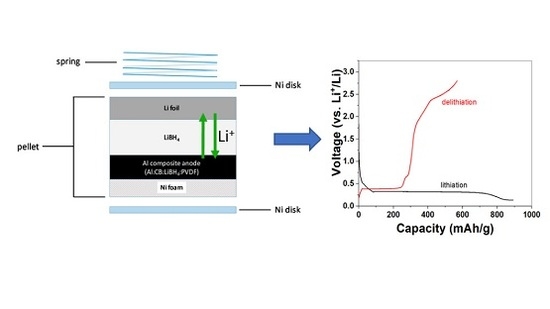
Investigation of the Reversible Lithiation of an Oxide Free Aluminum Anode by a LiBH4 Solid State Electrolyte
Abstract
:1. Introduction
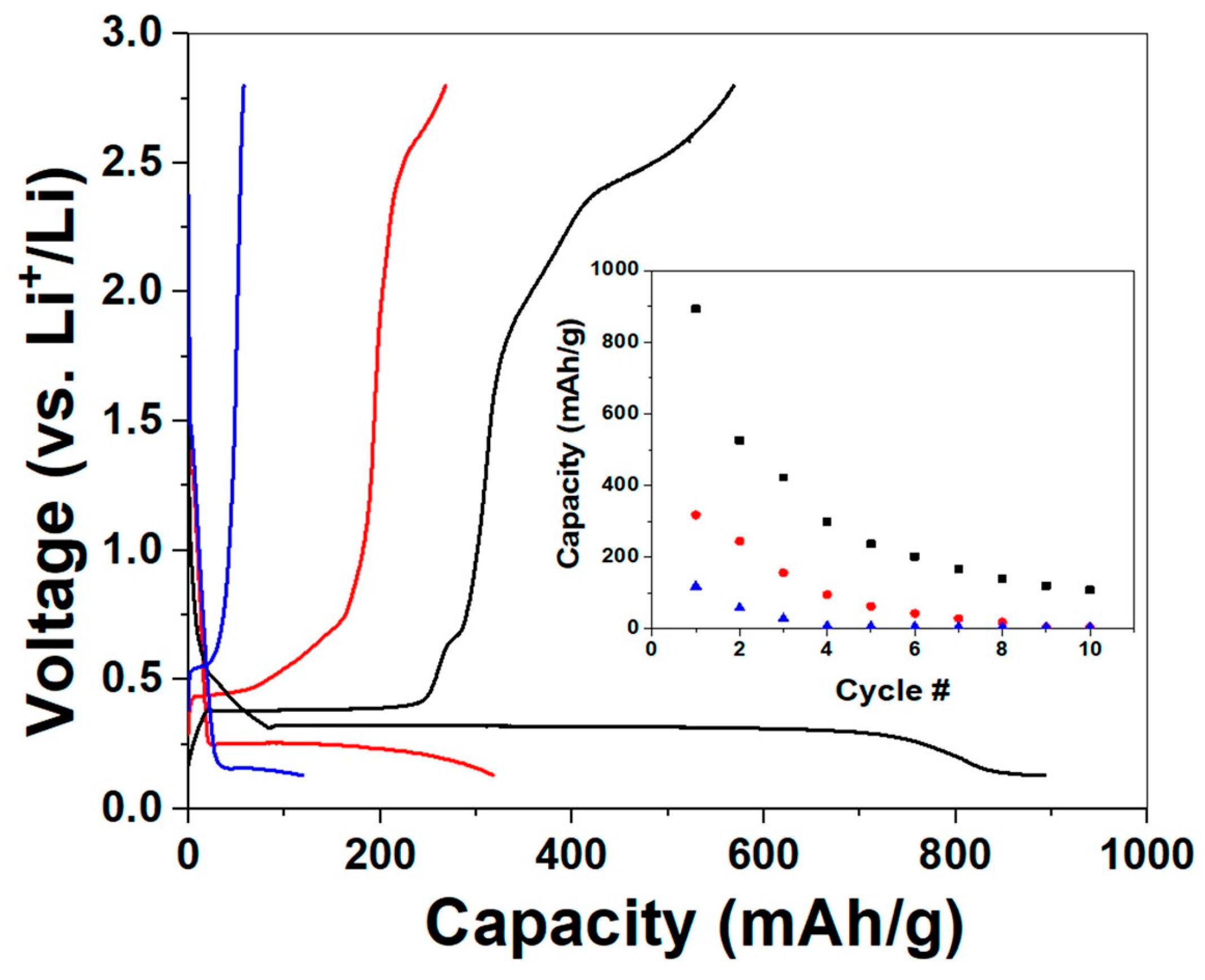
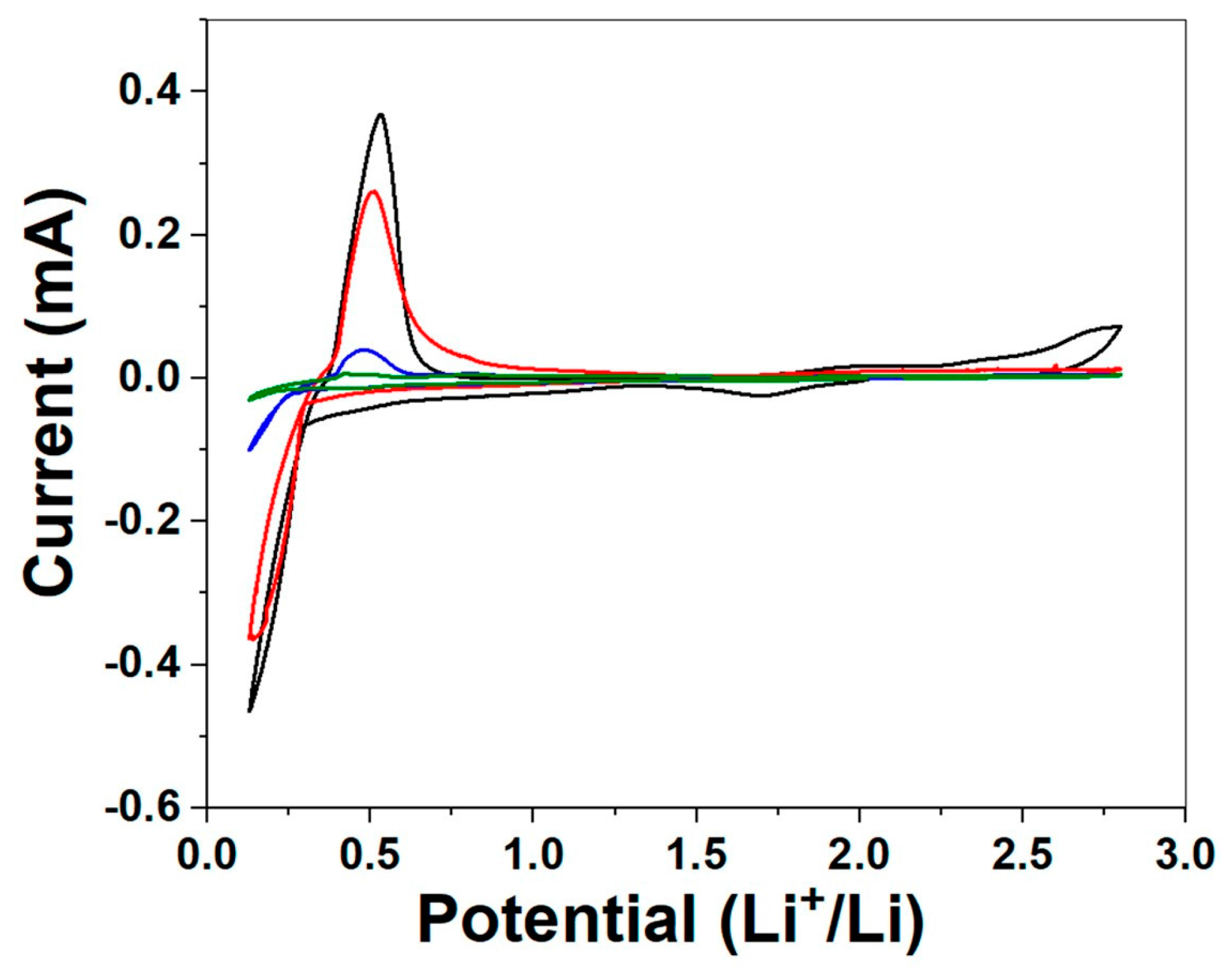
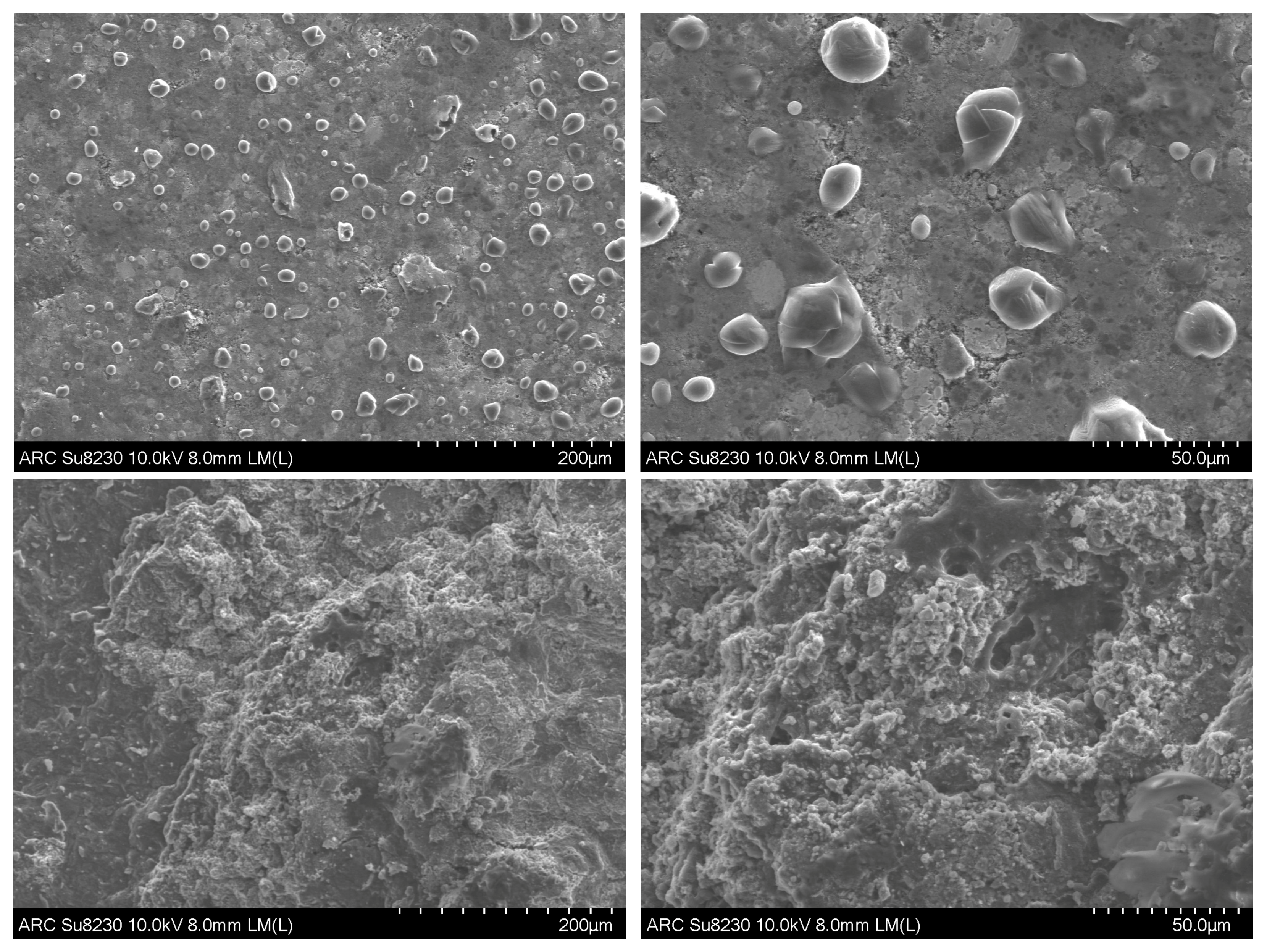
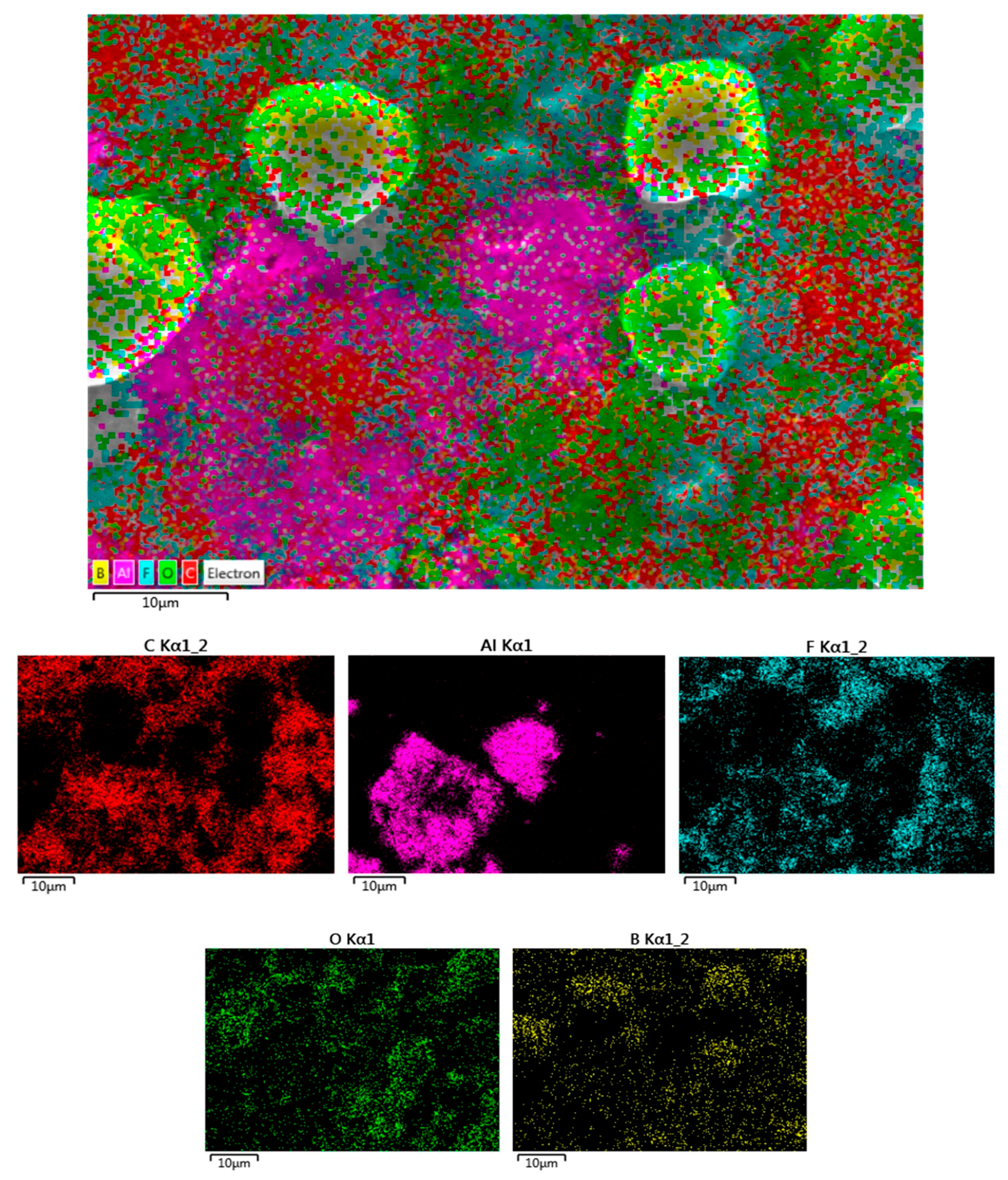
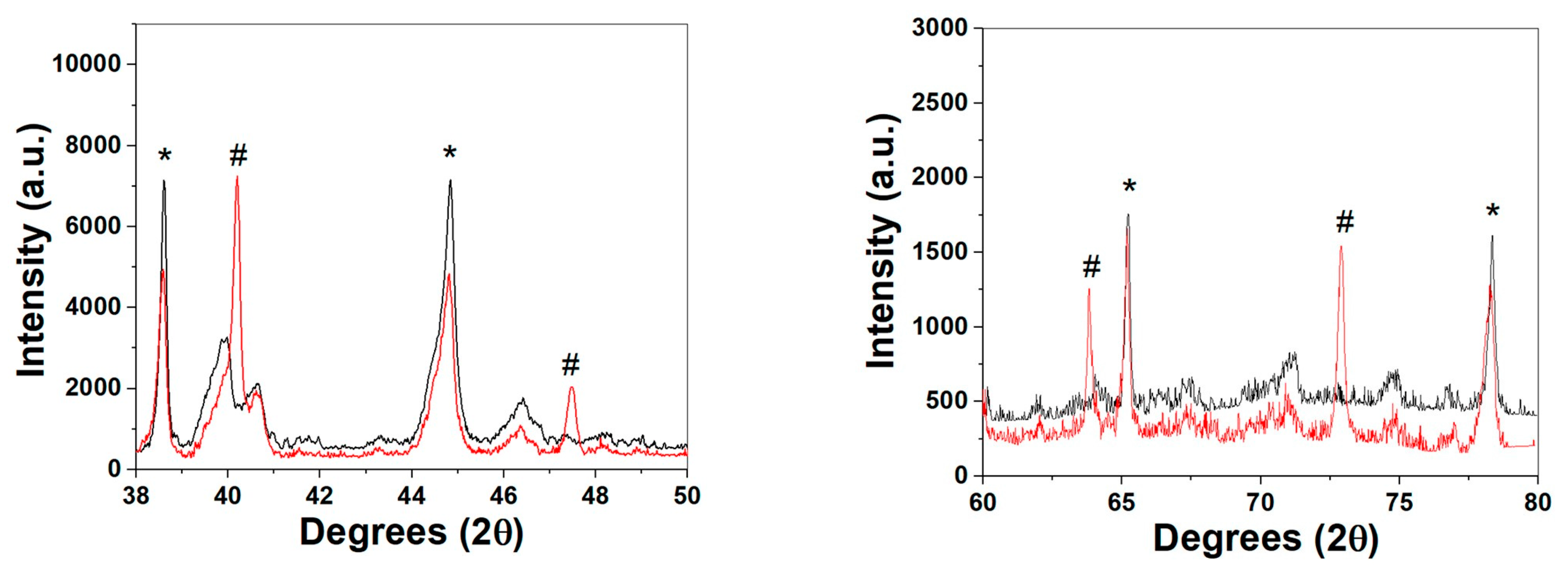
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Electrolyte and Anode Preparation
3.3. Cell Assembly and Electrochemical Characterization
3.4. Ex-Situ XRD, SEM-EDS, and Raman Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, X.; Bates, J.B.; Jellison, G.E.; Hart, F.X. A Stable Thin-Film Lithium Electrolyte: Lithium Phosphorus Oxynitride. J. Electrochem. Soc. 1997, 144, 524–532. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, W.; Payzant, E.A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous High Ionic Conductivity of Nanoporous β Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Nakamori, Y.; Orimo, S.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Gorbunov, V.E.; Gavrichev, K.S.; Zalukaev, V.L.; Sharpataya, G.A.; Bakum, S.I. Heat capacity and phase transition of lithium borohydride. Zhurnal Neorganicheskoj Khimii 1984, 29, 2333–2337. [Google Scholar]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S. Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Takamura, H.; Maekawa, H.; Li, H.; Orimo, S. Stabilization of lithium superionic conduction phase and enhancement of conductivity of LiBH4 by LiCl addition. Appl. Phys. Lett. 2009, 94, 084103. [Google Scholar] [CrossRef]
