Biomechanical Effects of Different Load Cases with an Implant-Supported Full Bridge on Four Implants in an Edentulous Mandible: A Three-Dimensional Finite Element Analysis (3D-FEA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Patient Treatment Characteristics
2.2. Ethical Considerations
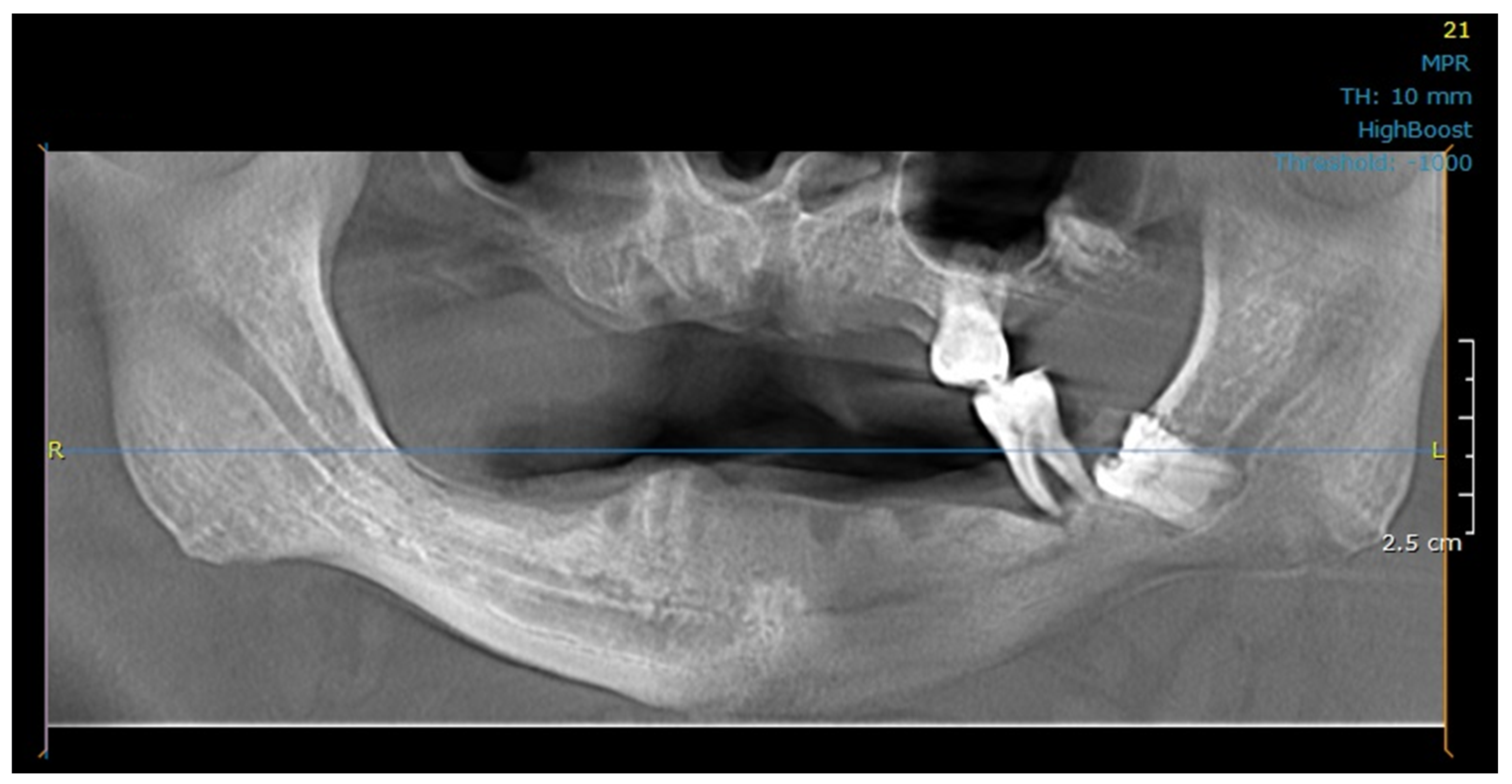
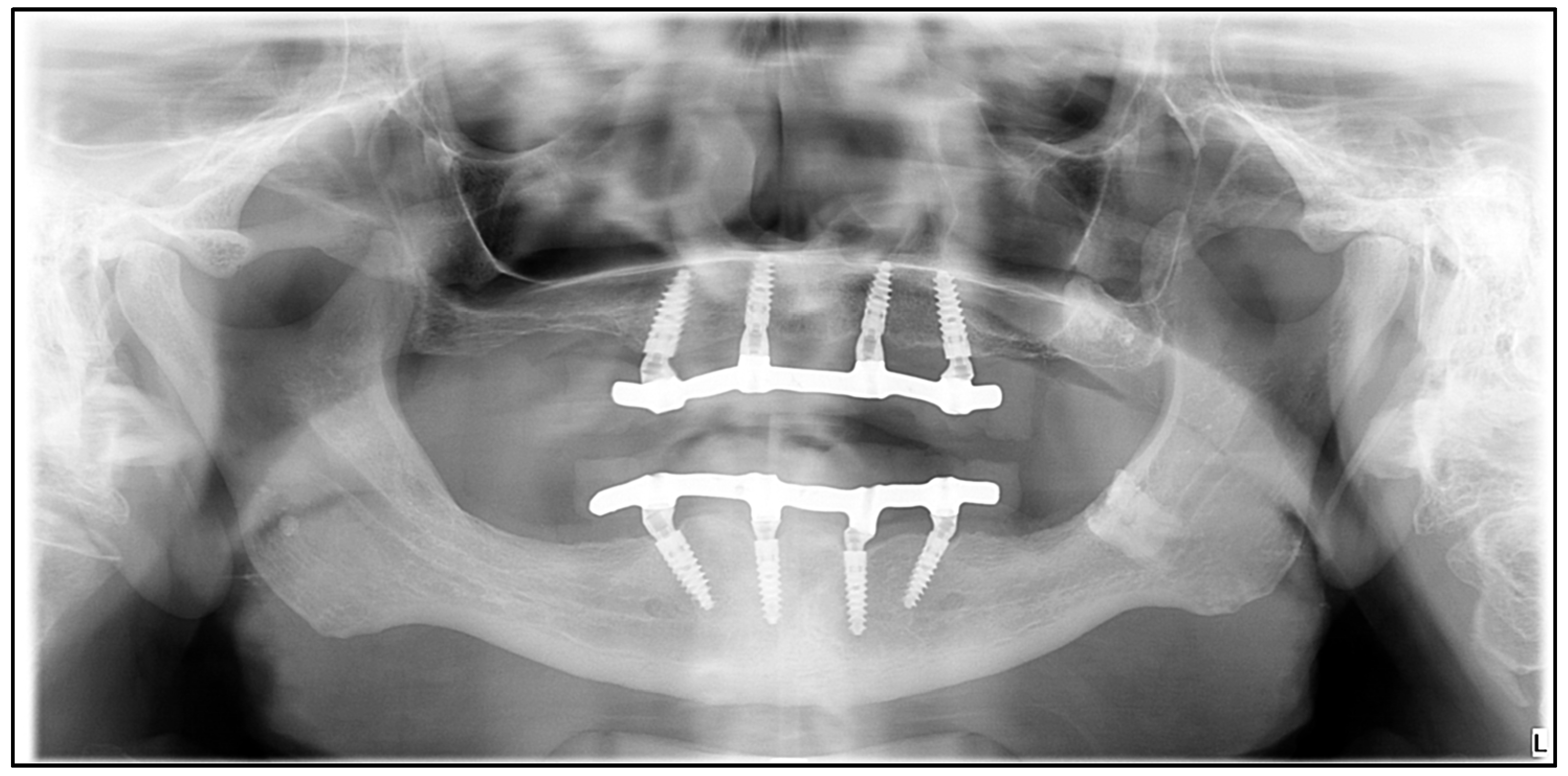
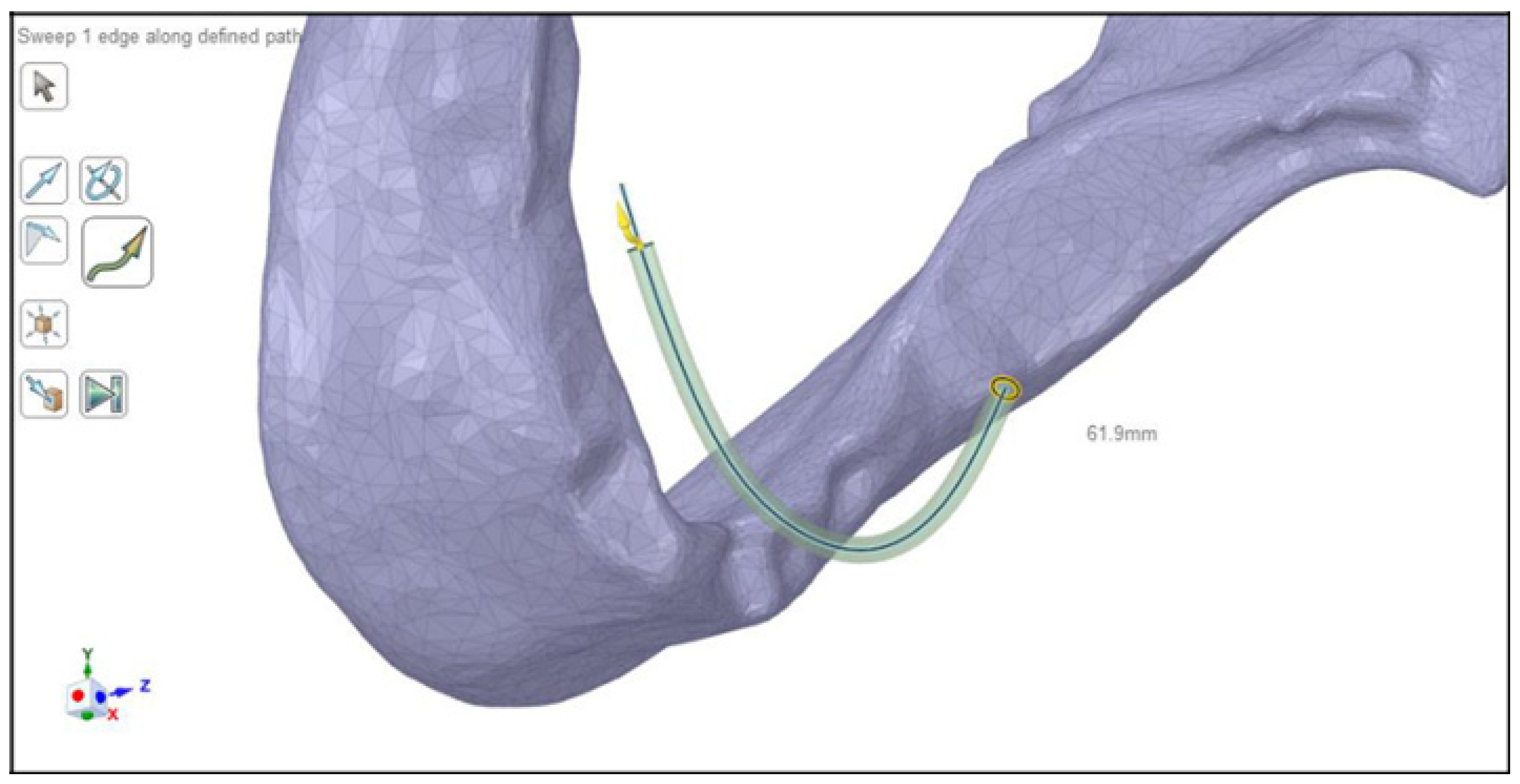
2.3. Modeling
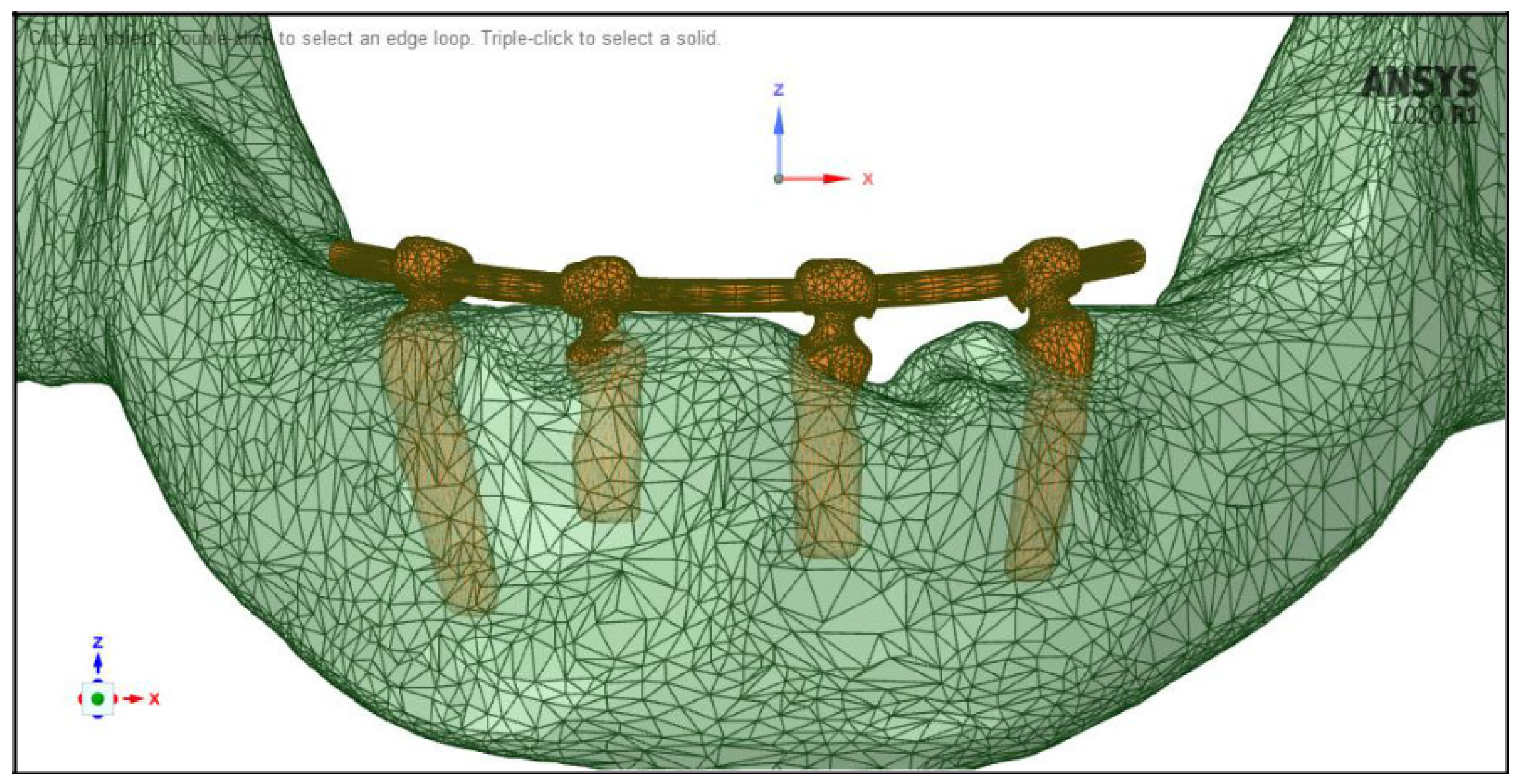
2.4. Meshing, Boundary Conditions
2.5. Material Properties
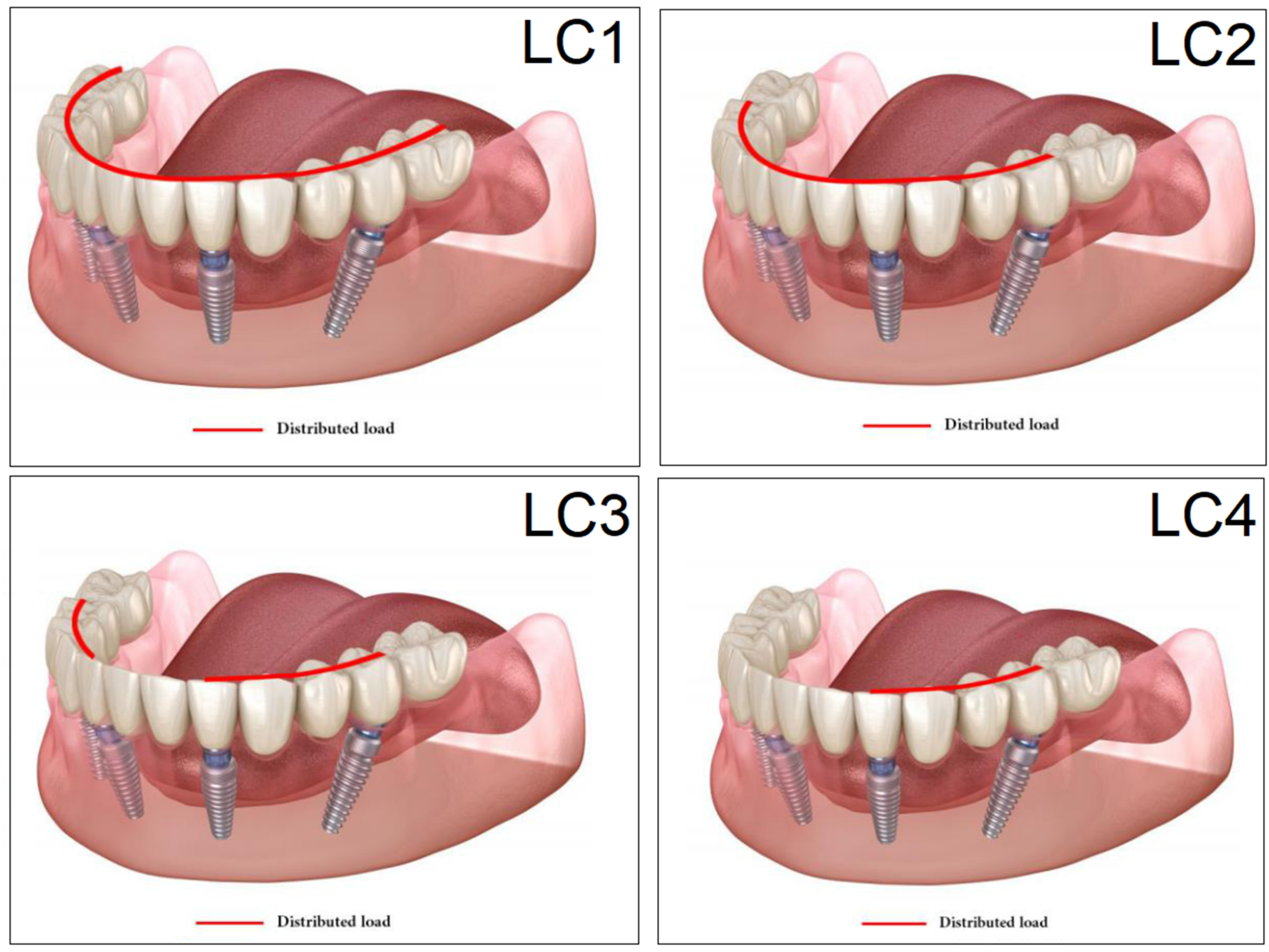
2.6. Loading, Occlusal Cases
2.7. Statistical Analysis
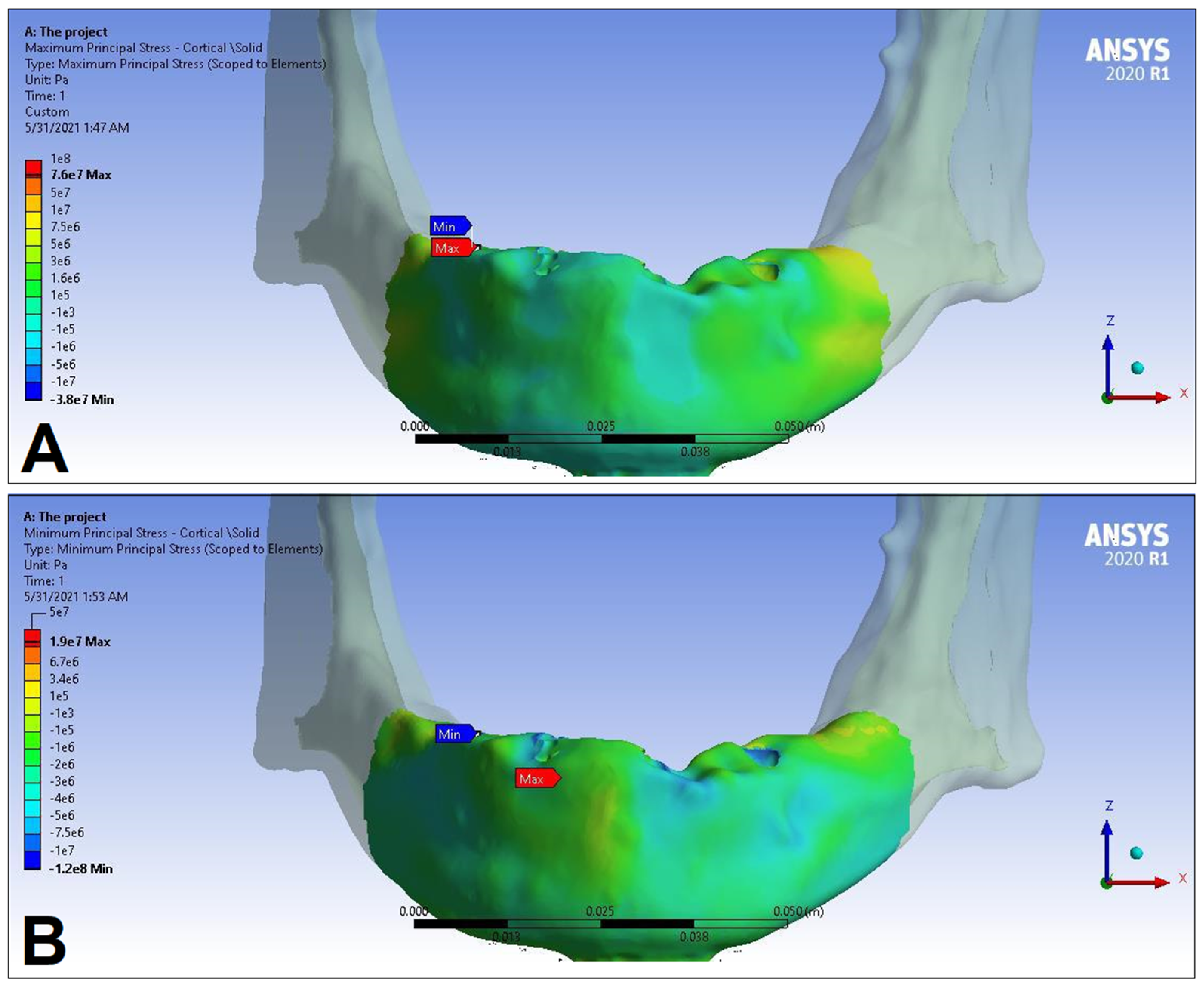
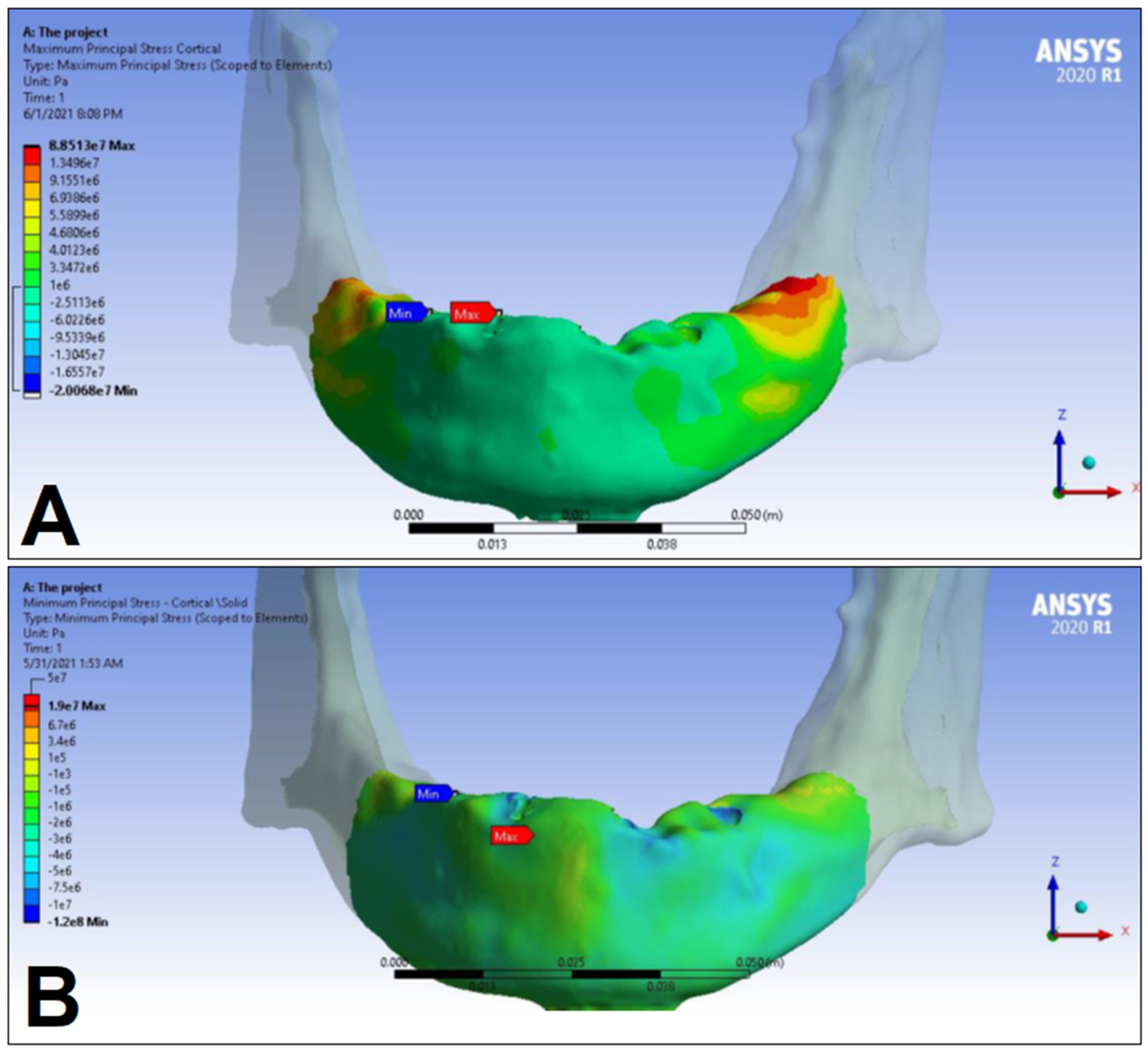
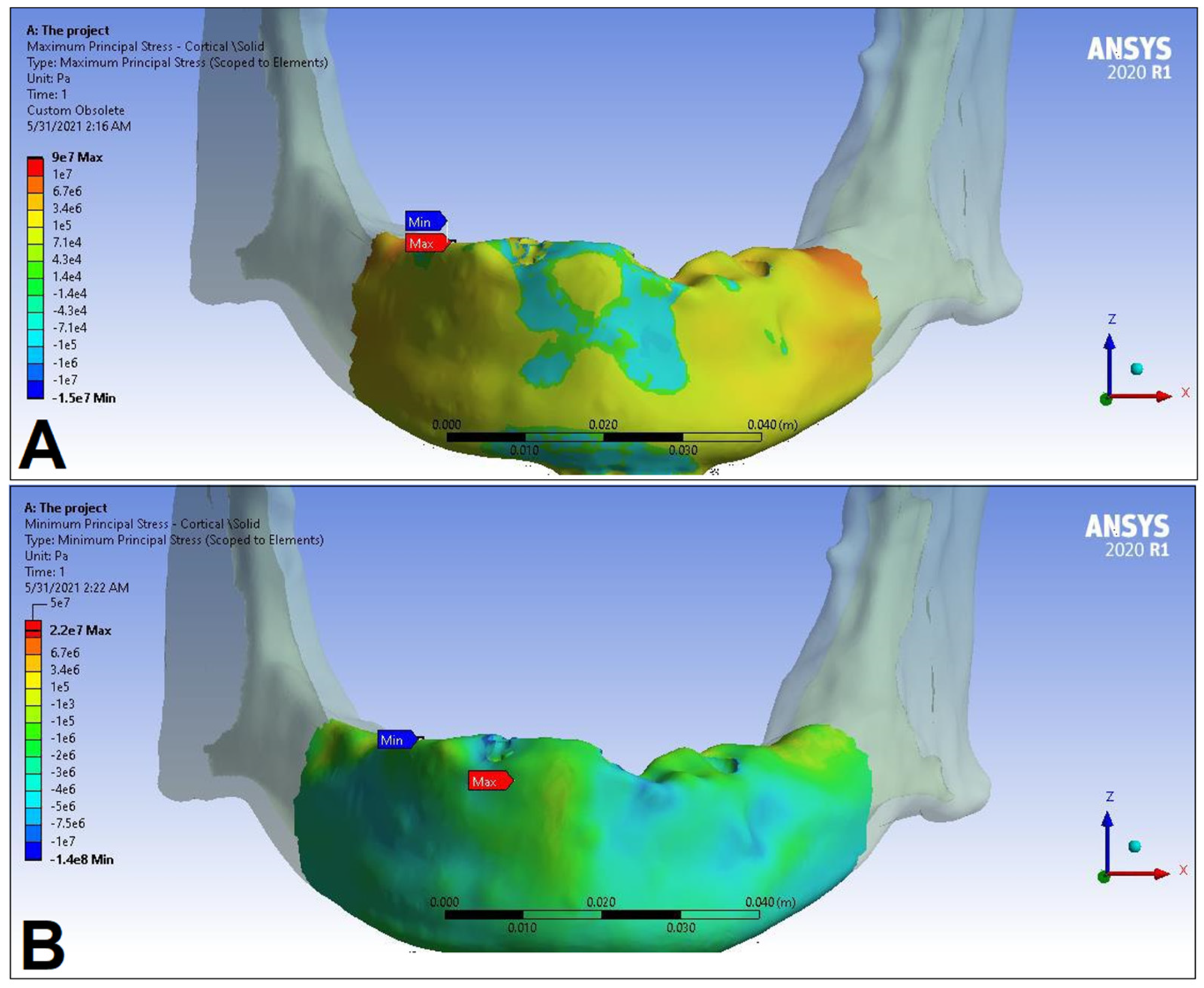
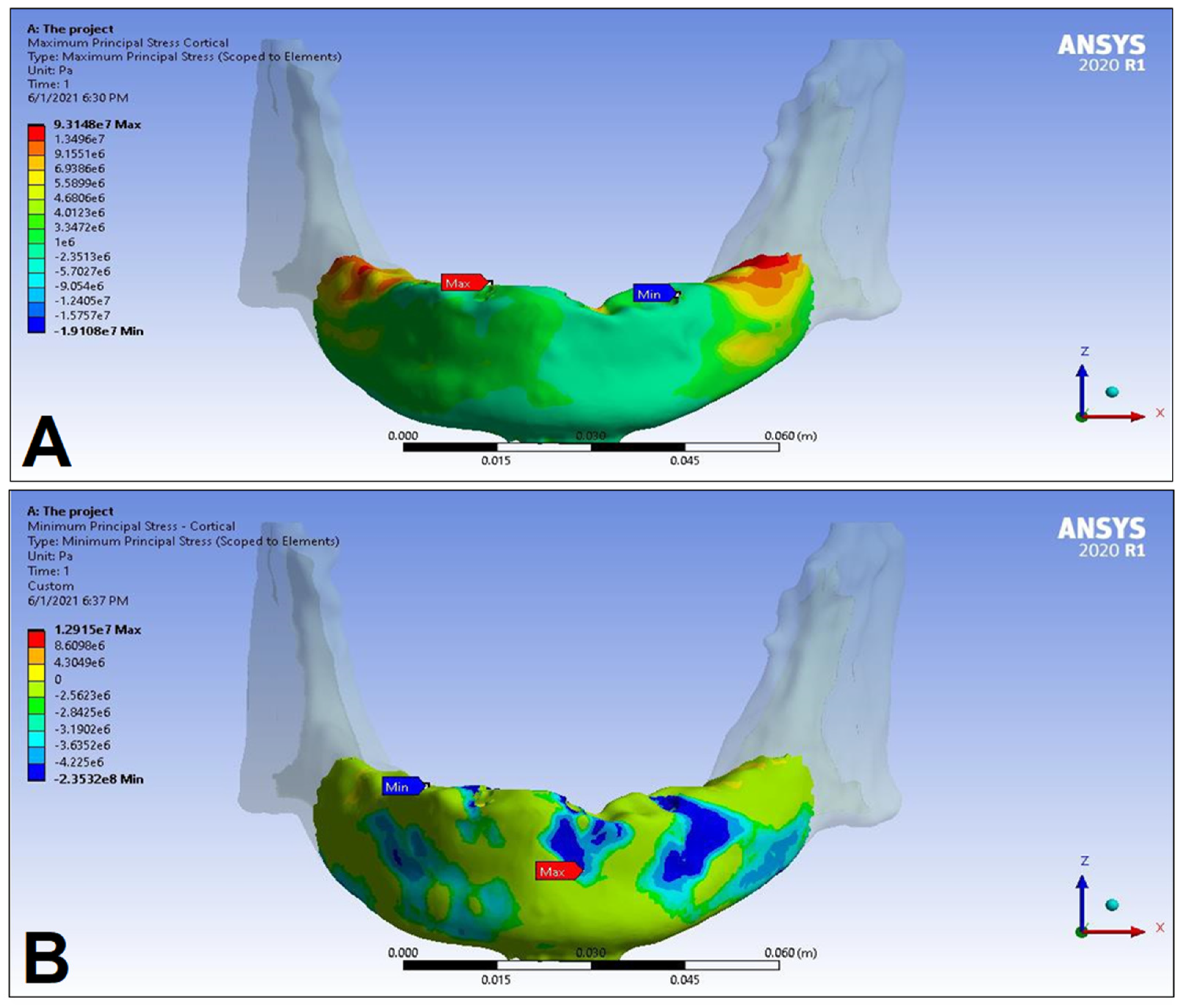
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gowd, M.S.; Shankar, T.; Ranjan, R.; Singh, A. Prosthetic Consideration in Implant-supported Prosthesis: A Review of Literature. J. Int. Soc. Prev. Community Dent. 2017, 7, S1–S7. [Google Scholar] [PubMed]
- Yoon, D.; Pannu, D.; Hunt, M.; Londono, J. Occlusal considerations for full-arch implant-supported prostheses: A guideline. Dent. Rev. 2022, 2, e100042. [Google Scholar] [CrossRef]
- Dos Santos, M.B.F.; Meloto, G.O.; Bacchi, A.; Correr-Sobrinho, L. Stress distribution in cylindrical and conical implants under rotational micromovement with different boundary conditions and bone properties: 3-D FEA. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.M.; Thumati, P. A 3-D finite element analysis of strain around end osseous threaded and non-threaded implant-opposing natural teeth with regular occlusion and altered occlusion: An in vitro study. J. Dent. Implant. 2014, 4, 53–61. [Google Scholar]
