2. Advantages of the MSS Technology
There is a large range of methods to carry out nano-particles synthesis, and all of them have some advantages and disadvantages. Wet chemical synthesis, including precipitation or sol-gel methods, is widely diffused. Additionally, these methods seem quite appropriate for low-cost production, and also for production of alloyed or stoichiometric NPs. However, a basic requirement for nanoparticles, especially oxide nanoparticles, is that their synthesis reaction is fully completed. If that is not the case, a layer of hydroxides or other precursors may be present, which negatively affect the nanoparticles properties. Both for the sol-gel or solvothermal synthesis this is achieved by raising the reaction temperature above certain level.
If the synthesis temperature is too low, part of the material remains in the amorphous form of hydroxides, no matter the reaction time. The surface layer of imperfectly crystallised nanoparticles (
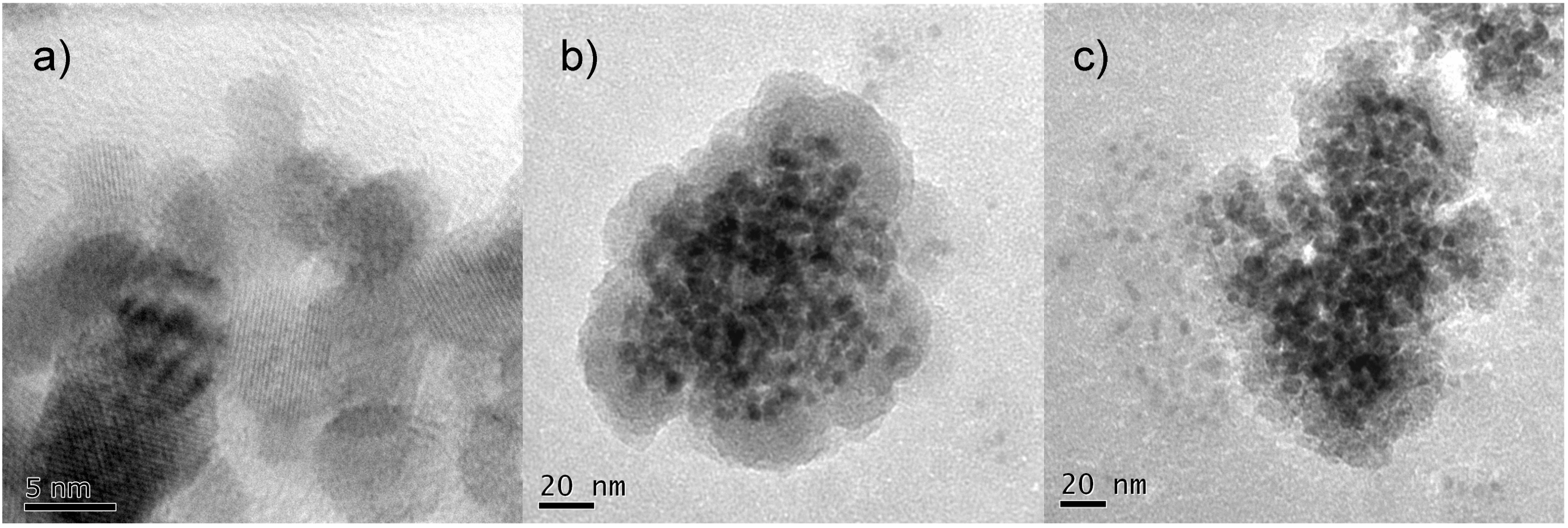
Figure 1) inevitably leads to a decrease of their density, deteriorating optical properties, or leading to poor dispersion capacity [
9].
Figure 1.
Examples of nano-sized zirconia particles differing in the degree of crystallinity: (a) fully crystallized ZrO2/calcined 400 °C, 40 min, in air; (b) partially crystallized ZrO2-Eu3+ 8 wt% doped, short and (c) long heating treatments (MSS).
Figure 1.
Examples of nano-sized zirconia particles differing in the degree of crystallinity: (a) fully crystallized ZrO2/calcined 400 °C, 40 min, in air; (b) partially crystallized ZrO2-Eu3+ 8 wt% doped, short and (c) long heating treatments (MSS).
In a solvothermal environment, high temperatures correspond to high pressures, as illustrated in
Figure 2, which illustrates the effect of synthesis temperature and pressure on the density of ZrO
2 NPs [
10]. Thus, the hydrothermal or solvothermal synthesis may permit to obtain non aggregated NPs and reduce the treatment temperature, which by (“rule of thumb”) is about 50% (measured in °C) with respect to calcination temperature.
Figure 2.
Effect of synthesis pressure on the density of zirconia nanoparticles produced in hydrothermal process [
10]. At the pressure of 5.5 MPa, corresponding to 270 °C, the particles were transformed from non-fully reacted to fully reacted.
Figure 2.
