Six-Year Survival and Early Failure Rate of 2918 Implants with Hydrophobic and Hydrophilic Enossal Surfaces
Abstract
:1. Introduction
2. Materials and Methods
- immediate loading;
- immediate temporization with bone grafting, mainly for simultaneous and two-stage sinus lifting using DBBM (Bio-Oss, Geistlich, Switzerland) or β calcium triphosphate (TCP, CEROS, Thommen Medical AG, Grenchen, Switzerland);
- guided bone regeneration using either autogenous bone from the retromolar area or autologous bone (TBF, Mions, France). Augmentation surgery has been performed prior to the implant placement. The bone healing time was 4 months when autogenous bone was used, 5 months when patients received allogeneic bone, and 6 months or more with the bovine bone.
3. Results
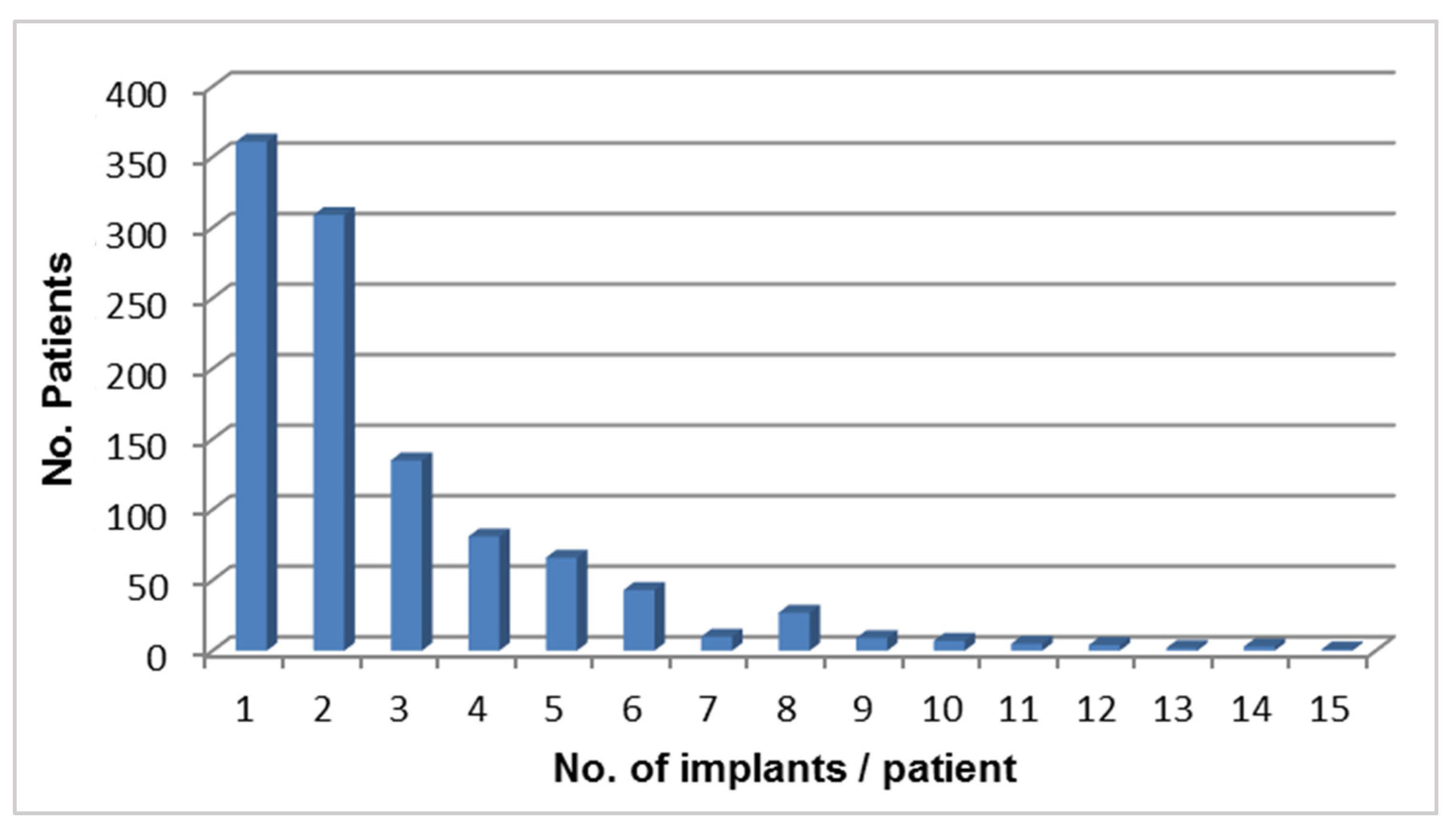
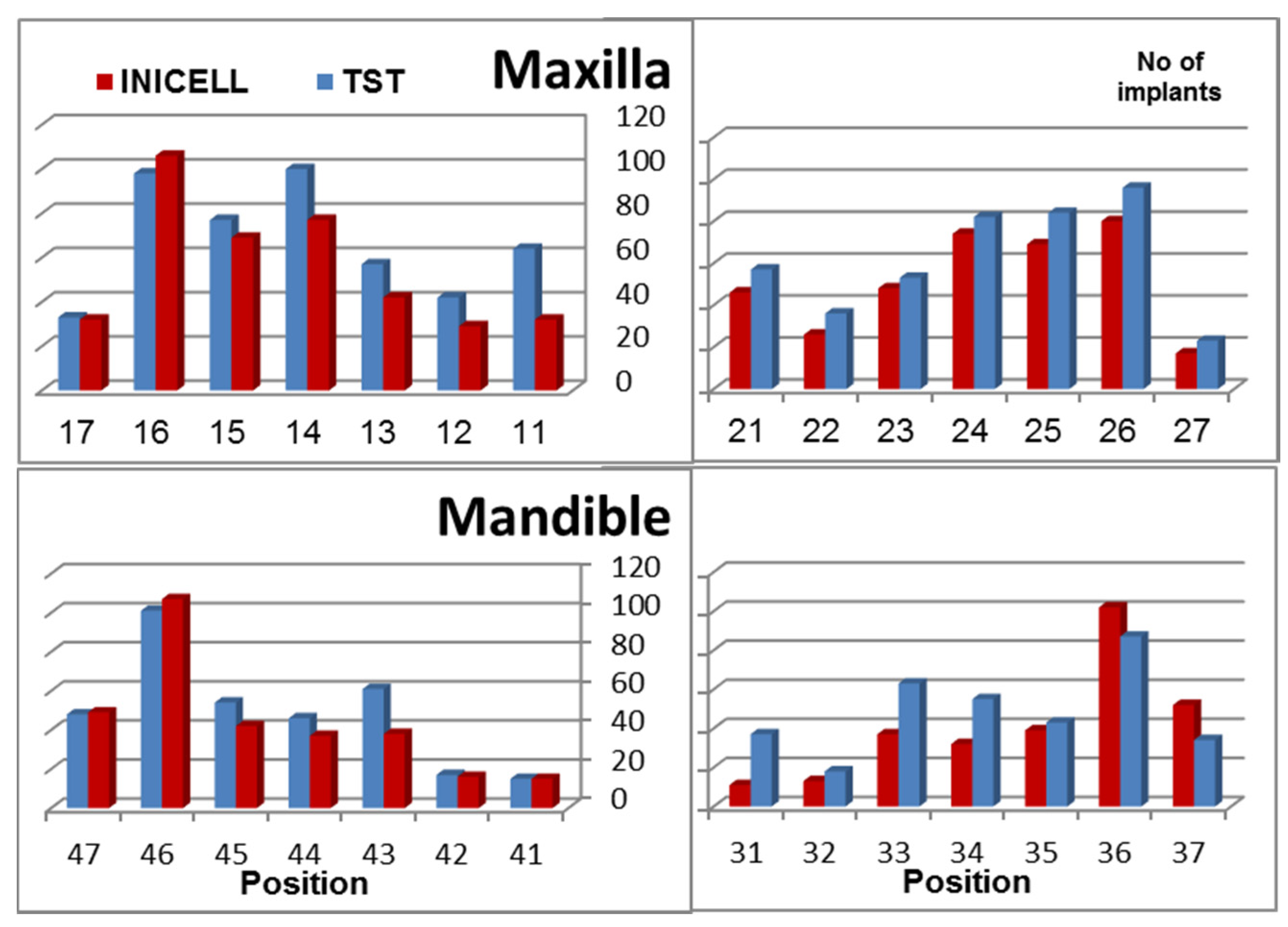

| No. of implants | Failed Implants | CSR | Observation Period |
|---|---|---|---|
| Total | 30 | 99.0 | 4.0 (1.1–5.9) years |
| INICELL | 7 * | 99.5 | 2.1 (1.1–5.4) years |
| TST | 23 * | 98.5 | 4.5 (1.3–5.9) years |
| Contributing Factor | Number of Failed Implants |
|---|---|