- Carinci, F.; Farina, A.; Zanetti, U.; Vinci, R.; Negrini, S.; Calura, G.; Laino, G.; Piattelli, A. Alveolar ridge augmentation: A comparative longitudinal study between calvaria and iliac crest bone grafts. J. Oral Implantol. 2005, 31, 39–45. [Google Scholar] [CrossRef]
- Piero, B.; Mario, V.; Niccolo, N.; Marco, F. Implant placement in combination with sinus membrane elevation without biomaterials: A 1-year study on 15 patients. Clin. Implant Dent. Relat. Res. 2012, 14, 682–689. [Google Scholar]
- Sakkas, A.; Schramm, A.; Winter, K.; Wilde, F. Risk factors for post-operative complications after procedures for autologous bone augmentation from different donor sites. J. Craniomaxillofac. Surg. 2018, 46, 312–322. [Google Scholar] [CrossRef]
- Murdoch-Kinch, C.A.; McLean, M.E. Minimally invasive dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef]
- Menini, M.; Pesce, P.; Delucchi, F.; Ambrogio, G.; Canepa, C.; Carossa, M.; Pera, F. One-stage versus two-stage technique using two splinted extra-short implants: A multicentric split-mouth study with a one-year follow-up. Clin. Implant Dent. Rel. Res. 2022, 24, 602–610. [Google Scholar] [CrossRef]
- Málo, P.; Rangert, B.; Nobre, M. “All-on-four” immediate-function concept with Brånemark system® implants for completely edentulous mandibles: A retrospective clinical study. Clin. Implant Dent. Relat. Res. 2003, 5, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; Rangert, B.; Nobre, M. All-on-4 