Autologous Platelet Rich Plasma (PRGF) Preserves Genomic Stability of Gingival Fibroblasts and Alveolar Osteoblasts after Long-Term Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Blood Cell and Platelet Counting in WP and F2 Preparations
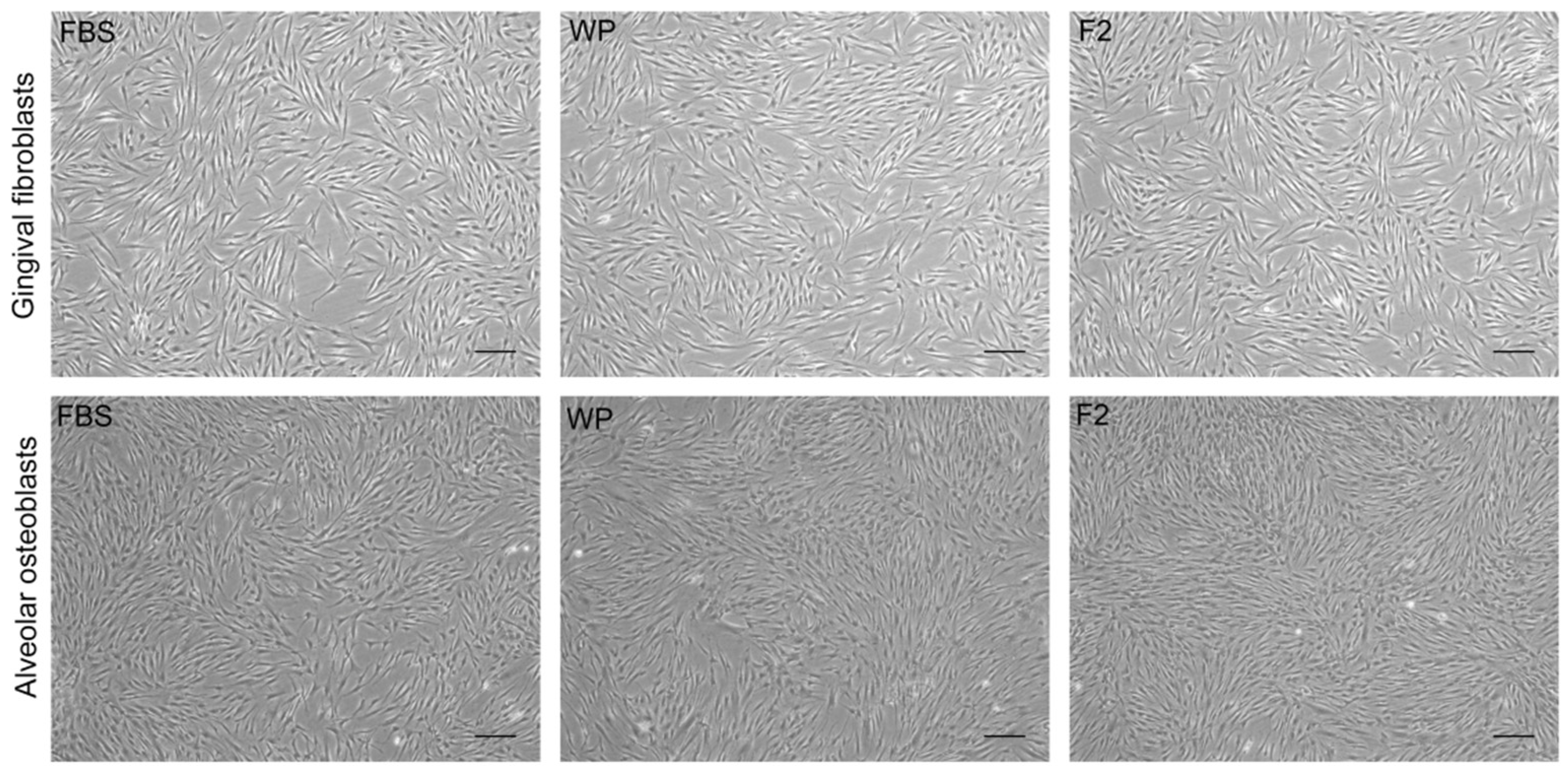
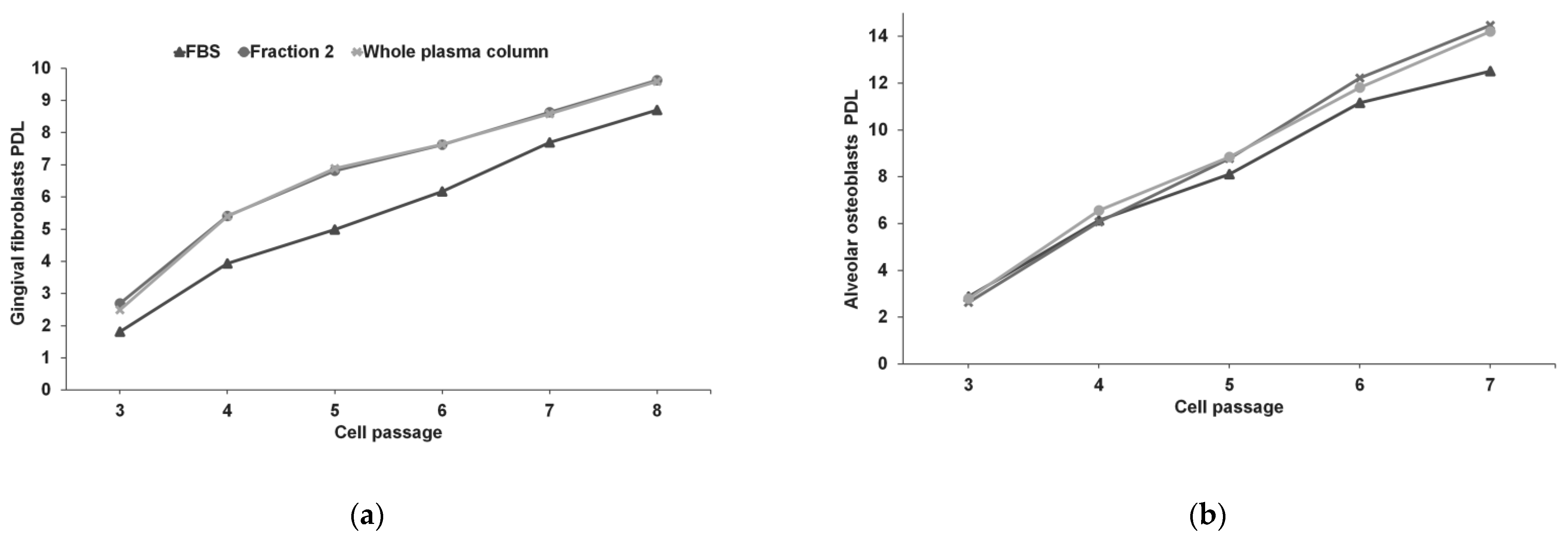
3.2. Gingival Fibroblasts and Alveolar Osteoblasts Cultures
3.3. Genetic Stability of hGFs and hABCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRGF | Plasma rich in growth factors |
| FBS | Foetal bovine serum |
| CGH | Comparative genomic hybridization |
| PRP | Platelet rich plasma |
| hGFs | Human gingival fibroblasts |
| hABCs | Human alveolar osteoblasts |
| ObM | Osteoblasts culture medium |
| OGSs | Osteoblast growth supplements |
| WP | Whole plasma column |
| F2 | Fraction 2 |
| PDL | Population doubling level |
| D-PBS | Dulbecco’s phosphate buffered saline |
| CNVs | Copy number variations |
| COX2 | Cyclooxygenase 2 |
| CXCR4 C-X-C | Chemokine receptor type 4 |
| GMPs | Good manufacturing practices |
| MSCs | Mesenchymal stem/progenitor cells |
References
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef] [PubMed]
- Elnawam, H.; Abdelmougod, M.; Mobarak, A.; Hussein, M.; Aboualmakarem, H.; Girgis, M.; El Backly, R. Regenerative Endodontics and Minimally Invasive Dentistry: Intertwining Paths Crossing Over Into Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 837639. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Ahadian, S.; Darabi, M.A.; El Tahchi, M.; Lee, J.; Suthiwanich, K.; Sheikhi, A.; Dokmeci, M.R.; Oklu, R.; Khademhosseini, A. Minimally invasive and regenerative therapeutics. Adv. Mater. 2019, 31, 1804041. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Amaral, R.J.F.C.d.; Balduino, A. Platelets in Tissue Regeneration. In The Non-Thrombotic Role of Platelets in Health and Disease; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.M.; Alkhraisat, M.H.; Orive, G. Release kinetics of platelet-derived and plasma-derived growth factors from autologous plasma rich in growth factors. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2013, 195, 461–466. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and methods of preparation of platelet-rich plasma: A review and author’s perspective. J. Cutan. Aesthetic Surg. 2014, 7, 189. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.; Troya, M.; Alkhraisat, M.H. The influence of sodium citrate on the characteristics and biological activity of plasma rich in growth factors. Regen. Med. 2020, 15, 2181–2192. [Google Scholar] [CrossRef]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. A J. Virtual Libr. 2008, 13, 3532–3548. [Google Scholar] [CrossRef] [PubMed]
- Odrljin, T.M.; Shainoff, J.R.; Lawrence, S.O.; Simpson-Haidaris, P.J. Thrombin cleavage enhances exposure of a heparin binding domain in the N-terminus of the fibrin beta chain. Blood 1996, 88, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Aoki, N. Cross-linking of alpha 2-plasmin inhibitor and fibronectin to fibrin by fibrin-stabilizing factor. Biochim. Biophys. Acta 1981, 661, 280–286. [Google Scholar] [CrossRef]