Effect of synthesis pressure on the density of zirconia nanoparticles produced in hydrothermal process [
10]. At the pressure of 5.5 MPa, corresponding to 270 °C, the particles were transformed from non-fully reacted to fully reacted.
In ref. [
9] this situation is further discussed. It is shown that the zirconia nanoparticles are coated with a monolayer of hydroxide even after high temperature calcination: this skin layer is an inherent part of the particle. It causes the nanoparticles’ density to be lower than the density of bulk materials, especially for particle sizes less than 50 nm. However, if the synthesis pressure or temperature is too low, the unreacted part of the nanoparticle is thicker than one monolayer, and the density of NPs is not optimal.
Crystallisation of NPs of pure oxides, mixed oxides, phosphates, carbonates and other inorganic compounds produced at low temperatures (
i.e., below 100 °C) as a rule, requires a second reaction step: annealing. For yttrium-aluminium garnet, Y
3Al
5O
12 or YAG, nanoparticles, the annealing temperature is above 1000 °C [
11], for ZrO
2, about 500 °C, and for ZnO, about 200 °C. This calcination process leads to nano-particles sintering and aggregation. Such a two-step synthesis route can be substituted with a single step, a solvo or hydrothermal synthesis, which is carried out at temperatures much lower than calcinations [
12]. As an example, in
Figure 3 we show YAG NPs produced in a solvothermal process at 450 °C [
13] and in a sol-gel process (followed by annealing at 1100 °C [
11]). The sol-gel particles form hard aggregates of NPs, while those prepared with solvothermal process are not strongly bonded to each other.
Figure 3.
Comparison of aggregation for nano-YAG powder produced using (a) the precipitation method followed by calcination at 1100 °C and (b) solvothermal process at 450 °C.
Figure 3.
Comparison of aggregation for nano-YAG powder produced using (a) the precipitation method followed by calcination at 1100 °C and (b) solvothermal process at 450 °C.
Additionally, it is not only the maximum possible crystallinity level that is required, but also a narrow size distribution and controlled dimension. To achieve this goal, the solution of reagents needs to be uniformly heated at a high rate until a given degree of oversaturation is achieved, and then the heating should be stopped to avoid grain growth. This procedure should lead to uniform nucleation of NPs in the whole volume of the liquid [
4]. An extension of the heating time will lead to Ostwald ripening and widening of the particle size dispersion.
How to achieve such a rapid overheating in a liquid? Conventional heating methods will inevitably lead to a situation where the hot heater contacts a cold liquid; hence, the reactive liquid will experience strong temperature gradients. A possible method is to heat a small volume of a liquid flowing through a narrow tube. This is the so-called continuous solvothermal or hydrothermal synthesis [
11,
13]. Another possible route is to use electromagnetic radiation, which penetrates deep enough in the reaction vessel, so that the heating rate is much more uniform than during conventional synthesis. This approach permits also the fast heating of the total volume of liquid due to turbulences caused by local microwave energy “hot spots”. The fundamental difference of the two processes is that in the continuous process it is difficult to control the time the NPs spend in the reaction zone, while during the MSS process, time of reaction can be precisely controlled. So, the use of microwave energy as a heat source is advantageous if control of reaction time is needed.
Application of MW energy in chemical synthesis is well established [
14,
15]. In the case of solvothermal synthesis it allows for additional control of the synthesis process, besides fast heating solely. The subject was reviewed recently in the paper [
16] and in the monograph edited by Horikoshi and Serperone [
8].