immediate-function concept with Brånemark System implants for completely edentulous maxillae: A 1-year retrospective clinical study. Clin. Implant Dent. Relat. Res. 2005, 7, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; Nobre, M.A.; Lopes, A.; Rodrigues, R. Double Full-Arch versus Single Full-Arch, Four Implant-Supported Rehabilitations: A Retrospective, 5-Year Cohort Study. J. Prosthodont. 2015, 24, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Taruna, M.; Chittarajan, B.; Sudheer, N.; Tella, S.; Abusaad, M. Prosthodontic Perspective to All-On-4® Concept for Dental Implants. J. Clin. Diagn. Res. 2014, 8, ZE16–ZE19. [Google Scholar] [CrossRef]
- Cattoni, F.; Chirico, L.; Merlone, A.; Manacorda, M.; Vinci, R.; Gherlone, E.F. Digital Smile Designed Computer-Aided Surgery versus Traditional Workflow in “All on Four” Rehabilitations: A Randomized Clinical Trial with 4-Years Follow-Up. Int. J. Environ. Res. Public Health 2021, 18, 3449. [Google Scholar] [CrossRef]
- Pera, P.; Menini, M.; Pesce, P.; Bevilacqua, M.; Pera, F.; Tealdo, T. Immediate Versus Delayed Loading of Dental Implants Supporting Fixed Full-Arch Maxillary Prostheses: A 10-year Follow-up Report. Int. J. Prostodont. 2019, 32, 27–31. [Google Scholar] [CrossRef]
- Pera, P.; Menini, M.; Bevilacqua, M.; Pesce, P.; Signori, A.; Tealdo, T. Factors Affecting the Outcome in the Immediate Loading Rehabilitation of the Maxilla: A 6-year Prospective Study. Int. J. Periodont. Restor. Dent. 2014, 34, 657–665. [Google Scholar] [CrossRef]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Moss, S.M.; Molina, G.J. A longitudinal study of the survival of All-on-4 implants in the mandible with up to 10 Years of follow-up. J. Am. Dent. Assoc. 2011, 142, 310–320. [Google Scholar] [CrossRef]
