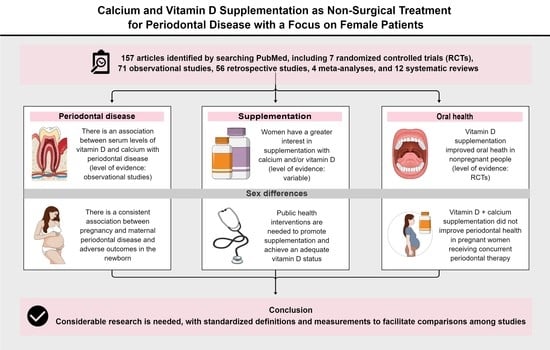
Calcium and Vitamin D Supplementation as Non-Surgical Treatment for Periodontal Disease with a Focus on Female Patients: Literature Review
Abstract
:1. Introduction
2. Methods
3. Theory Supporting Vitamin D and Calcium Supplementation as Non-Surgical Treatment for Periodontal Disease
3.1. Key Physiological Roles of Vitamin D and Calcium
3.2. Relationships of Serum Vitamin D and Calcium Levels with Systemic and Periodontal Inflammation
3.3. Vitamin D and Calcium Supplementation in the General Population
4. Effects of Vitamin D and Calcium Supplementation on Periodontal Disease
5. Sex Differences in Vitamin D and Calcium Supplementation
5.1. Investigations of Sex Differences in Supplementation
5.2. Interactions of Pregnancy with Periodontal and Systemic Health
5.3. Vitamin D and Calcium Supplementation in Pregnancy
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helal, O.; Göstemeyer, G.; Krois, J.; Fawzy El Sayed, K.; Graetz, C.; Schwendicke, F. Predictors for tooth loss in periodontitis patients: Systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 699–712. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 12, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Mougeot, F.K.; Saunders, S.E.; Brennan, M.T.; Lockhart, P.B. Associations between bacteremia from oral sources and distant-site infections: Tooth brushing versus single tooth extraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 430–435. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.N.; Hildebolt, C.F.; Miley, D.D.; Dixon, D.A.; Couture, R.A.; Spearie, C.L.; Langenwalter, E.M.; Shannon, W.D.; Deych, E.; Mueller, C.; et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J. Periodontol. 2011, 82, 25–32. [Google Scholar] [CrossRef]
- Assaf, M.; Aboelsaad, N. The effectiveness of vitamin D supplementation in chronic periodontitis patients: A randomized controlled clinical trial. Egypt. Dent. J. 2019, 65, 1311–1321. [Google Scholar] [CrossRef]
- Imrey, P.B.; Chilton, N.W.; Pihlstrom, B.L.; Proskin, H.M.; Kingman, A.; Listgarten, M.A.; Zimmerman, S.O.; Ciancio, S.G.; Cohen, M.E.; D’Agostin, R.B. Proposed guidelines for American Dental Association acceptance of products for professional, non-surgical treatment of adult periodontitis. Task Force on Design and Analysis in Dental and Oral Research. J. Periodontal. Res. 1994, 29, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Vandenbulcke, W.; Cosyn, J.; De Bruyn, H. Guidelines for non-surgical treatment of chronic periodontitis in Belgium. Rev. Belg. Med. Dent. 2008, 63, 86–90. [Google Scholar]
- Greenwell, H.; Committee on Research, Science and Therapy; American Academy of Periodontology. Position paper: Guidelines for periodontal therapy. J. Periodontol. 2001, 72, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- American Dental Association. Nonsurgical Treatment of Chronic Periodontitis Clinical Practice Guideline. 2015. Available online: https://www.ada.org/resources/research/science-and-research-institute/evidence-based-dental-research/nonsurgical-treatment-of-periodontitis-guideline (accessed on 28 February 2022).
- Könönen, E.; Klausen, B.; Verket, A.; Derk, J. Non-Surgical Periodontal Therapy: Recommendations by the European Federation of Periodontology and Guidelines in Nordic Countries. Available online: https://www.tannlegetidende.no/journal/2022/1/m-657/Recommendations_by_the_European_Federation_of_Periodontology_and_guidelines_in_Nordic_countries (accessed on 28 February 2022).