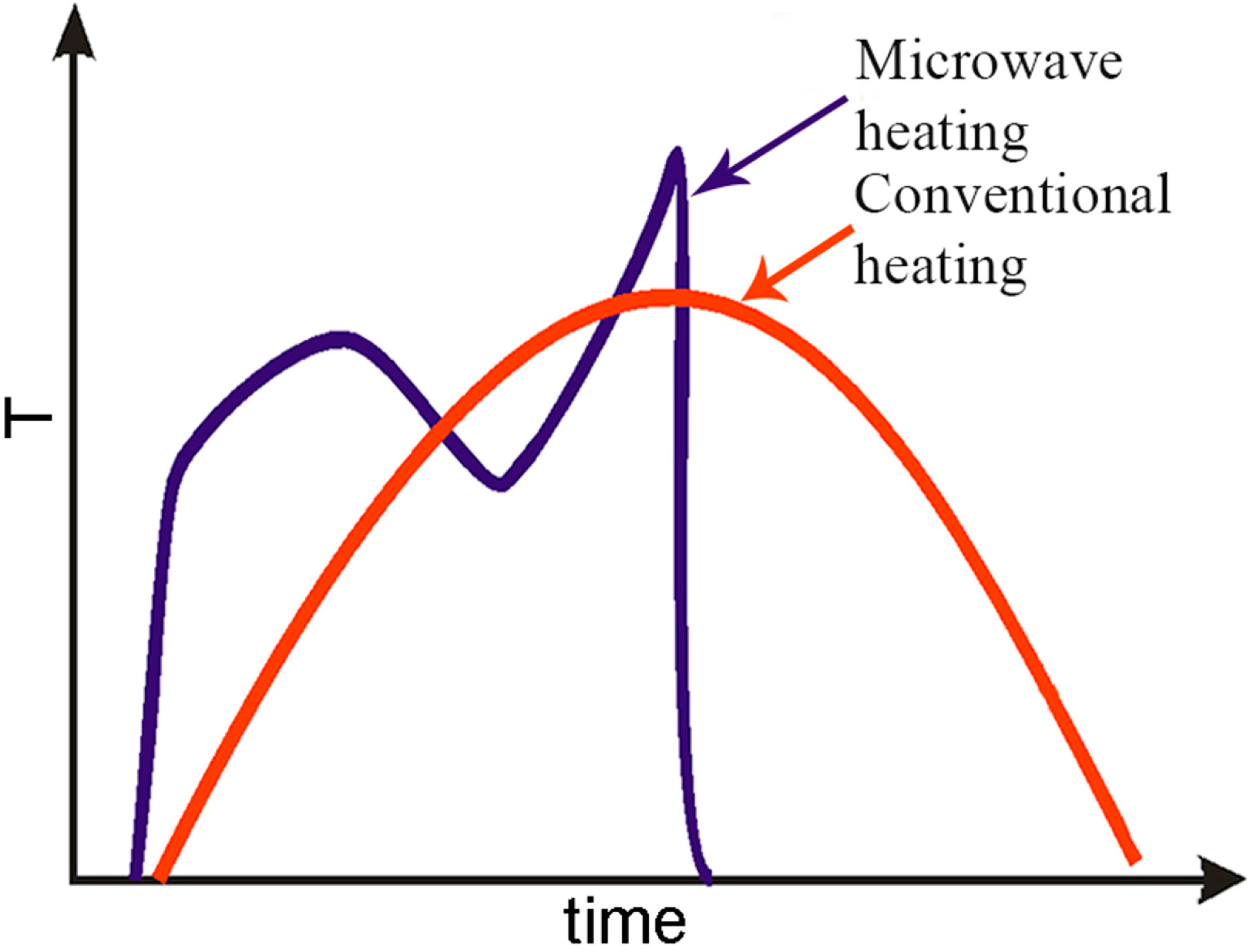
Figure 4 shows schematically further opportunities MW heating offers during NPs synthesis. In case of conventional heating, the thermal inertial of the system limits the heating rate and cooling rate. In the case of MW heating, one can not only rapidly heat, but can also change the temperature during the process, and at the end also rapidly cool the product, since the vessel walls temperature need not to be coupled with the temperature of the vessel content.
Further, it may be advantageous to have a controlled temperature gradient by focussing the MW energy in a particular part of the vessel, and further shorten the time the nucleated NP spend in the high temperature zone. As it is seen, the details of the microwave heating process strongly depend on the MW reactor design, which sometimes makes it difficult to compare results from various research groups. A thorough review of various reactors for MW synthesis of nanoparticles can be found in [
8].
Figure 4.
Schematic presentation of the thermal microwave effects as variation of temperature in time.
Figure 4.
Schematic presentation of the thermal microwave effects as variation of temperature in time.
There is also the wide area of research concerning specific MW effects, where it is expected that in a strong electromagnetic field the course of the chemical reactions is changed [
17], or where MW interact with individual NPs influencing their nucleation and growth process. This subject is outside the scope of the present paper, and more information can be found in [
18].
In the MW hydrothermal process, the strong variation of the dielectric properties of water with temperature has to be accounted for, since they affect MW absorption, reactants’ solubility and supersaturation. It is well known that water can easily dissolve ionic compounds, typically the salts of metals used in the preparation of oxidic NPs. It is also known that its polarity decreases with increasing temperature and the dielectric constant may become very close to that of unpolar solvents in near supercritical conditions [
19]. Such a decrease in polarity is accompanied by the sudden commencement of supersaturation, which leads to nuclei formation, the first step for NPs’ preparation. The supersaturation in water/aqueous solutions is very well known also in common hydrothermal treatment, but the uniformity of temperature profile, which can be reached in thick walled reaction vessels, is a peculiar feature in MW heating.
The above advantages of the MSS process are called MW thermal effects [
20], since they result from thermal schedules that are impossible to achieve using other heating methods.
A major limitation when comparing MW synthesis in the liquid phase and conventional heating is that it is quite difficult or even impossible to carry out two syntheses, one using MW and the other without, and follow the same temperature-time schedule. Thus, even if the results of the two processes are different, the reason of this difference could be either a divergence in the heating process or a real modification of the reaction path. A thorough review of application of MW in NPs’ synthesis is given in the already mentioned monograph [
8].