- Blombäck, B.; Hessel, B.; Hogg, D.; Therkildsen, L. A two-step fibrinogen–fibrin transition in blood coagulation. Nature 1978, 275, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C.; Bader, R.; Mannucci, P.M.; Edgington, T.S. Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J. Cell Biol. 1988, 107, 1893–1900. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.M.; Prado, R.; Alkhraisat, M.H.; Orive, G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: Evaluation of the effect of leukocyte inclusion. J. Biomed. Mater. Res. A 2015, 103, 1011–1020. [Google Scholar] [CrossRef]
- Novak, B.; Heldt, F.S.; Tyson, J.J. Genome Stability during Cell Proliferation: A Systems Analysis of the Molecular Mechanisms Controlling Progression through the Eukaryotic Cell Cycle. Curr. Opin. Syst. Biol. 2018, 9, 22–31. [Google Scholar] [CrossRef]
- Yao, Y.; Dai, W. Genomic Instability and Cancer. J. Carcinog. Mutagen. 2014, 5, 163. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Sanchez, M.; Azofra, J.; del Mar Zalduendo, M.; de la Fuente, M.; Nurden, P.; Nurden, A.T. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2005, 23, 281–286. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; de la Fuente, M.; Riestra, A.; Merayo-Lloves, J.; Orive, G. PRGF exerts more potent proliferative and anti-inflammatory effects than autologous serum on a cell culture inflammatory model. Exp. Eye Res. 2016, 151, 115–121. [Google Scholar] [CrossRef]
- Pinas, L.; Alkhraisat, M.H.; Suarez-Fernandez, R.; Anitua, E. Biomolecules in the treatment of lichen planus refractory to corticosteroid therapy: Clinical and histopathological assessment. Ann. Anat. 2018, 216, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Fernandez-de-Retana, S.; Alkhraisat, M.H. Platelet rich plasma in oral and maxillofacial surgery from the perspective of composition. Platelets 2021, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Gavino-Orduna, J.F.; Fernandez-Guallart, I.; Caviedes-Bucheli, J.; Espadas-Garcia, M.; Lopez-Lopez, J. Regenerative endodontic procedure combined with apical surgery of a necrotic permanent incisor with extensive periapical lesion using plasma rich in growth factors (PRGF): A Case report with 6 years post-op evaluation using CBCT. J. Clin. Exp. Dent. 2021, 13, e620–e625. [Google Scholar] [CrossRef]
- Solakoglu, O.; Heydecke, G.; Amiri, N.; Anitua, E. The use of plasma rich in growth factors (PRGF) in guided tissue regeneration and guided bone regeneration. A review of histological, immunohistochemical, histomorphometrical, radiological and clinical results in humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef]