- Skripov, A.V.; Soloninin, A.V.; Rude, L.H.; Jensen, T.R.; Filinchuk, Y. Nuclear Magnetic Resonance Studies of Reorientational Motion and Li Diffusion in LiBH4–LiI Solid Solutions. J. Phys. Chem. C 2012, 116, 26177–26184. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, Y.; Oh, K.H.; Cho, Y.W. Interface-enhanced Li ion conduction in a LiBH4–SiO2 solid electrolyte. Phys. Chem. Chem. Phys. 2016, 18, 22540–22547. [Google Scholar] [CrossRef] [PubMed]
- Shane, D.T.; Corey, R.L.; McIntosh, C.; Rayhel, L.H.; Bowman, R.C., Jr.; Vajo, J.J.; Gross, A.F.; Conradi, M.S. LiBH4 in Carbon Aerogel Nanoscaffolds: An NMR Study of Atomic Motions. J. Phys. Chem. C 2010, 114, 4008–4014. [Google Scholar] [CrossRef]
- Blanchard, D.; Nale, A.; Sveinbjornsson, D.; Eggenhuisen, T.M.; Verkuijlen, M.H.; Vegge, T.; Kentgens, A.P.; de Jongh, P.E. Nanoconfined LiBH4 as a fast lithium ion conductor. Adv. Funct. Mater. 2015, 25, 184–192. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; de Jongh, P.E.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4 Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef]
- Shane, D.T.; Corey, R.L.; Rayhel, L.H.; Wellons, M.; Teprovich, J.A., Jr.; Zidan, R.; Hwang, S.; Bowman, R.C., Jr.; Conradi, M.S. NMR Study of LiBH4 with C60. J. Phys. Chem. C 2010, 114, 19862–19866. [Google Scholar] [CrossRef]