- Durkan, R.; Oyar, P.; Deste, G. Maxillary and Mandibular All-on-Four Implant Designs: A Review. Niger. J. Clin. Pract. 2019, 22, 1033–1040. [Google Scholar]
- Szabó, Á.L.; Nagy, Á.L.; Lászlófy, C.; Gajdács, M.; Bencsik, P.; Kárpáti, K.; Baráth, Z. Distally Tilted Implants According to the All-on-Four® Treatment Concept for the Rehabilitation of Complete Edentulism: A 3.5-Year Retrospective Radiographic Study of Clinical Outcomes and Marginal Bone Level Changes. Dent. J. 2022, 10, 82. [Google Scholar] [CrossRef]
- Darwich, A.; Alammar, A.; Heshmeh, O.; Szabolcs, S.; Nazha, H. Fatigue Loading Effect in Custom-Made All-on-4 Implants System: A 3D Finite Elements Analysis. IRBM 2022, 43, 372–379. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J.J.P. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, e103574. [Google Scholar] [CrossRef] [PubMed]
- Steigenga, J.T.; al-Shammari, K.F.; Nociti, F.H.; Misch, C.E.; Wang, H.L. Dental implant design and its relationship to long-term implant success. Implant Dent. 2003, 12, 306–317. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, C.L.; Carvalho, M.A.; Bordin, D.; da Silva, W.J.; Cury, A.A.D.B.; Sotto-Maior, B.S. Biomechanical Behavior of the Dental Implant Macrodesign. Int. J. Oral Maxillofac. Implants 2017, 32, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of Implant Thread Geometry on Secondary Stability, Bone Density, and Bone-to-Implant Contact: A Biomechanical and Histological Analysis. Implant Dent. 2015, 24, 384–391. [Google Scholar] [CrossRef]
