Effect of the Surface Treatment Method Using Airborne-Particle Abrasion and Hydrofluoric Acid on the Shear Bond Strength of Resin Cement to Zirconia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zirconia Specimens and Surface Treatment Methods
2.2. Bonding and Thermocycling
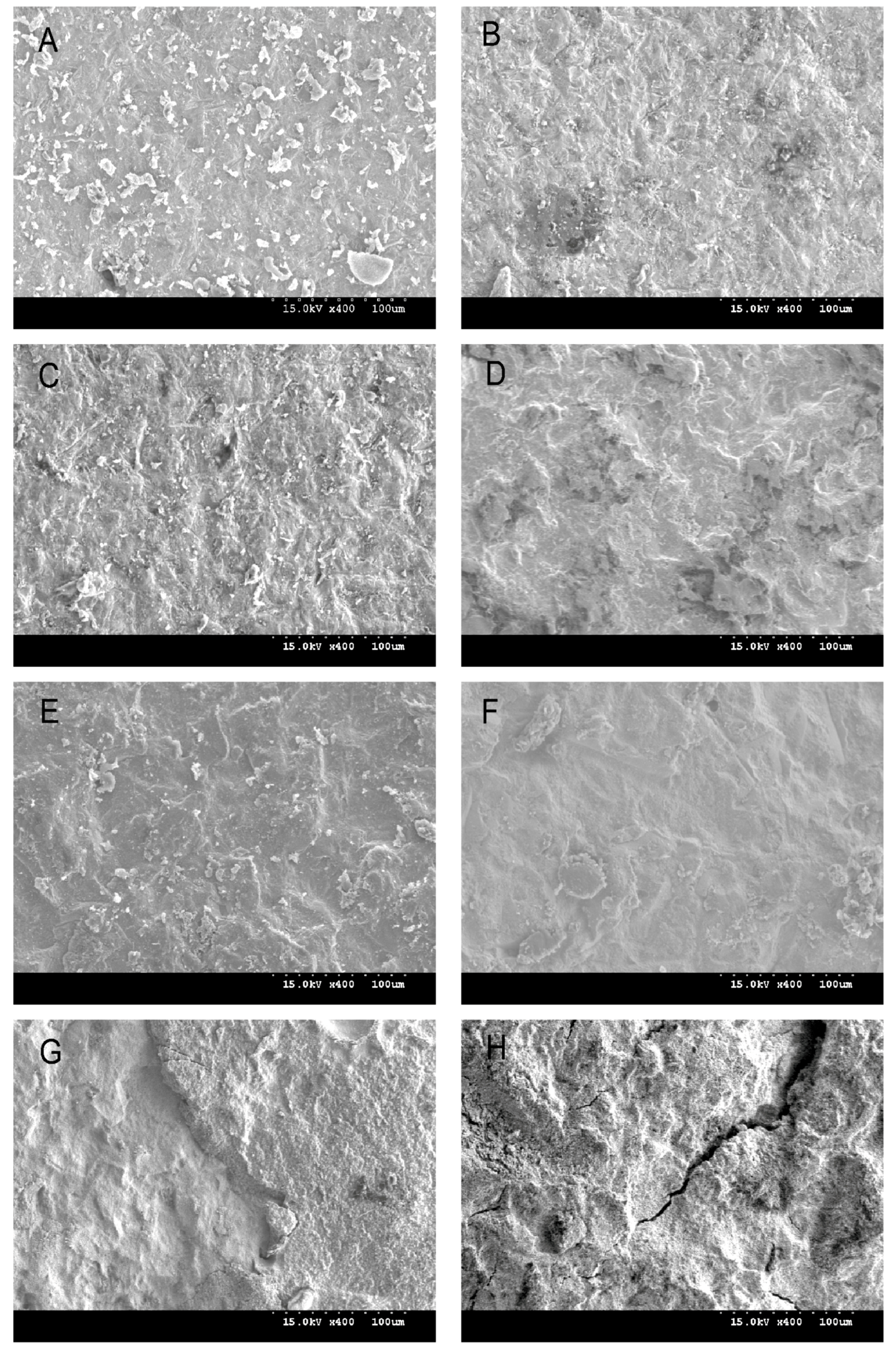
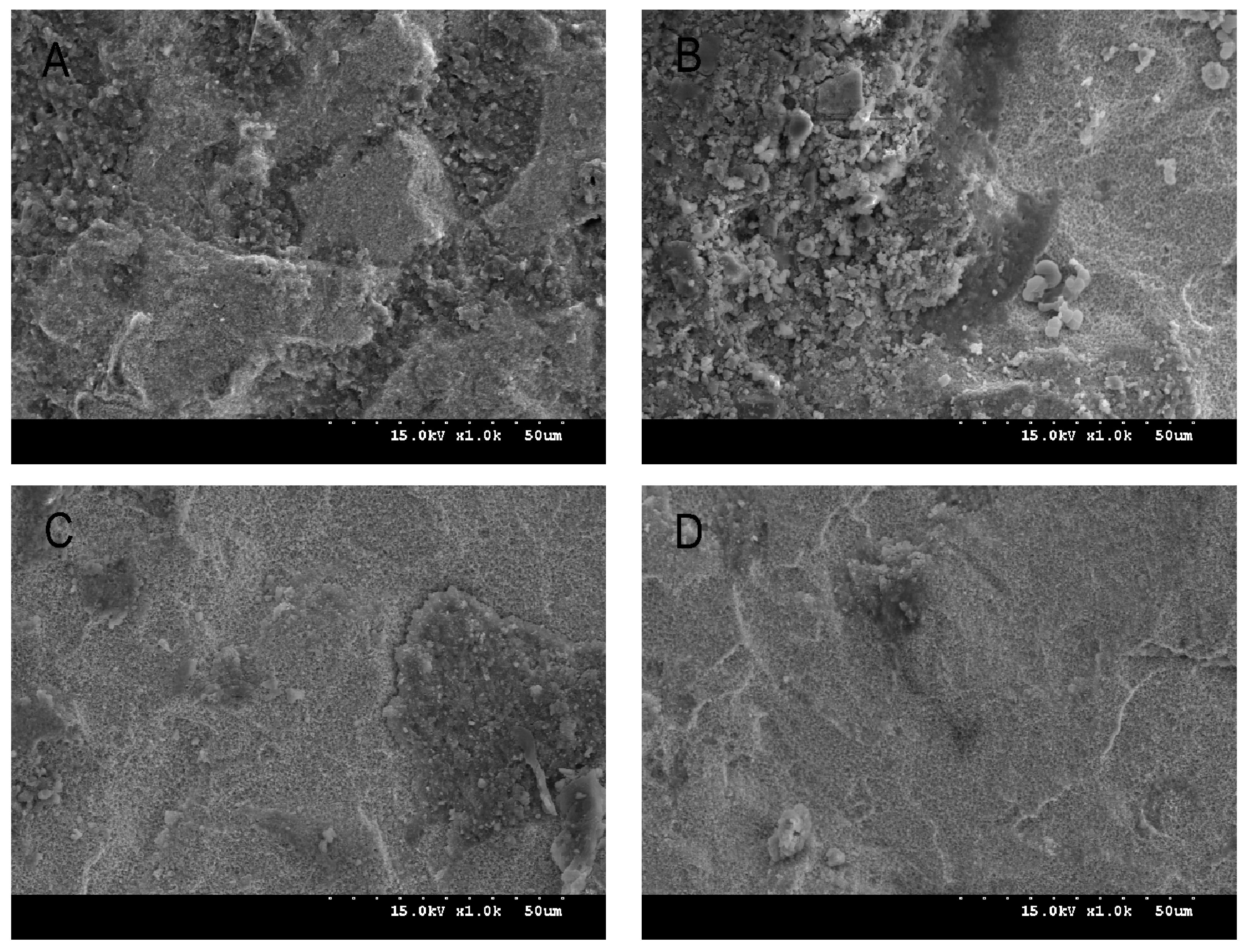
2.3. Shear Bond Strength Test and Debonded Zirconia Surface Observation