In the following sections, we will give several examples of MW reactors used for NPs synthesis and will show two examples where MSS of nanoparticles shows a clear advantage possibly due to MW indirect heating application.
3. Reactors for MSS Synthesis Developed at the Institute of High Pressure Physics—IHPP PAS
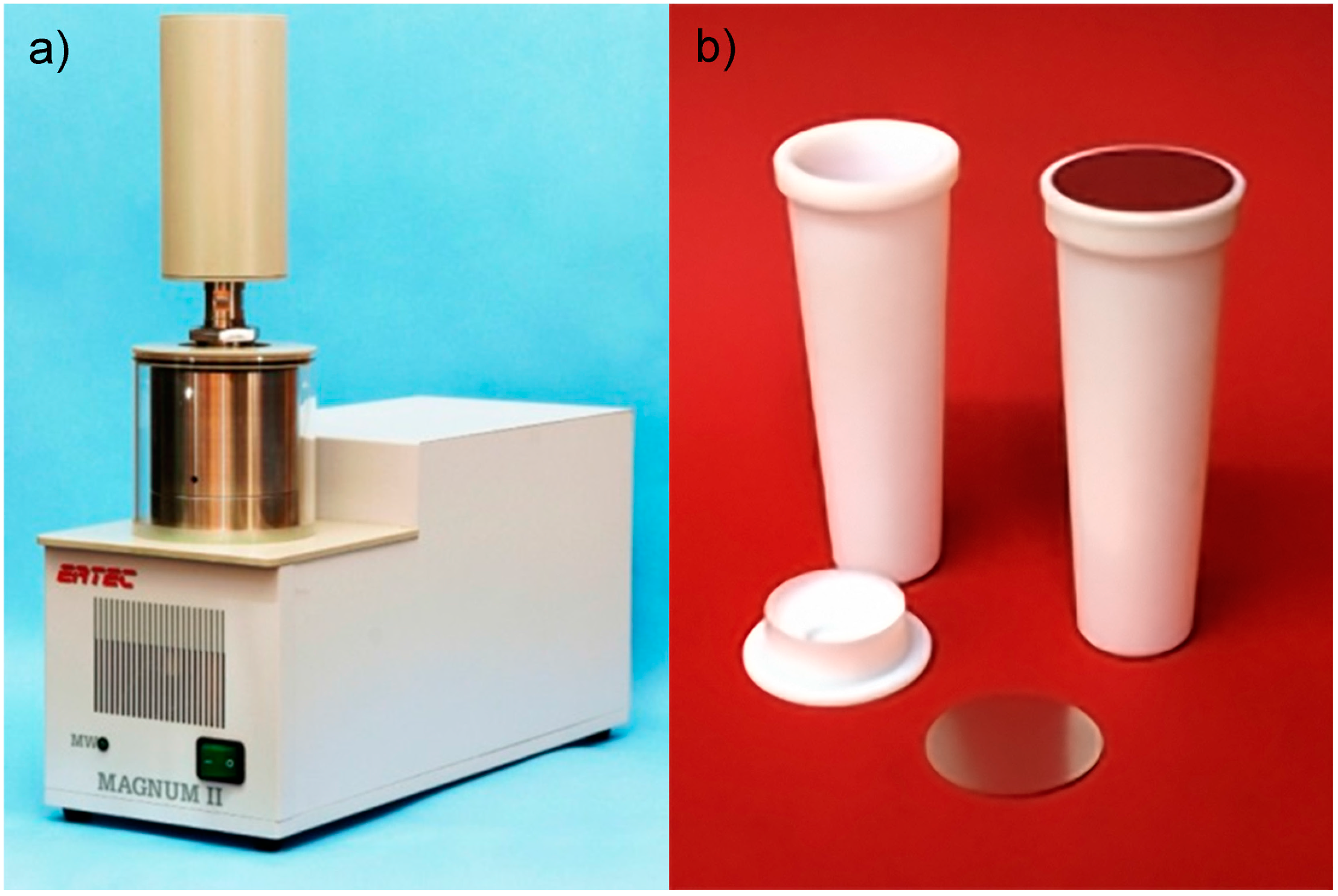
The MSS adventure at the Institute of High Pressure Physics, IHHP, started with the application of a simple MW reactor developed by the company Ertec-Poland, “Magnum II” (
Figure 5). A Teflon
® vessel with a volume of 10 mL is enclosed in a pressure vessel made of steel. A MW antenna focuses the energy at the bottom of the Teflon
® vessel. An energy density of about 5 W/mL is achieved at the bottom of the vessel, permitting to raise the temperature at a rate of approximately 1 K/s. The maximum temperature is 300 °C for short operation, and the maximum pressure is 10 MPa. All parameters—pressure and temperature—are controlled. The Teflon
® vessel can be changed for each reaction, which is essential if high purity of the product (no cross contamination) is required.