- Teprovich, J.A., Jr.; Colón-Mercado, H.R.; Ward, P.A.; Peters, B.; Giri, S.; Zhou, J.; Greenway, S.; Compton, R.N.; Jena, P.; Zidan, R. Experimental and Theoretical Analysis of Fast Lithium Ionic Conduction in a LiBH4–C60 Nanocomposite. J. Phys. Chem. C 2014, 118, 21755–21761. [Google Scholar] [CrossRef]
- Matsuo, M.; Remhof, A.; Martelli, P.; Caputo, R.; Ernst, M.; Miura, Y.; Sato, T.; Oguchi, H.; Maekawa, H.; Takamura, H.; et al. Complex Hydrides with (BH4)− and (NH2)− Anions as New Lithium Fast-Ion Conductors. J. Am. Chem. Soc. 2009, 131, 16389–16391. [Google Scholar] [CrossRef] [PubMed]
- GharibDoust, S.P.; Brighi, M.; Sadikin, Y.; Ravnsbæk, D.B.; Černý, R.; Skibsted, J.; Jensen, T.R. Synthesis, Structure, and Li-Ion Conductivity of LiLa(BH4)3X, X = Cl, Br, I. J. Phys. Chem. C 2017, 121, 19010–19021. [Google Scholar] [CrossRef]
- Skripov, A.V.; Soloninin, A.V.; Ley, M.B.; Jensen, T.R.; Filinchuk, Y. Nuclear Magnetic Resonance Studies of BH4 Reorientations and Li Diffusion in LiLa(BH4)3Cl. J. Phys. Chem. C 2013, 117, 14965–14972. [Google Scholar] [CrossRef]
- Roedern, E.; Lee, Y.; Ley, M.B.; Park, K.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. Solid state synthesis, structural characterization and ionic conductivity of bimetallic alkali-metal yttrium borohydrides MY(BH4)4 (M = Li and Na). J. Mater. Chem. A 2016, 4, 8793–8802. [Google Scholar] [CrossRef]