- Anitua, E.; Begona, L.; Orive, G. Treatment of hemimandibular paresthesia in a patient with bisphosphonate-related osteonecrosis of the jaw (BRONJ) by combining surgical resection and PRGF-Endoret. Br. J. Oral Maxillofac. Surg. 2013, 51, e272–e274. [Google Scholar] [CrossRef] [PubMed]
- Pardinas Lopez, S.; Iocca, O.; Khouly, I. Three-dimensional bone evaluation after surgical treatment with plasma rich in growth factors of Medication Related Osteonecrosis of the Jaw (MRONJ): A report of 3 cases. Bone Rep. 2019, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Arata, V.; Gallesio, G. Tooth extraction in patients on zoledronic acid therapy. Oral Oncol. 2012, 48, 817–821. [Google Scholar] [CrossRef]
- Scoletta, M.; Arduino, P.G.; Dalmasso, P.; Broccoletti, R.; Mozzati, M. Treatment outcomes in patients with bisphosphonate-related osteonecrosis of the jaws: A prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 46–53. [Google Scholar] [CrossRef]
- Astori, G.; Amati, E.; Bambi, F.; Bernardi, M.; Chieregato, K.; Schafer, R.; Sella, S.; Rodeghiero, F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: Present and future. Stem Cell Res. 2016, 7, 93. [Google Scholar] [CrossRef]
- Atashi, F.; Jaconi, M.E.; Pittet-Cuenod, B.; Modarressi, A. Autologous platelet-rich plasma: A biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng. Part C Methods 2015, 21, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.A.; Bolin, S.R.; Landgraf, J.G. Viral contamination of fetal bovine serum used for tissue culture: Risks and concerns. Dev. Biol. Stand. 1991, 75, 173–175. [Google Scholar] [PubMed]
- van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.G.; Fu, J.; Hickman, J.J.; Hohensee, C.; Kolar, R.; Liebsch, M.; et al. Fetal Bovine Serum (FBS): Past—Present—Future. Altex 2018, 35, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Zalduendo, M.; Troya, M. Autologous plasma rich in growth factors technology for isolation and ex vivo expansion of human dental pulp stem cells for clinical translation. Regen. Med. 2019, 14, 97–111. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, J.; Gstraunthaler, G. Fetal Bovine Serum (FBS)—A pain in the dish? Altern. Lab. Anim. ATLA 2017, 45, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Rauch, C.; Feifel, E.; Amann, E.-M.; Spötl, H.P.; Schennach, H.; Pfaller, W.; Gstraunthaler, G. Alternatives to the use of fetal bovine serum: Human platelet lysates as a serum substitute in cell culture media. ALTEX-Altern. Anim. Exp. 2011, 28, 305–316. [Google Scholar]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; de la Fuente, M.; Ferrando, M.; Quintana, F.; Larreategui, Z.; Matorras, R.; Orive, G. Biological effects of plasma rich in growth factors (PRGF) on human endometrial fibroblasts. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 125–130. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Zalduendo, M.M.; de la Fuente, M.; Prado, R.; Orive, G.; Andia, I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Zalduendo, M.; Tejero, R.; Orive, G. Progress in the use of autologous regenerative platelet-based therapies in implant dentistry. Curr. Pharm. Biotechnol. 2016, 17, 402–413. [Google Scholar] [CrossRef]