Figure 5.
(
a) The reactor Magnum II [
21] with its; (
b) Teflon
® vessels with a recommended amount of nanoparticles produced in single batch process of about 1.5 grams.
Figure 5.
(
a) The reactor Magnum II [
21] with its; (
b) Teflon
® vessels with a recommended amount of nanoparticles produced in single batch process of about 1.5 grams.
The two reactors shown below (
Figure 6 and
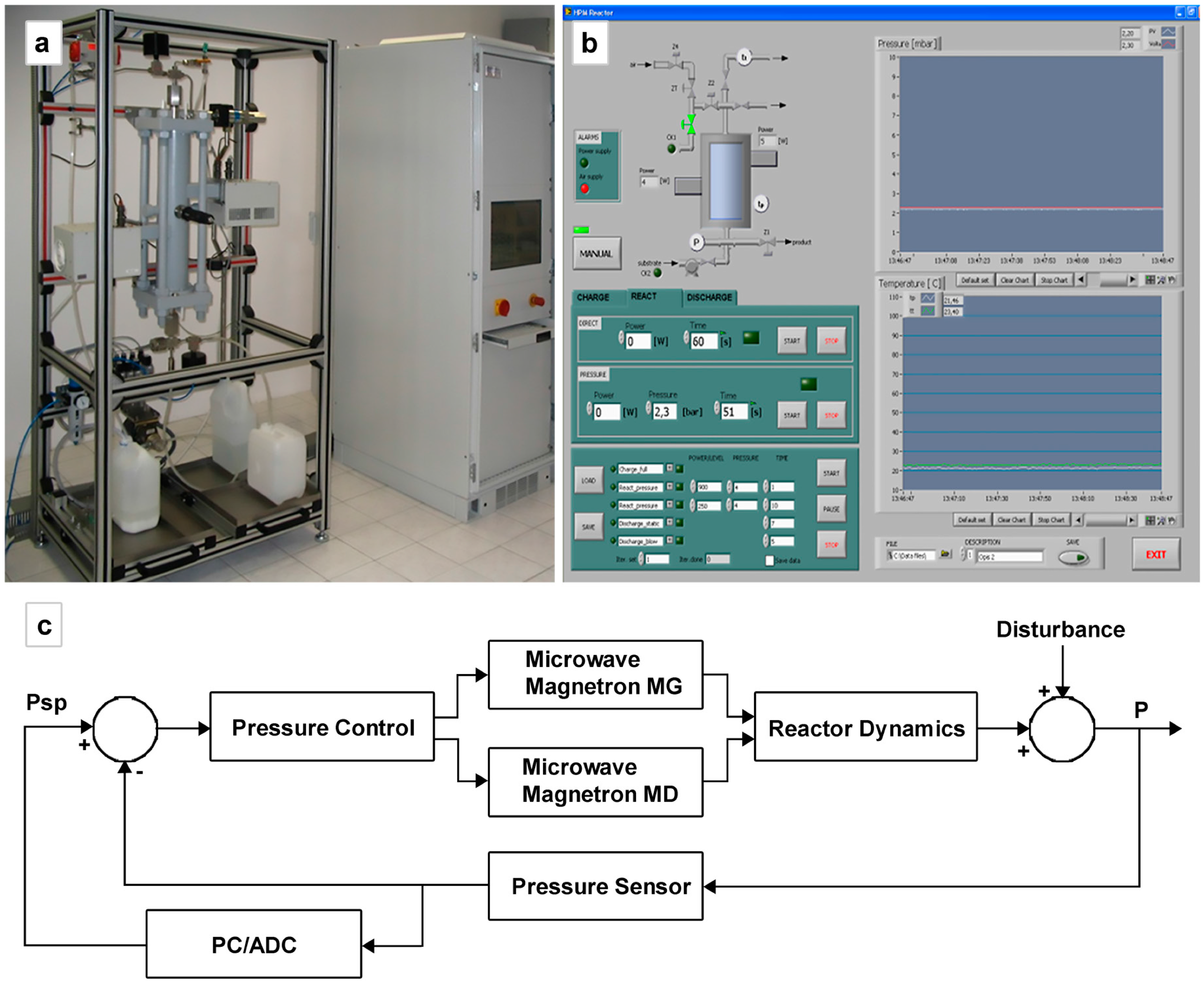
Figure 7) have been developed at IHPP in collaboration with the company Ertec—Poland and the Institute of Sustainable Technology in Radom, Poland. The reactor MSS-1 is a stop-flow reactor, automatically operating, with a chamber of volume 250 mL, maximum operating pressure 4 MPa and temperature 240 °C (
Figure 6). The reaction chamber is a Teflon
® tube, 3 cm in diameter. MW energy is supplied by means of two magnetrons with 1 kW output power. The magnetrons are placed at different levels in the chamber, opposite to each other. The temperature of the PTFE chamber is measured with the use of a pyrometer (TXP7, Raytek, Santa Cruz, CA, USA), which is placed between the magnetrons. The pressure is measured with a pressure sensor with a capillary and a separation membrane (SEN‑3291, Kobold, Pittsburgh, PA, USA). The circulator prevents reflected power damaging the magnetrons. The use of two magnetrons permits a uniform field distribution, but on demand one can use only one magnetron, and achieve a temperature gradient and/or microwave stirring effect.