- Ley, M.B.; Ravnsbæk, D.B.; Filinchuk, Y.; Lee, Y.; Janot, R.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. LiCe(BH4)3Cl, a New Lithium-Ion Conductor and Hydrogen Storage Material with Isolated Tetranuclear Anionic Clusters. Chem. Mater. 2012, 24, 1654–1663. [Google Scholar] [CrossRef]
- Lee, Y.; Ley, M.B.; Jensen, T.R.; Cho, Y.W. Lithium Ion Disorder and Conduction Mechanism in LiCe(BH4)3Cl. J. Phys. Chem. C 2016, 120, 19035–19042. [Google Scholar] [CrossRef]
- Yamauchi, A.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Preparation and ionic conductivities of (100 − x)(0.75Li2S·0.25P2S5)·xLiBH4 glass electrolytes. J. Power Sources 2013, 244, 707–710. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, S.; To, M.; Lee, Y.; Cho, Y.W. Discovery of Fluidic LiBH4 on Scaffold Surfaces and Its Application for Fast Co-confinement of LiBH4–Ca(BH4)2 into Mesopores. J. Phys. Chem. C 2015, 119, 9025–9035. [Google Scholar] [CrossRef]
- Takahashi, K.; Hattori, K.; Yamazaki, T.; Takada, K.; Matsuo, M.; Orimo, S.; Maekawa, H.; Takamura, H. All-Solid-State Lithium Battery with LiBH4 Solid Electrolyte. J. Power Sources 2013, 226, 61–64. [Google Scholar] [CrossRef]
- Suzuki, S.; Kawaji, J.; Yoshida, K.; Unemoto, A.; Orimo, S. Development of complex hydride-based allsolid-state lithium ion battery applying low melting point electrolyte. J. Power Sources 2017, 359, 97–103. [Google Scholar] [CrossRef]