- Journal of Clinical Periodontology. Clinical Guidelines for the Treatment of Periodontitis. Available online: https://onlinelibrary.wiley.com/doi/toc/10.1111/(ISSN)1600-051x.Clinical-Guidelines-for-the-treatment-of-Periodontitis (accessed on 28 February 2022).
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilhom, R.M.; Sanz, M.; Messora, M.R. New tendencies in non-surgical periodontal therapy. Braz. Oral Res. 2021, 24, e095. [Google Scholar] [CrossRef]
- Meqa, K.; Disha, M.; Dragidella, F.; Sllamniku-Dalipi, Z. Non-surgical periodontal treatment supplemented with photodynamic therapy. J. Int. Dent. Med. Res. 2016, 9, 139. [Google Scholar]
- Krall, E.A.; Wehler, C.; Garcia, R.I.; Harris, S.S.; Dawson-Hughes, B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001, 15, 452–456. [Google Scholar] [CrossRef]
- Hiremath, V.P.; Rao, C.B.; Naik, V.; Prasad, K.V. Anti-inflammatory effect of vitamin D on gingivitis: A dose-response randomised control trial. Oral Health Prev. Dent. 2013, 11, 61–69. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yokomichi, H.; Yokoyama, T. Difference and variance in nutrient intake by age for older adults living alone in Japan: Comparison of dietary reference intakes for the Japanese population. Nutrients 2021, 13, 1431. [Google Scholar] [CrossRef]
- Dixon, D.; Hildebolt, C.F.; Miley, D.D.; Garcia, M.N.; Pilgram, T.K.; Couture, R.; Anderson Spearie, C.; Civitelli, R. Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br. Dent. J. 2009, 206, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Teschemaker, A.R.; Daniel, M.; Maneno, M.K.; Johnson, A.A.; Wutoh, A.K.; Lee, E. Calcium and vitamin D use among older adults in U.S.: Results from national survey. J. Nutr. Health Aging 2016, 20, 300–305. [Google Scholar] [CrossRef]
- Aljefree, N.; Lee, P.; Ahmed, F. Exploring knowledge and attitudes about vitamin D among adults in Saudi Arabia: A qualitative study. Healthcare 2017, 5, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Sánchez, B.J.; Legorreta-Herrera, M.; Rodriguez-Sosa, M. Influence of gestational hormones on the bacteria-induced cytokine response in periodontitis. Mediat. Inflamm. 2021, 2021, 5834608. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, P.; Venugopal, S.; Shivakumar, M.A.; Sethuraman, S.; Ramaiah, S.K.; Mukundan, S. Maternal periodontal disease and preterm birth: A case-control study. J. Indian Soc. Periodontol. 2015, 19, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Guglielmetti, M.R.; Pannuti, C.M.; Chambrone, L.A. Evidence grade associating periodontitis to preterm birth and/or low birth weight: I. A systematic review of prospective cohort studies. J. Clin. Periodontol. 2011, 38, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.; Yitayeh, A. Relationship between periodontal disease and preterm low birth weight: Systematic review. Pan Afr. Med. J. 2016, 24, 215. [Google Scholar] [CrossRef]
- Katz, J.; Lee, A.C.; Kozuki, N.; Lawn, J.E.; Cousens, S.; Blencowe, H.; Ezzati, M.; Bhutta, Z.A.; Marchant, T.; Willey, B.A.; et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: A pooled country analysis. Lancet 2013, 382, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. BMC Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Perayil, J.; Menon, K.S.; Kurup, S.; Thomas, A.E.; Fenol, A.; Vyloppillil, R.; Bhaskar, A.; Megha, S. Influence of vitamin D & calcium supplementation in the management of periodontitis. J. Clin. Diagn. Res. 2015, 9, ZC35–ZC38. [Google Scholar] [CrossRef]
- Meghil, M.M.; Hutchens, L.; Raed, A.; Multani, N.A.; Rajendran, M.; Zhu, H.; Looney, S.; Elashiry, M.; Arce, R.M.; Peacock, M.E.; et al. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral Dis. 2019, 25, 1403–1413. [Google Scholar] [CrossRef]