Figure 6.
(a) The MSS-1 reactor. Stop-flow reactor, automatically operating, with a chamber of volume 250 mL, maximum operating pressure 4 MPa and temperature 240 °C; (b) The control panel; (c) Schematic presentation of the control system. Psp is the pressure set point. Magnetron MG/MD means the upper and lower magnetron, respectively.
Figure 6.
(a) The MSS-1 reactor. Stop-flow reactor, automatically operating, with a chamber of volume 250 mL, maximum operating pressure 4 MPa and temperature 240 °C; (b) The control panel; (c) Schematic presentation of the control system. Psp is the pressure set point. Magnetron MG/MD means the upper and lower magnetron, respectively.
An advanced control system allows for precise control of temperature, pressure, and the measurement of the reflected power. The control system of the device has two layers. The first one (
Figure 6b) is the layer of local regulation systems. The more important of the two is the process pressure regulator, in which hysteresis controlling is used, and ensures that the maximum speed of the batch pressure growth is maintained. The dynamics of the process is changeable. It depends mainly on the volume of the batch, its dielectric properties (temperature-related), and the level of the supplied microwave power. The hysteresis controller compensates for the changes in the dynamics at the level of pulsations, which is acceptable for the process. The second layer of the control system (
Figure 6c) concerns the superior control, whose objective is to set and monitor the process parameters, monitor the condition the device is in, and to record and read process data. The monitoring of pressure changes is much better due to the application of a quicker (than in the case of the controller in the sampling regulation layer) signal from the pressure sensor. The additional card of analogue to digital converter (ADC), (PCIe-6320, National Instruments), measures pressure with sampling speed 200,000 samplings/s. Such a high sampling speed ensures better analysis of the course of the process, particularly in the case of sudden pressure changes. The high-pressure valves and the system for product unload enable the chamber to be unloaded at any time during the process. Quick cooling of the product ejected from the reactor is another significant advantage.
MSS-2 is a batch reactor (
Figure 7). The pressure of reaction is up to 6 MPa, which is obtained through heating of the batch. However, operation at higher pressure (up to 10 MPa), obtained with the application of the suitable dosing pump or the external source of compressed air, is also possible. The allowed temperature of continuous operation is 270 °C. The capacity of the chamber is 410 mL. A unique structure of a process chamber, uncommon for other solutions of this type, was applied. The structure is closed on both sides with moveable plungers, which in consecutive stages of the process position the batch at the level of load slots of the microwave waveguide and chamber unload slots.
Figure 7.
The MSS-2 batch reactor. (a) MSS2 reactor general view; (b) Control panel and a process diagram; (c) Schematic presentation of the reactor operation: loading, processing, unloading.
Figure 7.
The MSS-2 batch reactor. (a) MSS2 reactor general view; (b) Control panel and a process diagram; (c) Schematic presentation of the reactor operation: loading, processing, unloading.
All the elements that are in contact with the substrate and the product are made of chemically resistant materials—PTFE (connection lines, head of the dosing pump, the middle part of the process chamber, seals) and Al
2O
3 ceramics (plungers, the top and bottom part of the process chamber). The reactor is cooled down with the forced airflow [
22].
The dimensions of the chamber were defined with the use of computer simulations (commercial software: QuickWave, QWED, Warsaw, Poland), so that the best adjustment of the chamber to the microwave generation path could be achieved, leakage of microwaves at the ends of the plungers prevented, and a homogenous arrangement of the electromagnetic field is ensured (
Figure 8).