- Unemoto, A.; Chen, C.L.; Wang, Z.; Matsuo, M.; Ikeshoji, T.; Orimo, S. Pseudo-binary electrolyte, LiBH4–LiCl, for bulk-type all-solid-state lithium-sulfur battery. Nanotechnology 2015, 26, 254001. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Aranguren, P.; Berti, N.; Dao, A.H.; Zhang, J.; Cuevas, F.; Latroche, M.; Jordy, C. An all-solid state metal hydride—Sulfur lithium-ion battery. J. Power Sources 2017, 357, 56–60. [Google Scholar] [CrossRef]
- Unemoto, A.; Ikeshoji, T.; Yasaku, S.; Matsuo, M.; Stavila, V.; Udovic, T.J.; Orimo, S. Stable Interface Formation between TiS2 and LiBH4 in Bulk-Type All-Solid-State Lithium Batteries. Chem. Mater. 2015, 27, 5407–5416. [Google Scholar] [CrossRef]
- Unemoto, A.; Wu, H.; Udovic, T.J.; Matsuo, M.; Ikeshoji, T.; Orimo, S. Fast lithium-ionic conduction in a new complex hydride–sulphide crystalline phase. Chem. Commun. 2016, 52, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Hamon, Y.; Brousse, T.; Jousse, F.; Topart, P.; Buvat, P.; Schleich, D.M. Aluminum negative electrode in lithium ion batteries. J. Power Sources 2001, 97–98, 185–187. [Google Scholar] [CrossRef]
- Buiel, E.; Dahn, J.R. Li-insertion in hard carbon anode materials for Li-ion batteries. Electrochim. Acta 1999, 45, 121–130. [Google Scholar] [CrossRef]