- Gao, W.; Tang, H.; Wang, D.; Zhou, X.; Song, Y.; Wang, Z. Effect of short-term vitamin D supplementation after nonsurgical periodontal treatment: A randomized, double-masked, placebo-controlled clinical trial. J. Periodontal Res. 2020, 55, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Rabbani, R.; Moffatt, M.E.K.; Kelekis-Cholakis, A.; Schroth, R.J. The relation between periodontal disease and vitamin D. J. Can. Dent. Assoc. 2019, 84, j4. [Google Scholar] [PubMed]
- Hildebolt, C.F. Effect of vitamin D and calcium on periodontitis. J. Periodontol. 2005, 76, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Vitamin D deficiency: Defining, prevalence, causes, and strategies of addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- EFSA. Panel on Dietetic Products. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Deschasaux, M.; Souberbielle, J.C.; Partula, V.; Lécuyer, L.; Gonzalez, R.; Srour, B.; Guinot, C.; Malvy, D.; Latino-Martel, P.; Druesne-Pecollo, N. What do people know and believe about vitamin D? Nutrients 2016, 8, 718. [Google Scholar] [CrossRef] [Green Version]
- Kotta, S.; Gadhvi, D.; Jakeways, N.; Saeed, M.; Sohanpal, R.; Hull, S.; Famakin, O.; Martineau, A.; Griffiths, C. “Test me and treat me”—Attitudes to vitamin D deficiency and supplementation: A qualitative study. BMJ Open 2015, 5, e007401. [Google Scholar] [CrossRef] [Green Version]
- Boland, S.; Irwin, J.D.; Johnson, A.M. A survey of university students’ vitamin D-related knowledge. J. Nutr. Educ. Behav. 2015, 47, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kung, A.W.; Lee, K.K. Knowledge of vitamin D and perceptions and attitudes toward sunlight among Chinese middle-aged and elderly women: A population survey in Hong Kong. BMC Public Health 2006, 6, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, L.H.; van der Pols, J.C.; Whiteman, D.C.; Kimlin, M.G.; Neale, R.E. Knowledge and attitudes about vitamin D and impact on sun protection practices among urban office workers in Brisbane, Australia. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1784–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, S.S.; Alhalabi, H.B.; Alharbi, K.S.; Alghamdi, O.S.; Alghamdi, A.I.; Ajarem, M.A.; Alqarni, M.A. Knowledge attitude and practices of university students to Vitamin D and Vitamin D supplements during times of low sun exposure and post lockdown. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7297–7305. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, A.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. A systematic review on the implication of minerals in the onset, severity and treatment of periodontal disease. Molecules 2016, 21, 1183. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.P.N.S.; Goergen, J.; Muniz, F.W.M.G.; Haas, A.N. Vitamin D levels and risk for periodontal disease: A systematic review. J. Periodontal Res. 2018, 53, 298–305. [Google Scholar] [CrossRef]
- Van der Putten, G.J.; Vanobbergen, J.; De Visschere, L.; Schols, J.; de Baat, C. Association of some specific nutrient deficiencies with periodontal disease in elderly people: A systematic literature review. Nutrition 2009, 25, 717–722. [Google Scholar] [CrossRef]
- Amarasena, N.; Yoshihara, A.; Hirotomi, T.; Takano, N.; Miyazaki, H. Association between serum calcium and periodontal disease progression in non-institutionalized elderly. Gerodontology 2008, 25, 245–250. [Google Scholar] [CrossRef]
- Perić, M.; Cavalier, E.; Toma, S.; Lasserre, J.F. Serum vitamin D levels and chronic periodontitis in adult, Caucasian population-a systematic review. J. Periodontal Res. 2018, 53, 645–656. [Google Scholar] [CrossRef]
- Varela-López, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. Nutraceuticals in periodontal health: A systematic review on the role of vitamins in periodontal health maintenance. Molecules 2018, 23, 1226. [Google Scholar] [CrossRef] [Green Version]