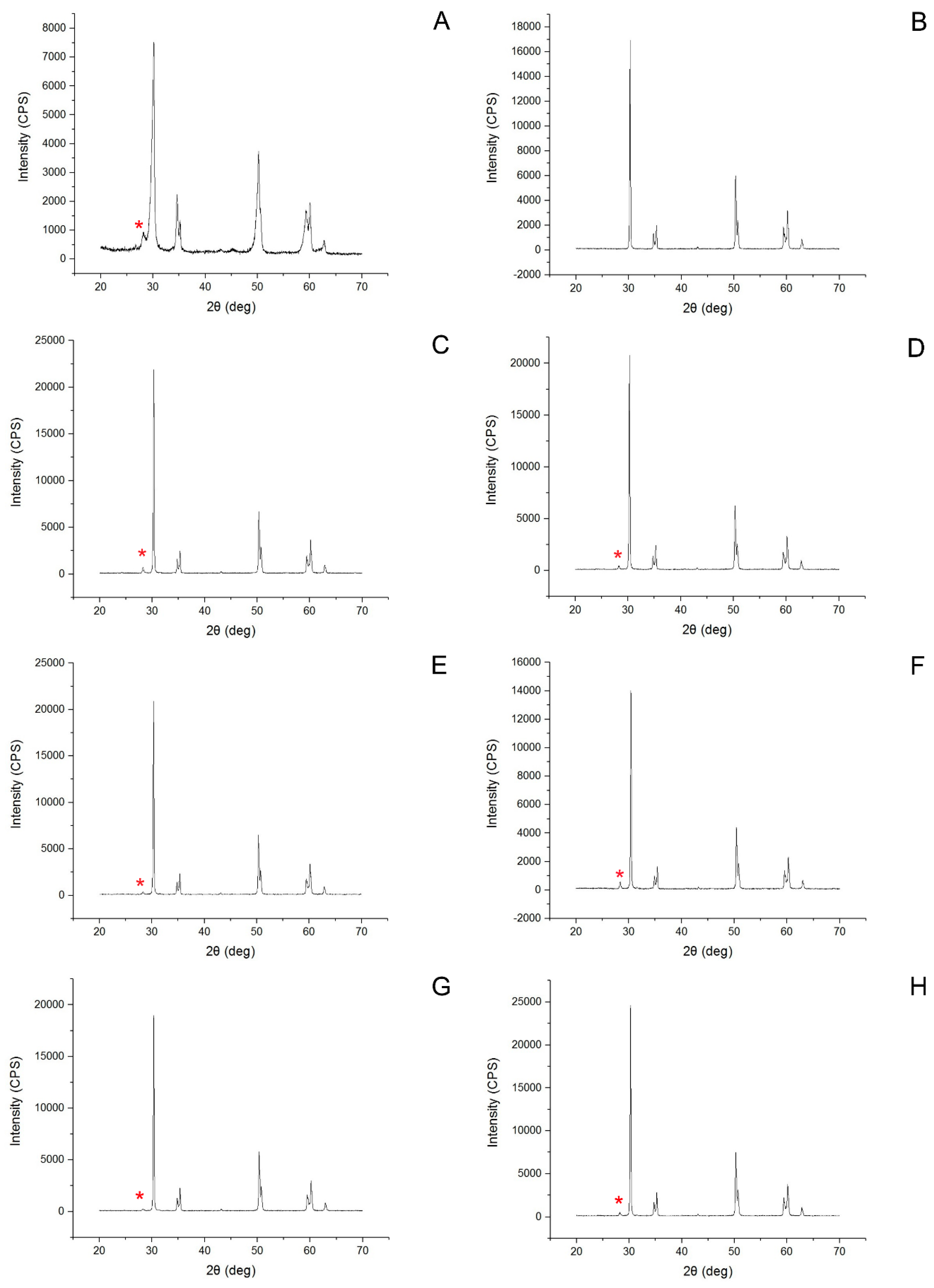
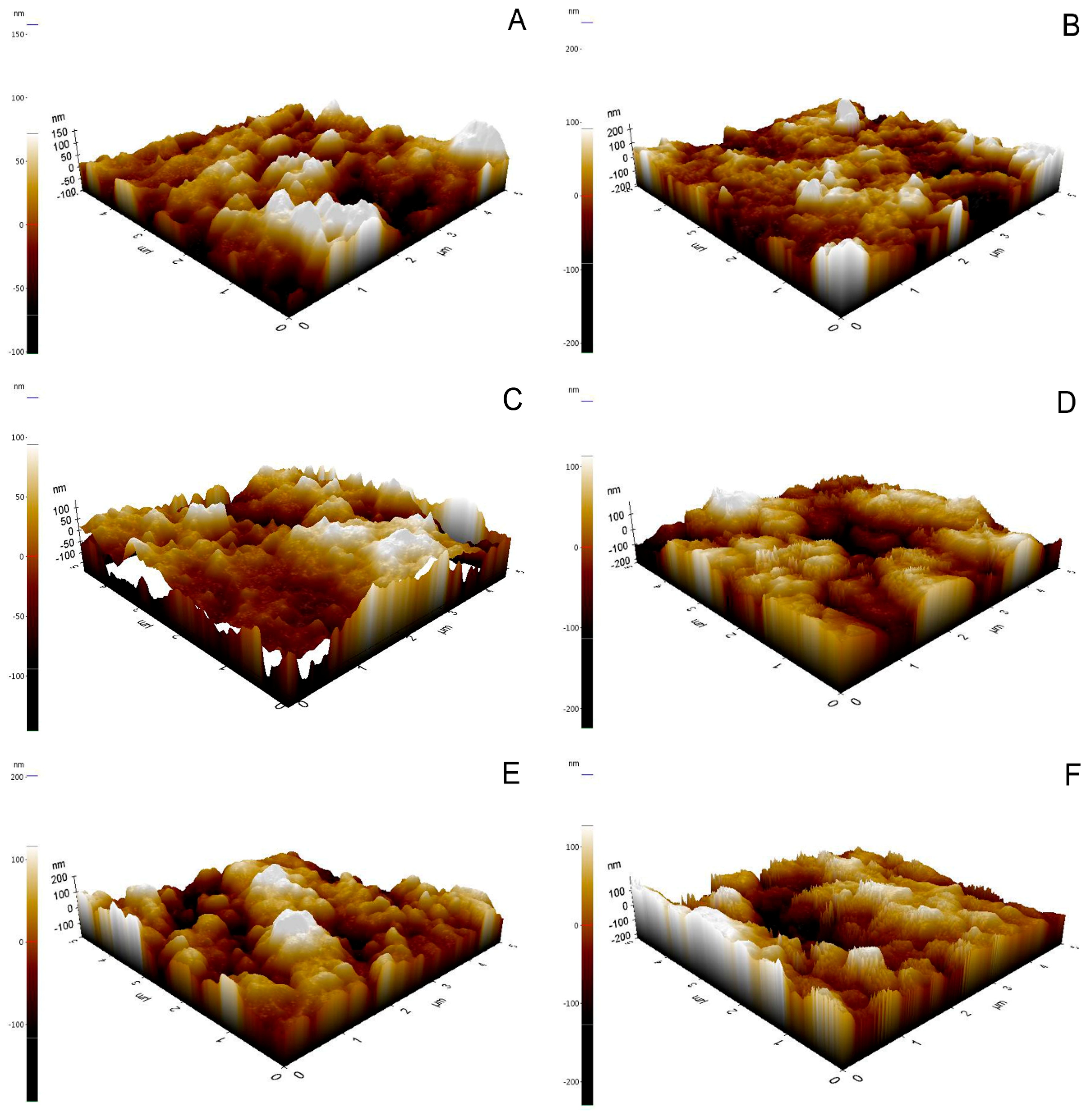
2.4. Phase Transformation Analysis and Morphological Analysis
2.5. Statistical Analysis
3. Results
3.1. Shear Bond Strength
3.2. Surface Characteristics, Zirconia Phase Transformation, and Debonded Zirconia Surface
4. Discussion
5. Conclusions
- (1)
- High-concentration HF etching and airborne-particle abrasion improved SBS.