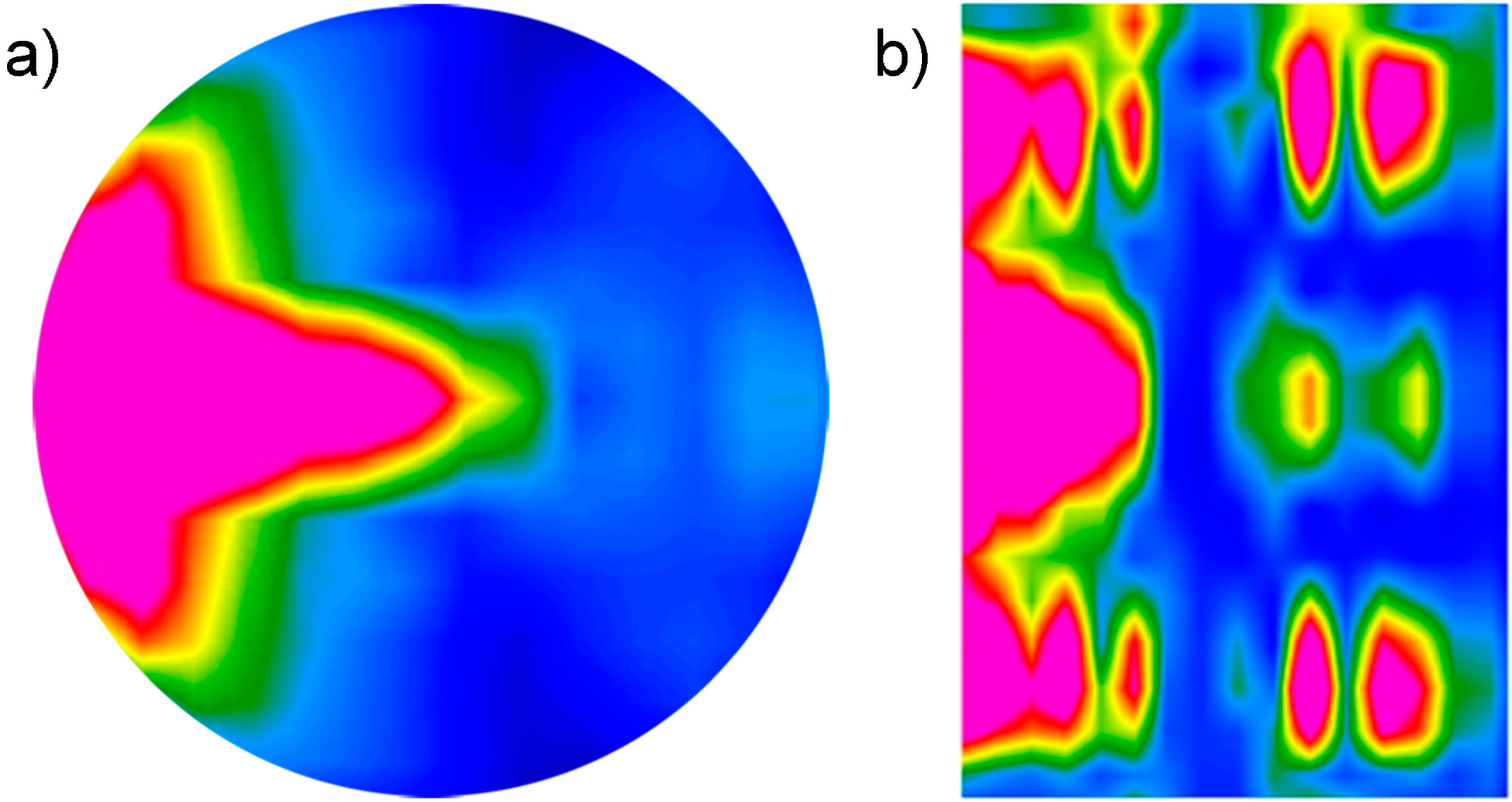
Figure 8.
Distribution of average bulk density of power losses within MSS‑2 reactor filled with water in the xy plane (a), and in (b) the xy section. Pink area shows more intense losses.
Figure 8.
Distribution of average bulk density of power losses within MSS‑2 reactor filled with water in the xy plane (a), and in (b) the xy section. Pink area shows more intense losses.
The microwave track of the device includes a generator with a 3 kW magnetron. The track is also equipped with two reeds located in the waveguide connecting the circulator with the process chamber, which—together with a system for the measurement of reflected power—allows for the system to be adjusted at the time of the process. The main tasks realised in the process control system include:
- -
the regulation of process parameters;
- -
the superior control over the processes;
- -
the monitoring and record of the processes;
- -
the control over the security and emergency states.
For the regulation of pressure (
Figure 9 and
Figure 10), due to the fact that the maximum speed of the pressure growth is required, a hysteresis controller, like in the case of the MSS‑1 reactor, was applied.
Figure 9.
Block diagram of pressure P regulation system and process temperature T determination.
Figure 9.
Block diagram of pressure P regulation system and process temperature T determination.
The superior process control is performed according to the PN-EN 61512 norm for the control of batch processes. In this control, the process takes place automatically with the use of a procedure (technological recipe) for single process phases, which is the input for the control PC. The process is divided into four phases: (1) chamber load; (2) process execution; (3) chamber unload; (4) chamber overflow.
