Camelus dromedarius). Int. J. Dairy Technol. 2018, 71, 27–35. [Google Scholar] [CrossRef]
- Jafar, S.; Kamal, H.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel whey protein hydrolysates displayed enhanced cholesteryl esterase and lipase inhibitory, anti-hypertensive and anti-haemolytic properties. LWT 2018, 98, 212–218. [Google Scholar] [CrossRef]
- Salami, M.; Moosavi-Movahedi, A.A.; Moosavi-Movahedi, F.; Ehsani, M.R.; Yousefi, R.; Farhadi, M.; Niasari-Naslaji, A.; Saboury, A.A.; Chobert, J.-M.; Haertlé, T. Biological activity of camel milk casein following enzymatic digestion. J. Dairy Res. 2011, 78, 471–478. [Google Scholar] [CrossRef]
- Mudgil, P.; Baby, B.; Ngoh, Y.-Y.; Kamal, H.; Vijayan, R.; Gan, C.-Y.; Maqsood, S. Molecular binding mechanism and identification of novel anti-hypertensive and anti-inflammatory bioactive peptides from camel milk protein hydrolysates. LWT 2019, 112, 108193. [Google Scholar] [CrossRef]
- Moslehishad, M.; Ehsani, M.R.; Salami, M.; Mirdamadi, S.; Ezzatpanah, H.; Naslaji, A.N.; Moosavi-Movahedi, A.A. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int. Dairy J. 2013, 29, 82–87. [Google Scholar] [CrossRef]
- Elias, R.J.; McClements, D.J.; Decker, E.A. Antioxidant Activity of Cysteine, Tryptophan, and Methionine Residues in Continuous Phase β-Lactoglobulin in Oil-in-Water Emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef] [PubMed]
- Addar, L.; Bensouici, C.; Zennia, S.S.A.; Haroun, S.B.; Mati, A. Antioxidant, tyrosinase and urease inhibitory activities of camel αS-casein and its hydrolysate fractions. Small Rumin. Res. 2019, 173, 30–35. [Google Scholar] [CrossRef]
- Salami, M.; Moosavi-Movahedi, A.A.; Ehsani, M.R.; Yousefi, R.; Haertlé, T.; Chobert, J.-M.; Razavi, S.H.; Henrich, R.; Balalaie, S.; Ebadi, S.A.; et al. Improvement of the Antimicrobial and Antioxidant Activities of Camel and Bovine Whey Proteins by Limited Proteolysis. J. Agric. Food Chem. 2010, 58, 3297–3302. [Google Scholar] [CrossRef]
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin. Res. 2016, 139, 20–25. [Google Scholar] [CrossRef]
- Shanmugam, V.; Kapila, S.; Sonfack, T.K.; Kapila, R. Antioxidative peptide derived from enzymatic digestion of buffalo casein. Int. Dairy J. 2015, 42, 1–5. [Google Scholar] [CrossRef]
- A Kadir, F.; Kassim, N.M.; A Abdulla, M.; A Yehye, W. Effect of oral administration of ethanolic extract of Vitex negundo on thioacetamide-induced nephrotoxicity in rats. BMC Complement. Altern. Med. 2013, 13, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehul, D.; Varsha, G. Effect of polyherbal preparation on thioacetamide induced liver damage and hepatic encephalopathy in rats. Int. Res. J. Pharm. 2012, 3, 192–198. [Google Scholar]
- El-Hadary, A.E.A. Protective effect of ginger oil against thioacetamide- induced liver cirrhosis in male rats. J. Agric. Chem. Biotechnol. 2015, 6, 393–405. [Google Scholar] [CrossRef]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.-I.; Friedman, S.L. Regression of Fibrosis and Reversal of Cirrhosis in Rats by Galectin Inhibitors in Thioacetamide-Induced Liver Disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef] [Green Version]
- Kilari, B.P.; Mudgil, P.; Azimullah, S.; Bansal, N.; Ojha, S.; Maqsood, S. Effect of camel milk protein hydrolysates against hyperglycemia, hyperlipidemia, and associated oxidative stress in streptozotocin (STZ)-induced diabetic rats. J. Dairy Sci. 2021, 104, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Osman, A.; El-Hadary, A.; Romeih, E.; Sitohy, M.; Li, L. Hepatoprotective action of papain-hydrolyzed buffalo milk protein on carbon tetrachloride oxidative stressed albino rats. J. Dairy Sci. 2020, 103, 1884–1893. [Google Scholar] [CrossRef] [PubMed]