- (2)
- Panavia F 2.0 showed higher SBS than Variolink N, regardless of thermocycling.
- (3)
- An increase in the HF concentration produced a higher Ra value.
- (4)
- HF etching resulted in a lower rate of monoclinic phase transformation than airborne-particle abrasion.
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Fahmy, N.Z.; Salah, E. An in vitro assessment of a ceramic-pressed-to-metal system as an alternative to conventional metal ceramic systems. J. Prosthodont. 2011, 20, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J. Recent developments in restorative dental ceramics. J. Am. Dent. Assoc. 1993, 124, 72–74, 76–78, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Luthardt, R.G.; Holzhüter, M.; Sandkuhl, O.; Herold, V.; Schnapp, J.D.; Kuhlisch, E.; Walter, M. Reliability and properties of ground Y-TZP-zirconia ceramics. J. Dent. Res. 2002, 81, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Russo, S.; Sorrentino, R. From porcelain-fused-to-metal to zirconia: Clinical and experimental considerations. Dent. Mater. 2011, 27, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.Y.; Stoner, B.R.; Piascik, J.R.; Smith, R. Adhesion/cementation to zirconia and other non-silicate ceramics: Where are we now? Dent. Mater. 2011, 27, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Nijhuis, H.; Valandro, L.F. Effect of various surface conditioning methods on the adhesion of dual-cure resin cement with MDP functional monomer to zirconia after thermal aging. Dent. Mater. J. 2008, 27, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.P.; Johnson, G.H.; Phillips, K.M.; Raigrodski, A.J. Retention of zirconium oxide ceramic crowns with three types of cement. J. Prosthet. Dent. 2006, 96, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Vallittu, P.K. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent. Mater. 2003, 19, 725–731. [Google Scholar] [CrossRef]
- Kulunk, S.; Kulunk, T.; Ural, C.; Kurt, M.; Baba, S. Effect of air abrasion particles on the bond strength of adhesive resin cement to zirconia core. Acta Odontol. Scand. 2011, 69, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Karakoca, S.; Yilmaz, H. Influence of surface treatments on surface roughness, phase transformation, and biaxial flexural strength of Y-TZP ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Phark, J.H.; Duarte, S.D.; Blatz, S., Jr.; Sadan, A. An in vitro evaluation of the long term resin bond to a new densely sintered high-purity zirconium-oxide ceramic surface. J. Prosthet. Dent. 2009, 101, 29–38. [Google Scholar] [CrossRef]
- Kern, M.; Wegner, S.M. Bonding to zirconia ceramic: Adhesion methods and their durability. Dent. Mater. 1998, 14, 64–71. [Google Scholar] [CrossRef]