- Riestra, A.; Vazquez, N.; Chacón, M.; Berisa, S.; Sanchez-Avila, R.M.; Orive, G.; Anitua, E.; Meana, A.; Merayo-Lloves, J. Autologous method for ex vivo expansion of human limbal epithelial progenitor cells based on plasma rich in growth factors technology. Ocul. Surf. 2017, 15, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Orive, G. Plasma rich in growth factors promote gingival tissue regeneration by stimulating fibroblast proliferation and migration and by blocking transforming growth factor-beta1-induced myodifferentiation. J. Periodontol. 2012, 83, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Tejero, R.; Zalduendo, M.M.; Orive, G. Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation, migration, and autocrine secretion in primary human osteoblasts. J. Periodontol. 2013, 84, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Gisonni-Lex, L.; Azizi, A. New approaches for characterization of the genetic stability of vaccine cell lines. Hum. Vaccin Immunother. 2017, 13, 1669–1672. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control. Release Off. J. Control. Release Soc. 2012, 157, 29–38. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Orive, G.; Padilla, S. A biological therapy to osteoarthritis treatment using platelet-rich plasma. Expert Opin. Biol. Ther. 2013, 13, 1161–1172. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; Pino, A.; Merayo-Lloves, J.; Orive, G. Biological Stability of Plasma Rich in Growth Factors Eye Drops After Storage of 3 Months. Cornea 2013, 32, 1380–1386. [Google Scholar] [CrossRef]
- Anitua, E.; Aguirre, J.J.; Algorta, J.; Ayerdi, E.; Cabezas, A.I.; Orive, G.; Andia, I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 415–421. [Google Scholar] [CrossRef]
- Orcajo, B.; Muruzabal, F.; Isasmendi, M.C.; Gutierrez, N.; Sanchez, M.; Orive, G.; Anitua, E. The use of plasma rich in growth factors (PRGF-Endoret) in the treatment of a severe mal perforant ulcer in the foot of a person with diabetes. Diabetes Res. Clin. Pract. 2011, 93, e65–e67. [Google Scholar] [CrossRef]
- Sanchez, M.; Anitua, E.; Delgado, D.; Sanchez, P.; Prado, R.; Orive, G.; Padilla, S. Platelet-rich plasma, a source of autologous growth factors and biomimetic scaffold for peripheral nerve regeneration. Expert Opin. Biol. 2017, 17, 197–212. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Eguia, A.; Pinas, L. Oral Pemphigoid Recalcitrant Lesion Treated with PRGF Infiltration. Case Report. Dent. J. 2021, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Pinas, L.; Alkhraisat, M.H.; Fernandez, R.S.; Anitua, E. Biological Therapy of Refractory Ulcerative Oral Lichen Planus with Plasma Rich in Growth Factors. Am. J. Clin. Derm. 2017, 18, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Houben, R.; Vetter, C.S.; Brocker, E.B. The carcinogenic potential of tacrolimus ointment beyond immune suppression: A hypothesis creating case report. BMC Cancer 2006, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Tekkatte, C.; Gunasingh, G.P.; Cherian, K.M.; Sankaranarayanan, K. "Humanized" stem cell culture techniques: The animal serum controversy. Stem Cells Int. 2011, 2011, 504723. [Google Scholar] [CrossRef]