- Di Fiore, A.; Montagner, M.; Sivolella, S.; Stelini, E.; Yilmaz, B.; Brunello, G. Peri-Implant Bone Loss and Overload: A Systematic Review Focusing on Occlusal Analysis through Digital and Analogic Methods. J. Clin. Med. 2022, 11, 4812. [Google Scholar] [CrossRef]
- Soto-Penaloza, D.; Zaragozí-Alonso, R.; Penarrocha-Diago, M.; Penarrocha-Diago, M. The all-on-four treatment concept: Systematic review. J. Clin. Exp. Dent. 2017, 9, e474–e488. [Google Scholar] [CrossRef]
- Nagy, Á.L.; Tóth, Z.; Tarjányi, T.; Práger, N.T.; Baráth, Z.L. Biomechanical properties of the bone during implant placement. BMC Oral Health 2021, 21, e86. [Google Scholar] [CrossRef]
- Geng, J.P.; Tan, K.B.; Liu, G.R. Application of finite element analysis in implant dentistry: A review of the literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef]
- Trivedi, S. Finite element analysis: A boon to dentistry. J. Oral Biol. Craniofacial Res. 2014, 4, 200–203. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.M.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magnetic Res. Imag. 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Freedman, G.; Antal, M.; Afrashtehfar, K.I. [An Option for Guided Implantology: The SMART Guide]. Rev. Dent. Paciente 2020, 144, 10–19. [Google Scholar]
- Alpha-Bio Tec Ltd. Product Catalog. Available online: https://alpha-bio.net/media/10285/product-catalog-2021-r17-english_mail.pdf (accessed on 29 January 2023).