- Machado, V.; Lobo, S.; Proença, L.; Mendes, J.J.; Botelho, J. Vitamin D and periodontitis: A systematic review and meta-analysis. Nutrients 2020, 12, 2177. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Pavlesen, S. Could vitamin D influence risk for periodontal disease—To “D” or not to “D”? Curr. Oral Health Rep. 2020, 7, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Diachkova, E.; Trifonova, D.; Morozova, E.; Runova, G.; Ashurko, I.; Ibadulaeva, M.; Fadeev, V.; Tarasenko, S. Vitamin D and its role in oral diseases development. Scoping review. Dent. J. 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Mínguez, I.; Caffesse, R.; Llambés, F. Periodontal disease in pregnancy: The influence of general factors and inflammatory mediators. Oral Health Prev. Dent. 2019, 17, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Doucède, G.; Dehaynin-Toulet, E.; Kacet, L.; Jollant, B.; Tholliez, S.; Deruelle, P.; Subtil, D. Dents et grossesse, un enjeu de santé publique [Tooth and pregnancy, a public health issue]. Presse Med. 2019, 48, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.R.; Karras, S.N.; März, W. The role of vitamin D in fertility and during pregnancy and lactation: A review of clinical data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boggess, K.A.; Espinola, J.A.; Moss, K.; Beck, J.; Offenbacher, S.; Camargo, C.A., Jr. Vitamin D status and periodontal disease among pregnant women. J. Periodontol. 2011, 82, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.R.; Ahmad, T.; Hussain, R.; Bhutta, Z.A. Vitamin D status and periodontal disease among pregnant and non-pregnant women in an underdeveloped district of Pakistan. J. Int. Soc. Prev. Community Dent. 2016, 6, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.R.; Ahmad, T.; Hussain, R.; Bhutta, Z.A. Relationship among hypovitaminosis D, maternal periodontal disease, and low birth weight. J. Coll. Physicians Surg. Pak. 2018, 28, 36–39. [Google Scholar] [CrossRef]
- Rodrigues Amorim Adegboye, A.; Dias Santana, D.; Cocate, P.G.; Benaim, C.; Teixeira Dos Santos, P.P.; Heitmann, B.L.; da Veiga Soares Carvalho, M.C.; Schlüssel, M.M.; Trindade de Castro, M.B.; Kac, G. Vitamin D and calcium milk fortification in pregnant women with periodontitis: A feasibility trial. Int. J. Environ. Res. Public Health 2020, 17, 8023. [Google Scholar] [CrossRef]
- Rodrigues Amorim Adegboye, A.; Dias Santana, D.; Teixeira Dos Santos, P.P.; Cocate, P.; Benaim, C.; Trindade de Castro, M.B.; Schlüssel, M.M.; Kac, G.; Heitmann, B.L. Exploratory efficacy of calcium-vitamin D milk fortification and periodontal therapy on maternal oral health and metabolic and inflammatory profile. Nutrients 2021, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R. Who, what, where and when—Influences on cutaneous vitamin D synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchwierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D toxicity—A clinical perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sllamniku Dalipi, Z.; Dragidella, F. Calcium and Vitamin D Supplementation as Non-Surgical Treatment for Periodontal Disease with a Focus on Female Patients: Literature Review. Dent. J. 2022, 10, 120. https://doi.org/10.3390/dj10070120
Sllamniku Dalipi Z, Dragidella F. Calcium and Vitamin D Supplementation as Non-Surgical Treatment for Periodontal Disease with a Focus on Female Patients: Literature Review. Dentistry Journal. 2022; 10(7):120. https://doi.org/10.3390/dj10070120
Chicago/Turabian StyleSllamniku Dalipi, Zana, and Fatmir Dragidella. 2022. "Calcium and Vitamin D Supplementation as Non-Surgical Treatment for Periodontal Disease with a Focus on Female Patients: Literature Review" Dentistry Journal 10, no. 7: 120. https://doi.org/10.3390/dj10070120