- Mirmohammadi, H.; Aboushelib, M.N.; Salameh, Z.; Feilzer, A.J.; Kleverlaan, C.J. Innovations in bonding to zirconia based ceramics: Part III. Phosphate monomer resin cements. Dent. Mater. 2010, 26, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.P.; Cohnen, U.; Stender, E.; Willershausen, B. In vitro retentive strength of zirconium oxide ceramic crowns using different luting agents. J. Prosthet. Dent. 2005, 93, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Spohr, A.M.; Borges, G.A.; Júnior, L.H.; Mota, E.G.; Oshima, H.M. Surface modification of In-Ceram Zirconia ceramic by Nd:YAG laser, Rocatec system, or aluminum oxide sandblasting and its bond strength to a resin cement. Photomed. Laser Surg. 2008, 26, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Casucci, A.; Osorio, X.; Osorio, R.; Monticelli, F.; Toledano, M.; Mazzitelli, C.; Ferrari, M. Influence of different surface treatments on surface zirconia framework. J. Dent. 2009, 37, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Sriamporn, T.; Thamrongananskul, N.; Busabok, C.; Poolthong, S.; Uo, M.; Tagami, J. Dental zirconia can be etched by hydrofluoric acid. Dent. Mater. J. 2014, 33, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Smielak, B.; Klimek, L. Effect of hydrofluoric acid concentration and etching duration on select surface roughness parameters for zirconia. J. Prosthet. Dent. 2015, 113, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Son, J.S.; Kim, K.H.; Kwon, T.Y. Improved resin-zirconia bonding by room temperature hydrofluoric acid etching. Materials 2015, 3, 850–866. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. Shear bond strengths of various luting cements to zirconia ceramics: Surface chemical aspects. J. Dent. 2011, 39, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H. Effect of Sandblasting on the Surface Roughness of Presintered and Sintered Zirconia. Ph.D. Thesis, Kyungpook national university, Daegu, Korea, December 2014. [Google Scholar]
- Wada, T. Development of a new adhesive material and its properties. In Proceedings of the International Symposium on Adhesive Prosthodontics, Amsterdam, The Netherlands, 22–24 June 1986; Academy of Dental Materials: Chicago, IL, USA, 1986; pp. 9–18. [Google Scholar]
- Dentistry-Polymer-Based Crown and Bridge Materials; ISO 10477:2004; International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- Blatz, M.B.; Chiche, G.; Holst, S.; Sadan, A. Influence of surface treatment and simulated aging on bond strengths of luting agents to zirconia. Quintessence Int. 2007, 38, 745–753. [Google Scholar] [PubMed]
- Dérand, P.; Dérand, T. Bond strength of luting cements to zirconium oxide ceramics. Int. J. Prosthodont. 2000, 13, 131–135. [Google Scholar] [PubMed]
- Ortorp, A.; Kihl, M.L.; Carlsson, G.E. A 5-year retrospective study of survival of zirconia single crowns fitted in a private clinical setting. J. Dent. 2012, 40, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Oliveira, M.; Jansen, P.; Wehner, M.; Dohrn, A.; Bello-Silva, M.S.; Eduardo, C.P.; Meyer-Lueckel, H. Surface characterization and short-term adhesion to zirconia after ultra-short pulsed laser irradiation. J. Adhes. Dent. 2016, 18, 483–492. [Google Scholar] [PubMed]
- Moon, J.E.; Kim, S.H.; Lee, J.B.; Ha, S.R.; Choi, Y.S. The effect of preparation order on the crystal structure of yttria-stabilized tetragonal zirconia polycrystal and the shear bond strength of dental resin cements. Dent. Mater. 2011, 27, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, S.J.; Shim, J.S.; Lee, K.W. Effect of zirconia surface treatment using nitric acid-hydrofluoric acid on the shear bond strengths of resin cements. J. Adv. Prosthodont. 2017, 9, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lughi, V.; Sergo, V. Low temperature degradation-aging-of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef] [PubMed]