- Bathe, K.J.; Almeida, C.A. A Simple and Effective Pipe Elbow Element—Linear Analysis. J. Appl. Mech. 1980, 47, 93–100. [Google Scholar] [CrossRef]
- Hassan, A.A.F.; Saleh, S.A.; Jibar, Z.H. Experimental and Theoretical Study of Hardness and Grain Size Variation in Cold Upset for Pure Copper Cylinder. Univ. J. Mech. Eng. 2015, 3, 34–36. [Google Scholar] [CrossRef]
- Liu, T.; Mu, Z.; Wang, C.; Huang, Y. Biomechanical comparison of implant inclinations and load times with the all-on-four treatment concept: A three-dimensional finite element analysis. Comp. Methods Biomech. Biomed. Eng. 2019, 22, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.C.; Mendonca, J.A.; Lopes, L.R.; Landre, J. Stress patterns on implants in prostheses supported by four or six implants: A three-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2010, 25, 239–246. [Google Scholar]
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissue Integrated Prosthesis: Osseointegration in Clinical Dentistry; Branemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence: Chicago, IL, USA, 1985; pp. 199–208. [Google Scholar]
- Türker, N.; Büyükkaplan, U.S.; Sadowsky, S.J.; Özarslan, M.M. Finite element stress analysis of applied forces to implants and supporting tissues using the „All-on-four” concept with different occlusal scemes. J. Prosthodont. 2019, 18, 185–194. [Google Scholar] [CrossRef]
- Huang, H.L.; Fuh, L.J.; Hsu, J.T.; Tu, M.G.; Shen, Y.W.; Wu, C.L. Effects of implant surface roughness and stiffness of grafted bone on an immediately loaded maxillary implant: A 3D numerical analysis. J. Oral Rehabil. 2008, 35, 283–290. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanimoto, Y.; Maruyama, N.; Nagakura, M. A review of improved fixation methods for dental implants. Part II: Biomechanical integrity at bone-implant interface. J. Prosthodont. Res. 2015, 59, 84–95. [Google Scholar] [CrossRef]
- Lakatos, É.; Magyar, L.; Bojtár, I. Material Properties of the Mandibular Trabecular Bone. J. Med. Eng. 2014, 2014, e470539. [Google Scholar] [CrossRef]
- Grimal, Q.; Laugier, P. Quantitative Ultrasound Assessment of Cortical Bone Properties beyond Bone Mineral Density. IRBM 2019, 40, 16–24. [Google Scholar] [CrossRef]
- Deste, G.; Durkan, R. Effects of All-on-four implant designs in mandible on implants and the surrounding bone: A 3-D finite element analysis. Nig. J. Clin. Pract. 2020, 23, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Uddanwadiker, R.V.; Padole, P.M.; Arya, H. Effect of variation of root post in different layers of tooth: Linear vs. nonlinear finite element stress analysis. J. Biosci. Bioeng. 2007, 104, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Katada, H.; Arakawa, T.; Ichimura, K.; Sueishi, K.; Sameshima, G.T. Stress distribution in mandible and temporomandibular joint by mandibular distraction: A 3-dimensional finite-element analysis. Bull. Tokyo Dent. Coll. 2009, 50, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.S.; Postaire, M.; Lipinski, P. Biomechanical study of mandible bone supporting a four-implant retained bridge. Finite element analysis of the influence of bone anisotrophy and foodstuff position. Med. Eng. Phys. 2009, 31, 806–815. [Google Scholar] [CrossRef]
- Ayali, A.; Altagar, M.; Ozan, O.; Kurtulmus-Yilmaz, S. Biomechanical comparison of the All-on-4, M-4, and V-4 techniques in an atrophic maxilla: A 3D finite element analysis. Comp. Biol. Med. 2020, 123, e103880. [Google Scholar] [CrossRef]