- Hudak, N.; Huber, D. Size Effects in the Electrochemical Alloying and Cycling-of Electrodeposited Aluminum with Lithium. J. Electrochem. Soc. 2012, 159, A688–A695. [Google Scholar] [CrossRef]
- Shenouda, A.Y.; Murali, K.R. Electrochemical properties of doped lithium titanate compounds and their performance in lithium rechargeable batteries. J. Power Sources 2008, 176, 332–339. [Google Scholar] [CrossRef]
- Au, M.; McWhorter, S.; Ajo, H.; Adams, T.; Zhao, Y.; Gibbs, J. Free standing aluminum nanostructures as anodes for Li-ion rechargeable batteries. J. Power Sources 2010, 195, 3333–3337. [Google Scholar] [CrossRef]
- Oltean, G.; Tai, C.; Edstrom, K.; Nyholm, L. On the origin of the capacity fading for aluminum negative electrodes in Li-ion batteries. J. Power Sources 2014, 269, 266–273. [Google Scholar] [CrossRef]
- Lei, X.; Wang, C.; Yi, Z.; Liang, Y.; Sun, J. Effects of particle size on the electrochemical properties of aluminum powders as anode materials for lithium ion batteries. J. Alloys Compd. 2007, 429, 311–315. [Google Scholar] [CrossRef]
- Teprovich, J.A., Jr.; Motyka, T.; Zidan, R. Hydrogen System Using Novel Additives to Catalyze Hydrogen Release from the Hydrolysis of Alane and Activated Aluminum. Int. J. Hydrogen Energy 2012, 37, 1594–1603. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, M.J.; García-Díaz, B.L.; Teprovich, J.A., Jr.; Knight, D.A.; Zidan, R. Advances in the Electrochemical Regeneration of Aluminum Hydride. Appl. Phys. A 2012, 106, 545–550. [Google Scholar] [CrossRef]