| Samples | ACE-IA (IC50; µg/mL) | Radical Scavenging (SC50; µg/mL) |
|---|---|---|
| CWP | 576.7 ± 6.5 a | 489 ± 3.6 a |
| CWPH | 410.8 ± 2.7 c | 79.8 ± 1.9 c |
| SEC-F1 | 469.3 ± 4 b | 317.6 ± 2.5 b |
| SEC-F2 | 179.9 ± 2.6 d | 60.9 ± 2.9 d |
| Groups | MDA (mol/mg) | Glutathione Peroxidase (U/g Tissue) | Glutathione S-Transferase (U/g Tissue) |
|---|---|---|---|
| G1 | 3.55 ± 0.39 c | 2.64 ± 0.06 b | 3.50 ± 0.10 ab |
| G2 | 10.21 ± 0.39 a | 0.98 ± 0.06 e | 2.55 ± 0.10 c |
| G3 | 5.94 ± 0.39 b | 2.12 ± 0.06 d | 3.23 ± 0.10 b |
| G4 | 4.15 ± 0.39 c | 2.37 ± 0.06 c | 3.56 ± 0.10 a |
| G5 | 3.88 ± 0.39 c | 2.93 ± 0.06 a | 3.74 ± 0.10 a |
| Groups | ALT Activity (U/L) | AST Activity (U/L) | ALP (U/L) | Total Proteins (g/dL) | Albumin (g/dL) | Globulin (g/dL) | A/G Ratio |
|---|---|---|---|---|---|---|---|
| G1 | 45.00 ± 4.41 bc | 23.33 ± 4.89 c | 81.86 ± 14.30 b | 7.74 ± 0.31 a | 2.89 ± 0.34 ab | 4.85 ± 0.28 c | 0.60 ± 0.13 b |
| G2 | 160.00 ± 4.41 a | 151.67 ± 4.89 a | 335.86 ± 14.30 a | 5.35 ± 0.31 b | 3.94 ± 0.34 a | 1.41 ± 0.28 d | 2.86 ± 0.13 a |
| G3 | 55.00 ± 4.41 b | 45.00 ± 4.89 b | 109.54 ± 14.30 b | 8.65 ± 0.31 a | 2.60 ± 0.34 b | 6.05 ± 0.28 ab | 0.43 ± 0.13 b |
| G4 | 33.33 ± 4.41 c | 36.67 ± 4.89 bc | 94.38 ± 14.30 b | 8.48 ± 0.31 a | 2.89 ± 0.34 ab | 5.59 ± 0.28 bc | 0.53 ± 0.13 b |
| G5 | 28.33 ± 4.41 d | 31.67 ± 4.89 bc | 74.72 ± 14.30 b | 8.75 ± 0.31 a | 1.92 ± 0.34 b | 6.83 ± 0.28 a | 0.30 ± 0.13 b |
| Groups | Total Lipid (mg/dl) | Triglyceride (mg/dl) | Total Cholesterol (mg/dl) | HDL-C (mg/dl) | LDL-C (mg/dl) | VLDL-C (mg/dl) |
|---|---|---|---|---|---|---|
| G1 | 491.83 ± 12.54 b | 151.56 ± 7.65 b | 96.78 ± 1.87 c | 39.38 ± 2.24 b | 27.09 ± 1.27 c | 30.31 ± 1.53 b |
| G2 | 663.25 ± 12.54 a | 274.83 ± 7.65 a | 147.03 ± 1.87 a | 27.03 ± 2.24 c | 65.03 ± 1.27 a | 54.97 ± 1.53 a |
| G3 | 493.19 ± 12.54 b | 160.20 ± 7.65 b | 111.89 ± 1.87 b | 35.06 ± 2.24 b | 44.79 ± 1.27 b | 32.04 ± 1.53 b |
| G4 | 503.77 ± 12.54 b | 147.88 ± 7.65 b | 107.63 ± 1.87 bc | 37.12 ± 2.24 b | 40.93 ± 1.27 b | 29.58 ± 1.53 b |
| G5 | 464.76 ± 12.54 b | 143.26 ± 7.65 b | 100.42 ± 1.87 c | 50.13 ± 2.24 a | 21.64 ± 1.27 c | 28.65 ± 1.53 b |
| Groups | Uric Acid (mg/dL) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|
| G1 | 5.70 ± 0.58 b | 39.93 ± 4.72 c | 1.08 ± 0.16 c |
| G2 | 9.09 ± 0.58 a | 68.52 ± 4.72 a | 3.25 ± 0.16 a |
| G3 | 7.47 ± 0.58 b | 59.06 ± 4.72 ab | 2.20 ± 0.16 b |
| G4 | 6.74 ± 0.58 b | 48.36 ± 4.72 bc | 1.45 ± 0.16 c |
| G5 | 6.19 ± 0.58 b | 44.41 ± 4.72 bc | 1.19 ± 0.16 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, A.; El-Hadary, A.; Korish, A.A.; AlNafea, H.M.; Alhakbany, M.A.; Awad, A.A.; Abdel-Hamid, M. Angiotensin-I Converting Enzyme Inhibition and Antioxidant Activity of Papain-Hydrolyzed Camel Whey Protein and Its Hepato-Renal Protective Effects in Thioacetamide-Induced Toxicity. Foods 2021, 10, 468. https://doi.org/10.3390/foods10020468
Osman A, El-Hadary A, Korish AA, AlNafea HM, Alhakbany MA, Awad AA, Abdel-Hamid M. Angiotensin-I Converting Enzyme Inhibition and Antioxidant Activity of Papain-Hydrolyzed Camel Whey Protein and Its Hepato-Renal Protective Effects in Thioacetamide-Induced Toxicity. Foods. 2021; 10(2):468. https://doi.org/10.3390/foods10020468
Chicago/Turabian StyleOsman, Ali, Abdalla El-Hadary, Aida A. Korish, Haifa M. AlNafea, Manan A. Alhakbany, Awad A. Awad, and Mahmoud Abdel-Hamid. 2021. "Angiotensin-I Converting Enzyme Inhibition and Antioxidant Activity of Papain-Hydrolyzed Camel Whey Protein and Its Hepato-Renal Protective Effects in Thioacetamide-Induced Toxicity" Foods 10, no. 2: 468. https://doi.org/10.3390/foods10020468