- Chowdhary, R.; Kumararama, S.S. “Simpli5y” a novel concept for fixed rehabilitation of completely edentulous maxillary and mandibular edentulous arches: A 3-year randomized clinical trial, supported by a numerical analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 749–755. [Google Scholar] [CrossRef]
- Liu, C.; Xing, Y.; Li, Y.; Lin, Y.; Xu, J.; Wu, D. Bone quality effect on short implants in the edentulous mandible: A finite element study. BMC Oral Health 2022, 22, e139. [Google Scholar] [CrossRef]
- Omi, M.; Mishina, Y. Roles of osteoclasts in alveolar bone remodeling. Genesis 2022, 60, e23490. [Google Scholar] [CrossRef]
- Malo, P.; de Araujo Nobre, M.; Lopes, A.; Ferro, A. The All-on-4 treatment concept for the rehabilitation of the completely edentulous mandible: A longitudinal study with 10 to 18 years of follow-up. Clin. Implant Dent. Relat. Res. 2019, 21, 565–577. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.A.; Sánchez-Siles, M.; Guerrero-Sánchez, Y.; Pato-Mourelo, J.; Camacho-Alonso, F. Evaluation of Quality of Life and Satisfaction in Patients with Fixed Prostheses on Zygomatic Implants Compared with the All-on-Four Concept: A Prospective Randomized Clinical Study. Int. J. Environ. Res. Public Health 2021, 18, 3426. [Google Scholar] [CrossRef] [PubMed]
- Zincir, Ö.Ö.; Parlar, A. Comparison of stresses in monoblock tilted implants and conventional angled multiunit abutment-implant connection systems in the all-on-four procedure. BMC Oral Health 2021, 21, e646. [Google Scholar] [CrossRef] [PubMed]
- Brunski, J.B.; Puleo, D.A.; Nanci, A. Biomaterials and biomechanics of oral and maxillofacial implants: Current status and future developments. Int. J. Oral Maxillofac. Implant. 2000, 15, 15–46. [Google Scholar]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Tu, M.G.; Hsu, J.T.; Fuh, L.J.; Lin, D.J.; Huang, H.L. Effects of cortical bone thickness and implant length on bone strain and interfacial micromotion in an immediately loaded implant. Int. J. Oral Maxillofac. Implant. 2010, 25, 706–714. [Google Scholar]
- Beviacqua, M.; Tealdo, T.; Menini, M.; Mossolov, A.; Drago, C.; Pera, P. The influence of cantilever length and implant inclination on stress distribution in maxillary implant-supported fixed dentures. J. Prosthet. Dent. 2011, 105, 5–13. [Google Scholar] [CrossRef]
- Malhotra, A.O.; Padmananbhan, T.V.; Mohamed, K.; Natarajan, S.; Elavia, U. Load transfer in tilted implants with various cantilever lengths in an all-on-four situation. Austrailan Dent. J. 2012, 57, 440–445. [Google Scholar] [CrossRef]
- van Zyl, P.P.; Grundling, N.L.; Jooste, C.H.; Terblanche, E. Three-dimensional finite element model of a human mandible incorporating six osseointegrated implants for stress analysis of mandibular cantilever prostheses. Int. J. Oral Maxillofac. Implant. 1995, 10, 51–57. [Google Scholar]
- Almeida, E.O.; Rocha, E.P.; Freitas, A.M.J.; Anchieta, R.B.; Poveda, R.; Gupta, N.; Coelho, P.G. Tilted and short implants supporting fixed prosthesis in an atrophic maxilla: A 3D-FEA biomechanical evaluation. Clin. Implant Dent. Relat. Res. 2015, 17, e332–e342. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Kermani, M.E.; Sadafsaz, M.; Motlagh, A.M. Evaluation of stress and strain magnitude in tapered and cylindrical implant surrounding bone, used for restoration of mandibular teeth: Finite element analysis in All-on-Four concept. J. Long-Term Eff. Med. Implant. 2021, 31, 51–58. [Google Scholar] [CrossRef]