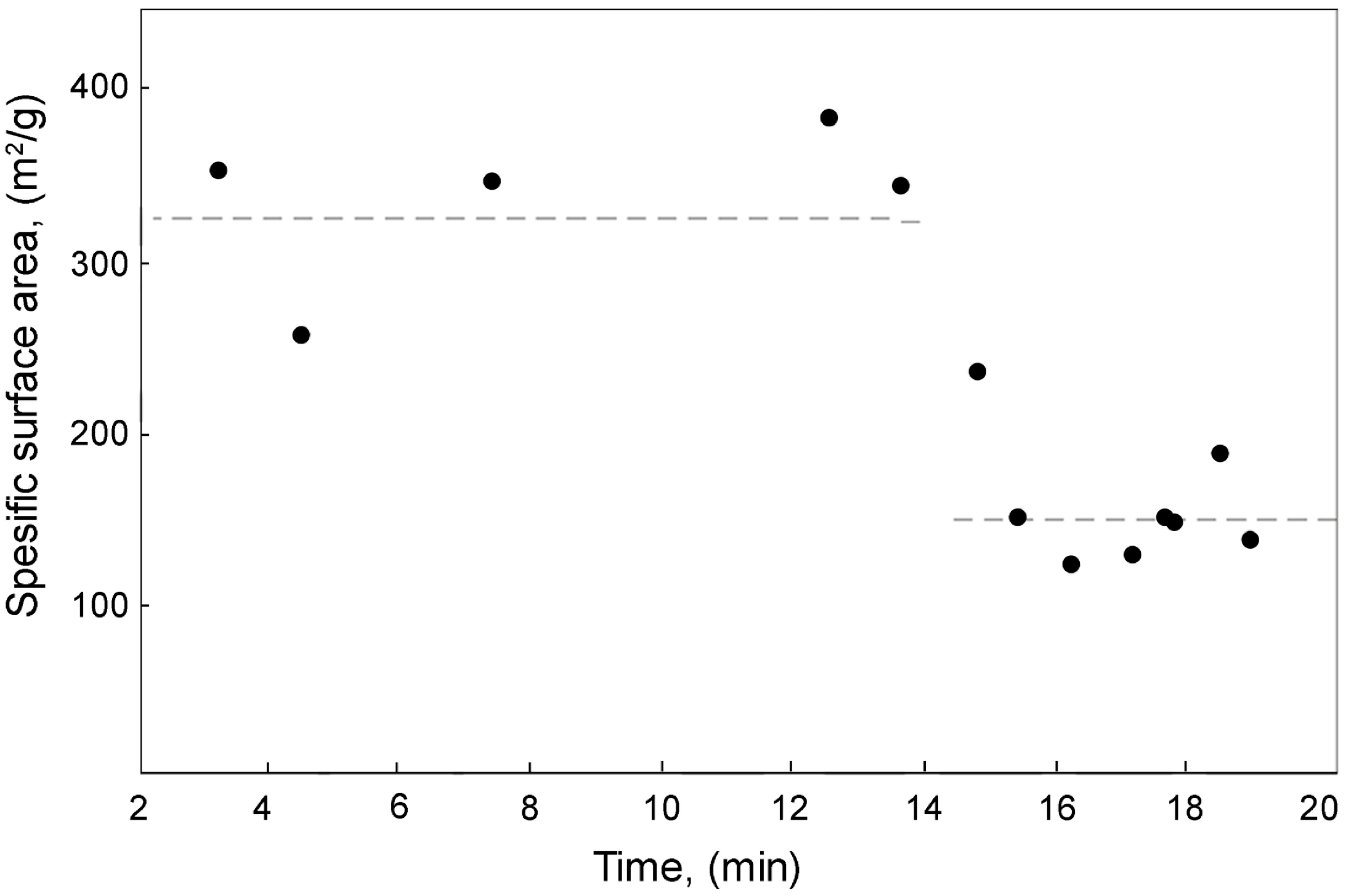
Figure 10.
Pressure in the chamber and average temperature from outside chamber, batch—deionised water 350 mL.
Figure 10.
Pressure in the chamber and average temperature from outside chamber, batch—deionised water 350 mL.
The main feature of the reactor is its high efficiency. The original architecture of the reactor allows for full automation of the process and the procurement of nanopowders in a significantly greater scale than in the case of the application of laboratory equipment. The device radically speeds up the entire process and makes it possible to obtain products measured in litres, not as in the case of laboratory apparatus, millilitres only. A significant advantage of the reactor concerns the high purity of the reaction process, which is obtained thanks to the application of chemically neutral materials, and low sensitivity to substrates of high density, including suspensions with sedimentation tendencies. The MSS‑2 reactor has a built-in system for the measurement of reflected power of microwave radiation. This enables the monitoring of changes in dielectric properties of reagents at the time of the process and helps to determine the dependencies between the absorption of the radiation and the state of reaction, and the on-line adjustment of the energy of the process chamber and the microwave generator. The control system enables automatic execution of processes with consideration of the aspect of the operator and environment safety, and makes it possible for the device to be easily included in a complex technological line.