| Group (n = 14) | Airborne-Particle Abrasion | HF Concentration and Dwelling Time |
|---|---|---|
| C | After sintering | Not applied |
| NoHF | Before sintering | Not applied |
| 20HF1 | Before sintering | 20% for 1 h |
| 20HF2 | Before sintering | 20% for 2 h |
| 30HF1 | Before sintering | 30% for 1 h |
| 30HF2 | Before sintering | 30% for 2 h |
| 40HF1 | Before sintering | 40% for 1 h |
| 40HF2 | Before sintering | 40% for 2 h |
| Group | Pre-thermocycling | Post-thermocycling | ||||
|---|---|---|---|---|---|---|
| Panavia F 2.0 | Variolink N | p-Value † | Panavia F 2.0 | Variolink N | p-Value † | |
| C | 3.96 ± 0.42 c | 1.45 ± 0.34 c | <0.001 * | 1.59 ± 0.21 b | 0 c | <0.001 * |
| NoHF | 1.88 ± 0.96 d | 1.09 ± 0.37 c | 0.067 | 0.21 ± 0.28 c | 0 c | 0.062 |
| 20HF1 | 4.46 ± 0.84 c | 3.55 ± 1.08 b | 0.103 | 1.78 ± 0.84 b | 0.48 ± 0.47 c | 0.004 * |
| 20HF2 | 5.84 ± 1.47 b | 3.87 ± 0.89 b | 0.011 * | 1.80 ± 0.68 b | 0.62 ± 0.24 c | 0.001 * |
| 30HF1 | 5.52 ± 1.07 b | 3.66 ± 0.59 b | 0.002 * | 2.28 ± 0.78 b | 1.95 ± 0.94 b | 0.489 |
| 30HF2 | 7.94 ± 1.05 a | 5.62 ± 1.14 a | 0.002 * | 4.89 ± 1.38 a | 3.71 ± 0.93 a | 0.085 |
| 40HF1 | 7.71 ± 0.64 a | 5.89 ± 1.00 a | 0.002 * | 5.08 ± 1.57 a | 2.56 ± 0.88 b | 0.003 * |
| 40HF2 | 7.62 ± 1.67 a | 6.53 ± 1.29 a | 0.196 | 4.30 ± 1.20 a | 2.21 ± 0.92 b | 0.003 * |
| p-value | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| Cement | Thermocyling | p-Value † | |
|---|---|---|---|
| Pre | Post | ||
| Panavia F 2.0 | 5.61 ± 2.26 a | 2.74 ± 1.91 a | <0.001 * |
| Variolink N | 3.95 ± 2.06 b | 1.44 ± 1.43 b | <0.001 * |
| p-value | <0.001 * | <0.001 * | |
| Group | Monoclinic Peak Intensity |
|---|---|
| C | 7.9 |
| NoHF | 0 |
| 20HF1 | 2.8 |
| 20HF2 | 1.8 |
| 30HF1 | 1.4 |
| 30HF2 | 3.5 |
| 40HF1 | 0.9 |
| 40HF2 | 1.6 |
| Group | 1 h Application | 2 h Application | p-Value † |
|---|---|---|---|
| 20HF | 26.03 ± 1.71 c | 33.67 ± 3.60 c | 0.030 * |
| 30HF | 40.60 ± 2.30 b | 44.19 ± 3.01 b | 0.176 |
| 40HF | 48.30 ± 1.52 a | 51.03 ± 1.53 a | 0.093 |
| p-value | <0.001 * | <0.001 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Lee, C.-H. Effect of the Surface Treatment Method Using Airborne-Particle Abrasion and Hydrofluoric Acid on the Shear Bond Strength of Resin Cement to Zirconia. Dent. J. 2017, 5, 23. https://doi.org/10.3390/dj5030023
Lee J-H, Lee C-H. Effect of the Surface Treatment Method Using Airborne-Particle Abrasion and Hydrofluoric Acid on the Shear Bond Strength of Resin Cement to Zirconia. Dentistry Journal. 2017; 5(3):23. https://doi.org/10.3390/dj5030023
Chicago/Turabian StyleLee, Ju-Hyoung, and Cheong-Hee Lee. 2017. "Effect of the Surface Treatment Method Using Airborne-Particle Abrasion and Hydrofluoric Acid on the Shear Bond Strength of Resin Cement to Zirconia" Dentistry Journal 5, no. 3: 23. https://doi.org/10.3390/dj5030023