- Wu, A.Y.J.; Hsu, J.T.; Fuh, L.J.; Huang, H.L. Biomechanical effect of implant design on four implants supporting mandibular full-arch fixed dentures: In vitro test and finite element analysis. J. Formosan Med. Assoc. 2020, 119, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Orsini, E.; Giavaresi, G.; Triré, A.; Ottani, V.; Salgarello, S. Dental implant thread pitch and its influence on the osseointegration process: An in vivo comparison study. Int. J. Oral Maxillofac. Implant. 2012, 27, 383–392. [Google Scholar]
- Horita, S.; Sugiura, T.; Yamamoto, K.; Murakami, K.; Imai, Y.; Kirita, T. Biomechanical analysis of immediately loaded implants according to the “All-on-Four” concept. J. Prosthodont. Res. 2017, 61, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bhering, C.L.B.; Mesquita, M.F.; Kemmoku, D.T.; Noritomi, P.Y.; Consani, R.L.X.; Barao, V.A.R. Comparison between all-on-four and all-on-six treatment concepts and framework material on stress distribution in atrophic maxilla: A prototyping guided 3D-FEA study. Mat. Sci. Eng. C 2016, 69, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Sigiura, T.; Yamamoto, K.; Horita, S.; Murakami, K.; Kirita, T. Micromotion analysis of different implant configuration, bone density, and crestal bone thickness in immediately loaded mandibular full-arch implant restorations: A nonlinear finite element study. Clin. Implant. Dent. Relat. Res. 2018, 20, 43–49. [Google Scholar] [CrossRef]
- Daas, M.; Dubois, G.; Bonnet, A.S.; Lipinski, P.; Rignon-Bret, C. A complete finite element model of a mandibular implant-retained overdenture with two implants: Comparison between rigid and resilient attachment configurations. Med. Eng. Phys. 2008, 30, 218–225. [Google Scholar] [CrossRef]
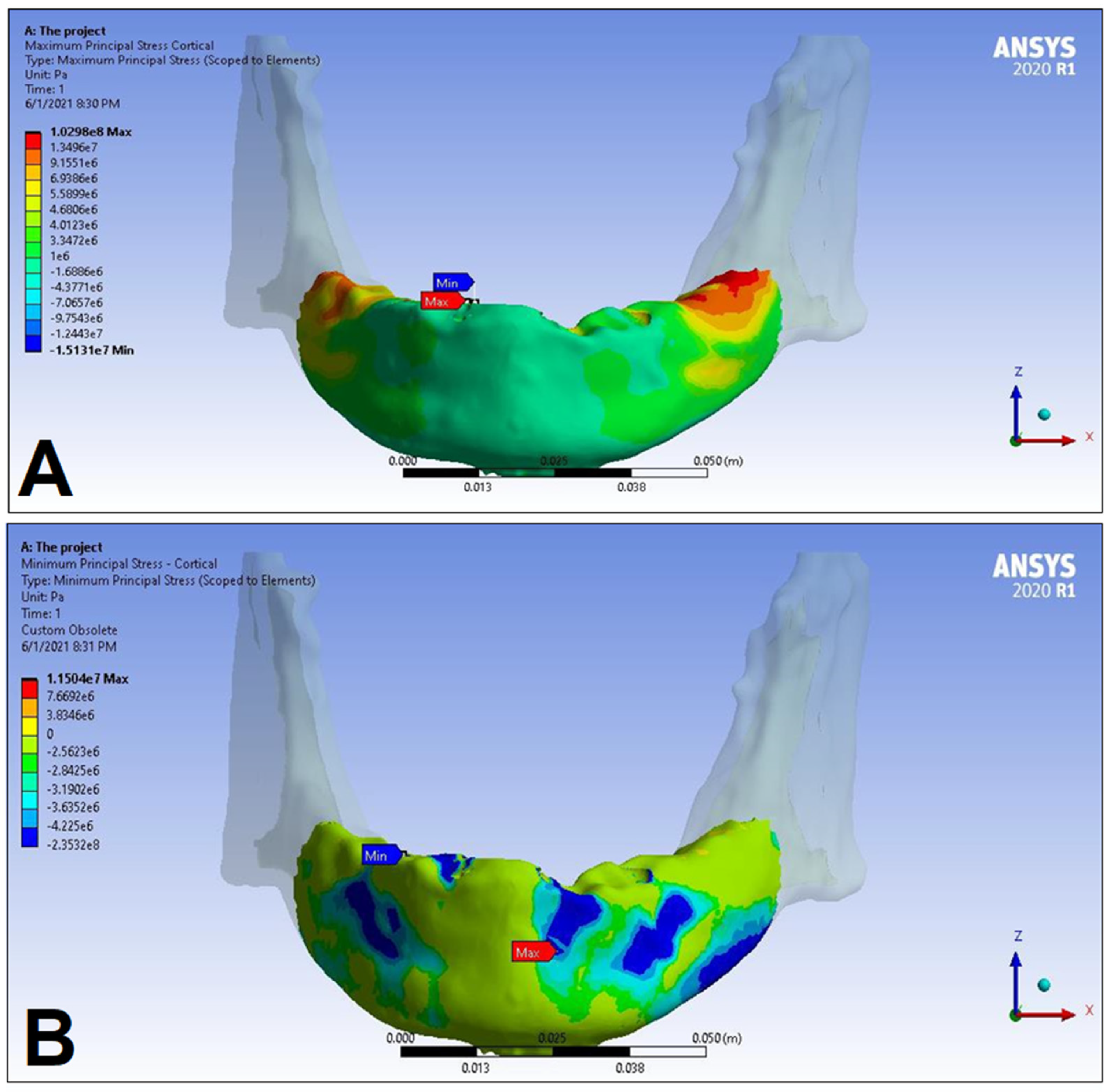
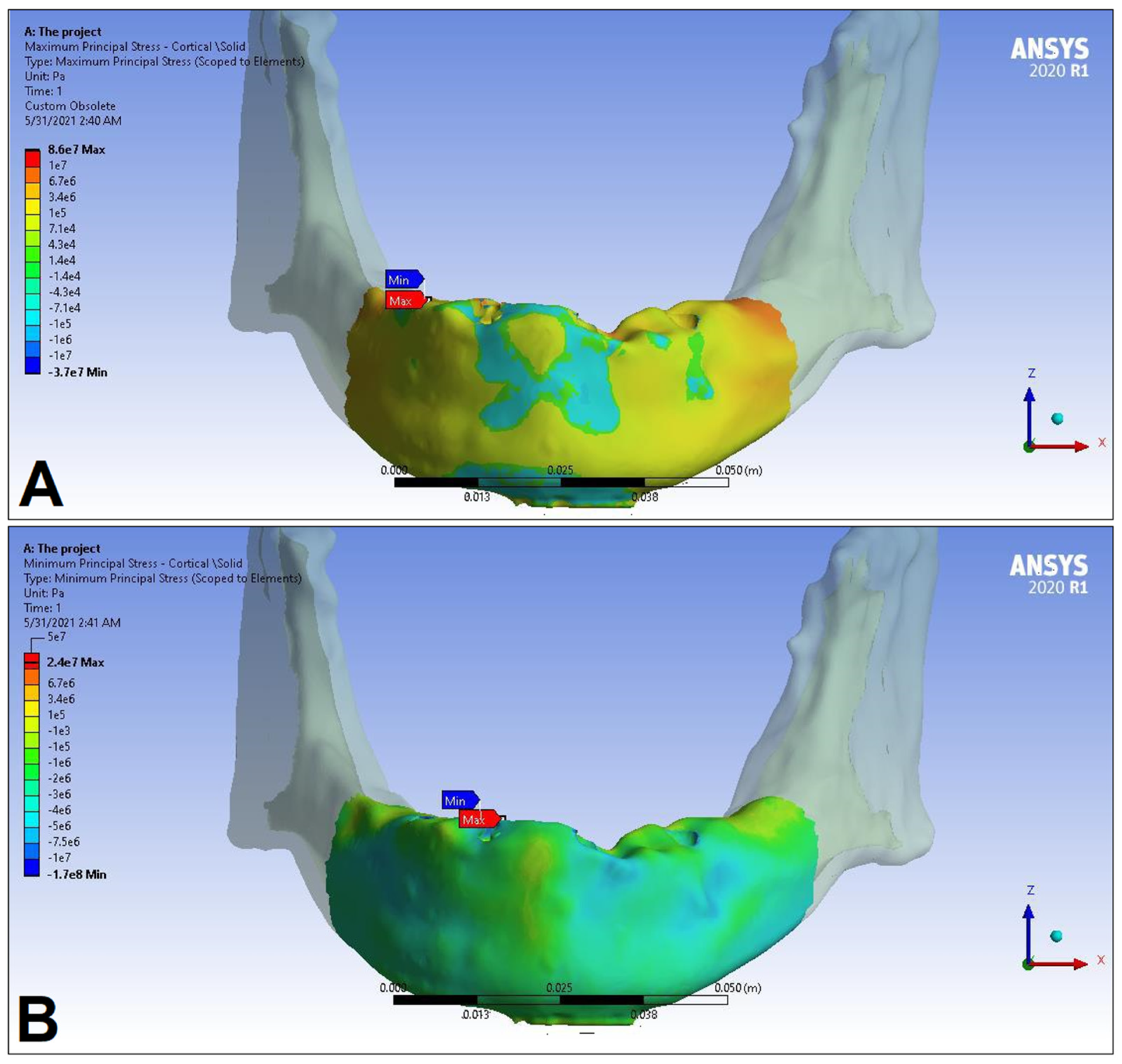
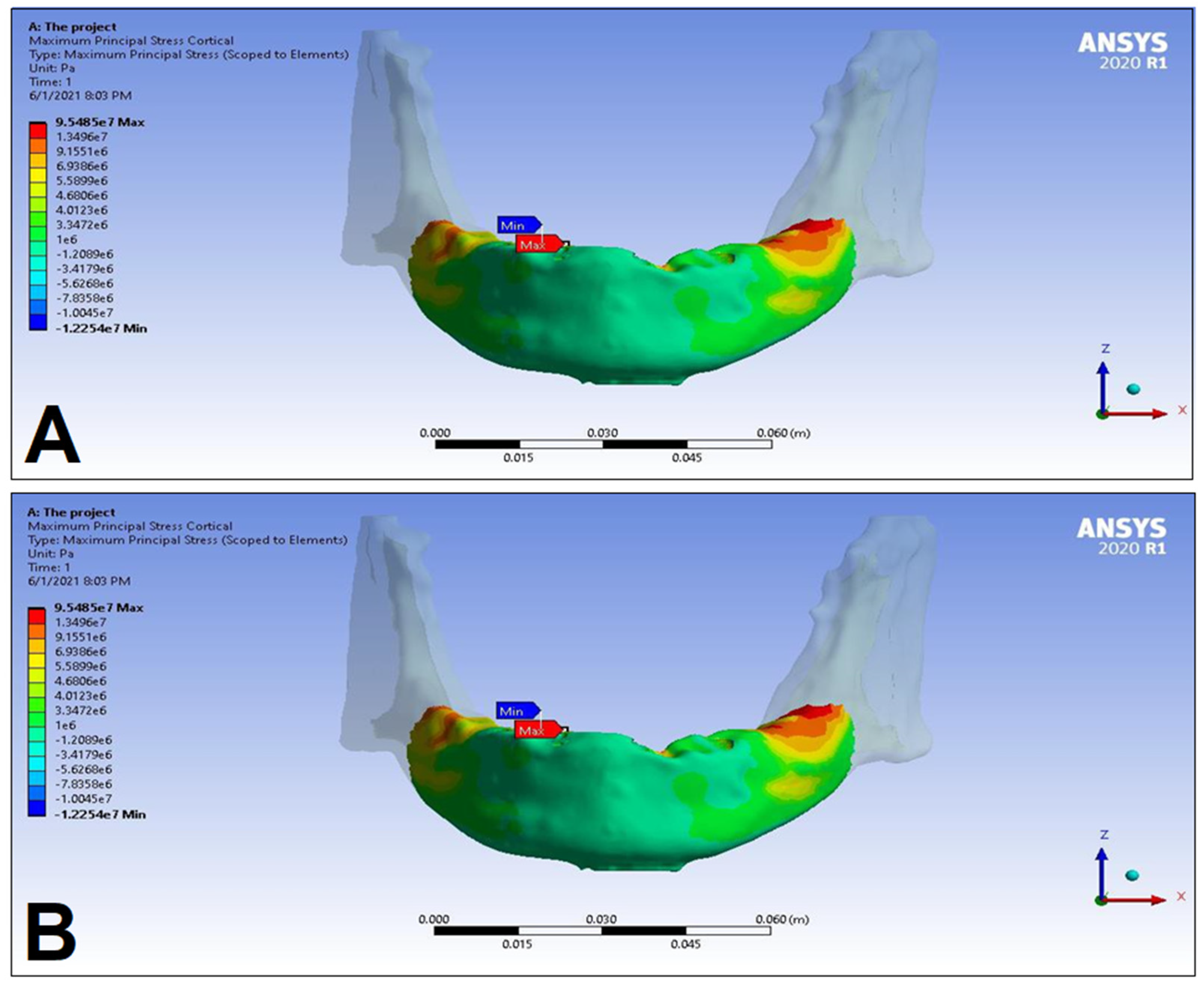
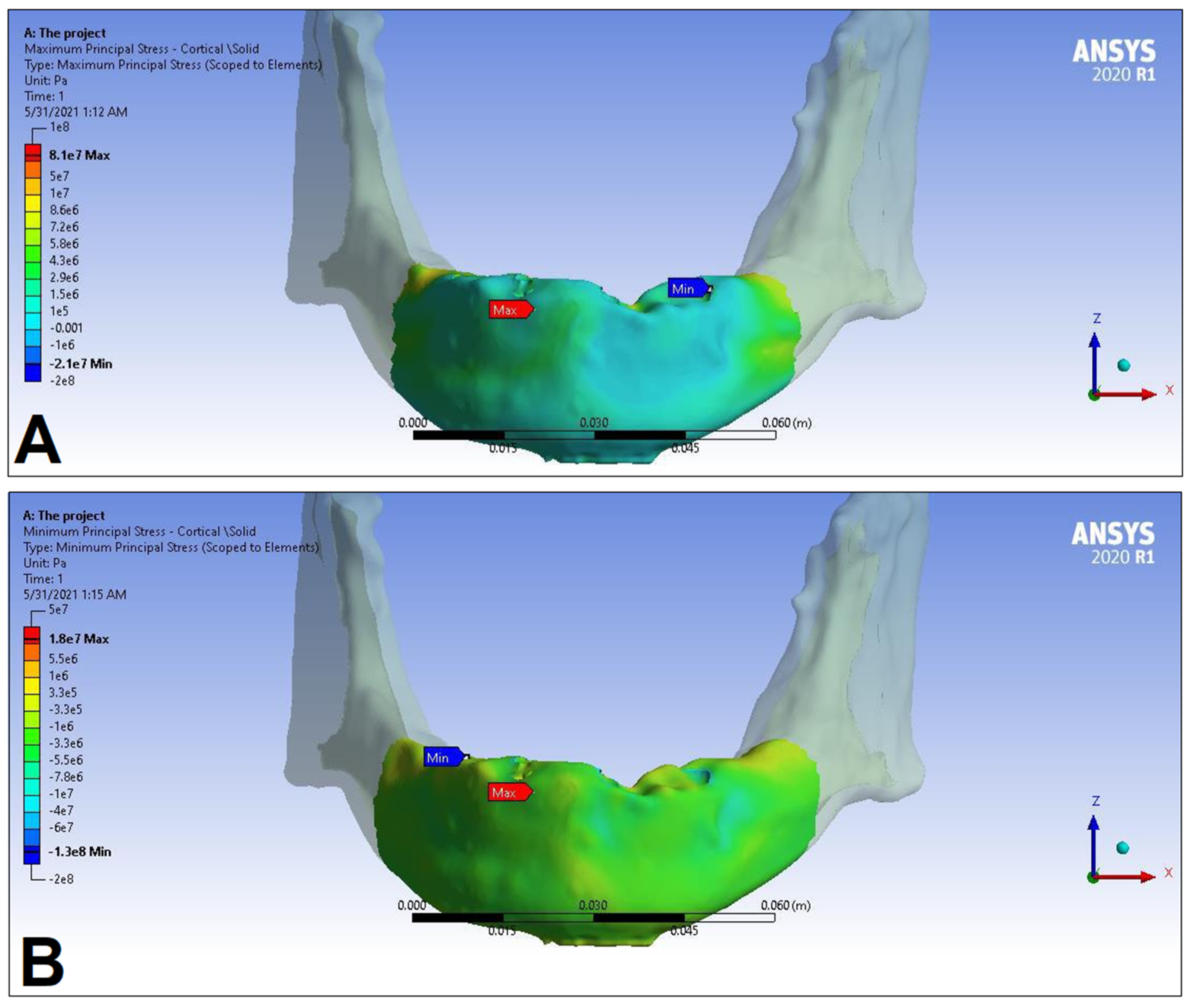
| Materials | Density ρ [g/cm3] | Young’s Modulus E [GPa] | Poisson’s Ratio v |
|---|---|---|---|
| Titanium [40,41] (TiAl6V4) | 4.51 | 102 | 0.36 |
| Cobalt−Chromium [40,41] (CO−CR-01-P.30CR) | 10 | 210 | 0.29 |
| Cortical bone [42,43] | 1.6 | 15 | 0.3 |
| Trabecular bone [42,43] | 0.2 | 0.096 | 0.3 |
| LC1 | LC2 | LC3 | LC4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | ||
| Cortical bone | Pmax [MPa] | 76.39 | 88.51 | 89.57 | 102.98 | 85.63 | 95.48 | 81.02 | 93.15 |
| Pmin [MPa] | −115.30 | −222.76 | −136.4 | −265.35 | −172.30 | −252.61 | −125.20 | −235.32 | |
| Trabecular bone | Pmax [MPa] | 2.49 | 2.24 | 3.03 | 2.62 | 2.95 | 2.52 | 2.92 | 2.59 |
| Pmin [MPa] | −2.81 | −2.89 | −3.34 | −3.38 | −3.25 | −3.25 | −3.49 | −3.52 | |
| Peqv [MPa] | 166.40 | 244.92 | 166.36 | 279.69 | 164.36 | 265.58 | 142.27 | 260.77 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, Á.L.; Matusovits, D.; Slyteen, H.; Lakatos, É.I.; Baráth, Z. Biomechanical Effects of Different Load Cases with an Implant-Supported Full Bridge on Four Implants in an Edentulous Mandible: A Three-Dimensional Finite Element Analysis (3D-FEA). Dent. J. 2023, 11, 261. https://doi.org/10.3390/dj11110261
Szabó ÁL, Matusovits D, Slyteen H, Lakatos ÉI, Baráth Z. Biomechanical Effects of Different Load Cases with an Implant-Supported Full Bridge on Four Implants in an Edentulous Mandible: A Three-Dimensional Finite Element Analysis (3D-FEA). Dentistry Journal. 2023; 11(11):261. https://doi.org/10.3390/dj11110261
Chicago/Turabian StyleSzabó, Árpád László, Danica Matusovits, Haydar Slyteen, Éva Ilona Lakatos, and Zoltán Baráth. 2023. "Biomechanical Effects of Different Load Cases with an Implant-Supported Full Bridge on Four Implants in an Edentulous Mandible: A Three-Dimensional Finite Element Analysis (3D-FEA)" Dentistry Journal 11, no. 11: 261. https://doi.org/10.3390/dj11110261