- Sveinbjörnsson, D.; Myrdal, J.S.G.; Blanchard, D.; Bentzen, J.J.; Hirata, T.; Mogensen, M.B.; Norby, P.; Orimo, S.; Vegge, T. Effect of Heat Treatment on the Lithium Ion Conduction of the LiBH4–LiI Solid Solution. J. Phys. Chem. C 2013, 117, 3249–3257. [Google Scholar] [CrossRef]
- Teprovich, J.A., Jr.; Colon-Mercado, H.; Washington, A.L., II; Ward, P.A.; Hartman, H.; Greenway, S.; Missimer, D.M.; Velten, J.; Christian, J.H.; Zidan, R. Bi-functional Li2B12H12 for energy storage and conversion applications: Solid-state electrolyte and luminescent down-conversion dye. J. Mater. Chem. A 2015, 3, 22853–22859. [Google Scholar] [CrossRef]
- Teprovich, J.A., Jr.; Zhang, J.; Colón-Mercado, H.; Cuevas, F.; Peters, B.; Greenway, S.; Zidan, R.; Latroche, M. Li-Driven Electrochemical Conversion Reaction of AlH3, LiAlH4, and NaAlH4. J. Phys. Chem. C 2015, 119, 4666–4674. [Google Scholar] [CrossRef]
- Liu, D.X.; Co, A.C. Revealing Chemical Processes Involved in Electrochemical (De)Lithiation of Al with in Situ Neutron Depth Profiling and X-ray Diffraction. J. Am. Chem. Soc. 2016, 138, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Besenhard, J.O.; Hess, M.; Komenda, P. Dimensionally stable Li-alloy electrodes for secondary batteries. Solid State Ion. 1990, 40–41, 525–529. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50. [Google Scholar] [CrossRef]