- Jochems, C.E.; van der Valk, J.B.; Stafleu, F.R.; Baumans, V. The use of fetal bovine serum: Ethical or scientific problem? Altern. Lab. Anim. ATLA 2002, 30, 219–227. [Google Scholar] [CrossRef]
- Kandoi, S.; Patra, B.; Vidyasekar, P.; Sivanesan, D.; Verma, R.S. Evaluation of platelet lysate as a substitute for FBS in explant and enzymatic isolation methods of human umbilical cord MSCs. Sci. Rep. 2018, 8, 12439. [Google Scholar] [CrossRef]
- Berndt, S.; Turzi, A.; Pittet-Cuenod, B.; Modarressi, A. Autologous Platelet-Rich Plasma (CuteCell PRP) Safely Boosts In Vitro Human Fibroblast Expansion. Tissue Eng. Part A 2019, 25, 1550–1563. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Goni, F.; Gomez, P.; Tierno, R.; Pino, A. A Novel Autologous Topical Serum Based on Plasma Rich in Growth Factors Technology Counteracts Ultraviolet Light-Derived Photo-Oxidative Stress. Ski. Pharm. Physiol. 2020, 33, 67–81. [Google Scholar] [CrossRef]
- Anitua, E.; Prado, R. Addressing Reproducibility in Stem Cell and PRP Therapies. Trends Biotechnol. 2019, 37, 340–344. [Google Scholar] [CrossRef]
- Lang, S.; Loibl, M.; Herrmann, M. Platelet-Rich Plasma in Tissue Engineering: Hype and Hope. Eur. Surg. Res. Eur. Chir. Forschung. Rech. Chir. Eur. 2018, 59, 265–275. [Google Scholar] [CrossRef]
| Platelets | Leukocytes | Erythrocytes | ||||
|---|---|---|---|---|---|---|
| (×103/µL) | (PRGF/Blood) | (×103/µL) | (PRGF/Blood) | (×106/µL) | (PRGF/Blood) | |
| Blood | 209 | 8.2 | 4.32 | |||
| PRGF-WP | 428 | 2.05 | 0.4 | 0.05 | 0.03 | 0.01 |
| PRGF-F2 | 465 | 2.22 | 0.3 | 0.04 | 0.04 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Fuente, M.d.l.; Troya, M.; Zalduendo, M.; Alkhraisat, M.H. Autologous Platelet Rich Plasma (PRGF) Preserves Genomic Stability of Gingival Fibroblasts and Alveolar Osteoblasts after Long-Term Cell Culture. Dent. J. 2022, 10, 173. https://doi.org/10.3390/dj10090173
Anitua E, Fuente Mdl, Troya M, Zalduendo M, Alkhraisat MH. Autologous Platelet Rich Plasma (PRGF) Preserves Genomic Stability of Gingival Fibroblasts and Alveolar Osteoblasts after Long-Term Cell Culture. Dentistry Journal. 2022; 10(9):173. https://doi.org/10.3390/dj10090173
Chicago/Turabian StyleAnitua, Eduardo, María de la Fuente, María Troya, Mar Zalduendo, and Mohammad Hamdan Alkhraisat. 2022. "Autologous Platelet Rich Plasma (PRGF) Preserves Genomic Stability of Gingival Fibroblasts and Alveolar Osteoblasts after Long-Term Cell Culture" Dentistry Journal 10, no. 9: 173. https://doi.org/10.3390/dj10090173