| INICELL: 7 failed implants | |
| Reduced implant diameter (PF Ø 3.5 mm) | 3 |
| Immediate loading | 2 |
| Provisional loading | 1 |
| Edentulous patient with immediate loading | 1 |
| TST: 23 failed implants | |
| Reduced implant diameter (PF Ø 3.5 mm) | 5 |
| Edentulous patient with immediate loading | 6 (in 4 patients) |
| Sinus lift | 3 (in 2 patients) |
| Immediate temporization | 9 |
- Standard cases: within the reported population 640 patients received 968 INICELL and 1064 TST implants. In standard cases implants have been placed after performing a full thickness flap and preparation of the implant bed by using the drilling sequence (recommended by the manufacturer). Most of the time a healing screw was placed and sutures done. 6 weeks later the permanent crown was fabricated and screw-attached with the torque recommended by the manufacturer (25 N for platforms between 4 and 6 mm and 15 N for the 3.5 mm). Early failures have occurred with only 1 INICELL and 9 TST implants. This difference was also statistically significant (p < 0.05; Fisher’s exact test).
- Edentulous cases: 75 edentulous patients received 493 immediately loaded implants (267 INICELL and 226 TST). Of these 2 INICELL and 6 TST implants have failed early. In some of these patients the implants have been placed with the help of navigated surgery [7].
- Immediate loading: immediate non-occlusal loading of single tooth was done in 74 patients with 35 INICELL and 39 TST implants. Only 1 INICELL implant failed early.
- Sinus floor elevation: Consecutive and simultaneous sinus lift was done in 240 patients that have received 86 INICELL and 141 TST implants. Only 3 TST implants failed early.
- Reduced diameter implants: from the 228 INICELL and 203 TST inserted reduced diameter (PF Ø 3.5 mm) implants early failures occurred with 5 TST and 3 INICELL implants (this difference is not statistically significant). This finding is in apparent contradiction to the outcome of a retrospective analysis of papers published until September 2012 [8]. In the presented study only a small proportion (13%) of the implants had a reduced diameter (3.5 mm). In this single center case series their survival rate was no different from that of wider diameter implants. Their outcome did not differ between INICELL or TST implants.