- Reynier, Y.; Yazami, R.; Fultz, B. XRD evidence of macroscopic composition inhomogeneities in the graphite–lithium electrode. J. Power Sources 2007, 165, 616–619. [Google Scholar] [CrossRef]
- Gomes, S.; Hagemann, H.; Yvon, K. Lithium boro-hydride LiBH4: II. Raman spectroscopy. J. Alloys Compd. 2002, 346, 206–210. [Google Scholar] [CrossRef]
- Li, S.; Niu, J.; Zhao, Y.C.; So, K.P.; Wang, C.; Wang, C.A.; Li, J. High-rate aluminum yolk-shell nanoparticle anode for Li-ion battery with long cycle life and ultrahigh capacity. Nat. Commun. 2015, 6, 7872. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weeks, J.A.; Tinkey, S.C.; Ward, P.A.; Lascola, R.; Zidan, R.; Teprovich, J.A. Investigation of the Reversible Lithiation of an Oxide Free Aluminum Anode by a LiBH4 Solid State Electrolyte. Inorganics 2017, 5, 83. https://doi.org/10.3390/inorganics5040083
Weeks JA, Tinkey SC, Ward PA, Lascola R, Zidan R, Teprovich JA. Investigation of the Reversible Lithiation of an Oxide Free Aluminum Anode by a LiBH4 Solid State Electrolyte. Inorganics. 2017; 5(4):83. https://doi.org/10.3390/inorganics5040083
Chicago/Turabian StyleWeeks, Jason A., Spencer C. Tinkey, Patrick A. Ward, Robert Lascola, Ragaiy Zidan, and Joseph A. Teprovich. 2017. "Investigation of the Reversible Lithiation of an Oxide Free Aluminum Anode by a LiBH4 Solid State Electrolyte" Inorganics 5, no. 4: 83. https://doi.org/10.3390/inorganics5040083