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Buser, D.; Janner, S.F.M.; Wittneben, J.-G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Östman, P.-O.; Hellman, M.; Sennerby, L. Ten years later. Results from a prospective single-centre clinical study on 121 oxidized (tiunitetm) brånemark implants in 46 patients. Clin. Implant Dent. Relat. Res. 2012, 14, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.-I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Stadlinger, B.; Lode, A.T.; Eckelt, U.; Range, U.; Schlottig, F.; Hefti, Th.; Mai, R. Surface-conditioned dental implants: An animal study on bone formation. J. Clin. Periodontol. 2009, 36, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Wittneben, J.G.; Brägger, U.; Buser, D. Early loading at 21 days of non-submerged titanium implants with a chemically modified sandblasted and acid-etched surface: 3-Year results of a prospective study in the posterior mandible. J. Periodontol. 2010, 81, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.B. Sinus floor elevation with osteotomes. J. Esthet. Restor. Dent. 1998, 10, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Le Gac, O.; Armand, S. Bridge complet implanto-porté. Présentation d’un protocole original utilisant la robotique passive (systéme Robodent). Implant 2012, 18, 109–120. [Google Scholar]
- Ortega-Oller, I.; Suárez, H.; Galindo-Moreno, P.; Torecillas-Martínez, L.; Monje, A.; Catena, A.; Wang, H.L. The influence of implant diameter on its survival: A meta-analysis based on prospective clinical trials. J. Periodontol. 2014, 85, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Halbritter, S.; Harnisch, H.; Weber, H.P.; Buser, D. A retrospective analysis of patients referred for implant placement to a specialty clinic regarding indications, surgical procedures and early failures. Int. J. Oral Maxillofac. Implants 2008, 23, 1109–1116. [Google Scholar] [PubMed]
- Wang, F.; Zhang, Z.; Monje, A.; Huang, W.; Wu, Y.; Wang, G. Intermediate long-term clinical performance of dental implants placed in sites with previous early implant failure: A retrospective analysis. Clin. Oral Implants Res. 2014. [Google Scholar] [CrossRef]
- Garcia-Bellosta, S.; Bravo, M.; Subirá, C.; Echeveria, J.J. Retrospective study of the long-term survival of 980 implants placed in a periodontal practice. Int. J. Oral Maxillofac. Implants 2010, 25, 613–619. [Google Scholar] [PubMed]
- Andreoni, C.; Meier, Th.; Minoretti, R.; Wehrli, C. Langzeiterfahrung mit durchmesser-reduzierten thommen-implantaten. Prakt. Implantol. Implant. 2011, 3, 30–34. [Google Scholar]
- Olate, S.; Lyrio, M.C.; de Morales, M.; Mazzonetto, R.; Moreira, R.W. Influence of diameter and length on early dental implant failure. J. Oral Maxillofac. Surg. 2010, 68, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pommer, B.; Mailath-Pokorny, G.; Haas, R.; Busenlechner, D.; Fürhauser, R.; Watzek, G. Patients’ preferences towards minimally invasive treatment alternatives for implant rehabilitation of edentulous jaws. Eur. J. Oral Implantol. 2014, 7, S91–S109. [Google Scholar] [PubMed]
- Stadlinger, B.; Ferguson, S.J.; Eckelt, U.; Mai, R.; Lode, A.T.; Loukota, R.; Schlottig, F. Biomechanical evaluation of a titanium implant surface conditioned by a hydroxide ion solution. Br. J. Oral Maxillofac. Surg. 2012, 50, 74–79. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gac, O.L.; Grunder, U. Six-Year Survival and Early Failure Rate of 2918 Implants with Hydrophobic and Hydrophilic Enossal Surfaces. Dent. J. 2015, 3, 15-23. https://doi.org/10.3390/dj3010015
Gac OL, Grunder U. Six-Year Survival and Early Failure Rate of 2918 Implants with Hydrophobic and Hydrophilic Enossal Surfaces. Dentistry Journal. 2015; 3(1):15-23. https://doi.org/10.3390/dj3010015
Chicago/Turabian StyleGac, Olivier Le, and Ueli Grunder. 2015. "Six-Year Survival and Early Failure Rate of 2918 Implants with Hydrophobic and Hydrophilic Enossal Surfaces" Dentistry Journal 3, no. 1: 15-23. https://doi.org/10.3390/dj3010015
APA StyleGac, O. L., & Grunder, U. (2015). Six-Year Survival and Early Failure Rate of 2918 Implants with Hydrophobic and Hydrophilic Enossal Surfaces. Dentistry Journal, 3(1), 15-23. https://doi.org/10.3390/dj3010015





