Nanostructured Boron Nitride: From Molecular Design to Hydrogen Storage Application
Abstract
:1. Introduction
2. Results and Discussion
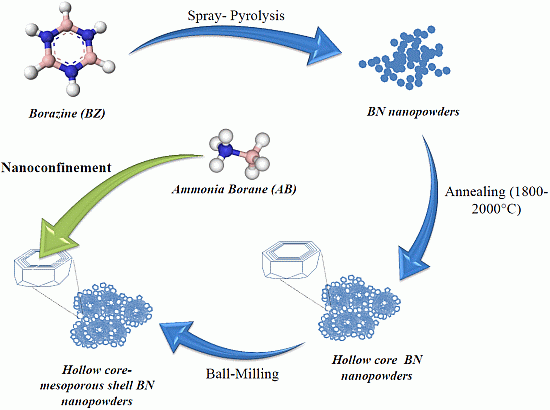
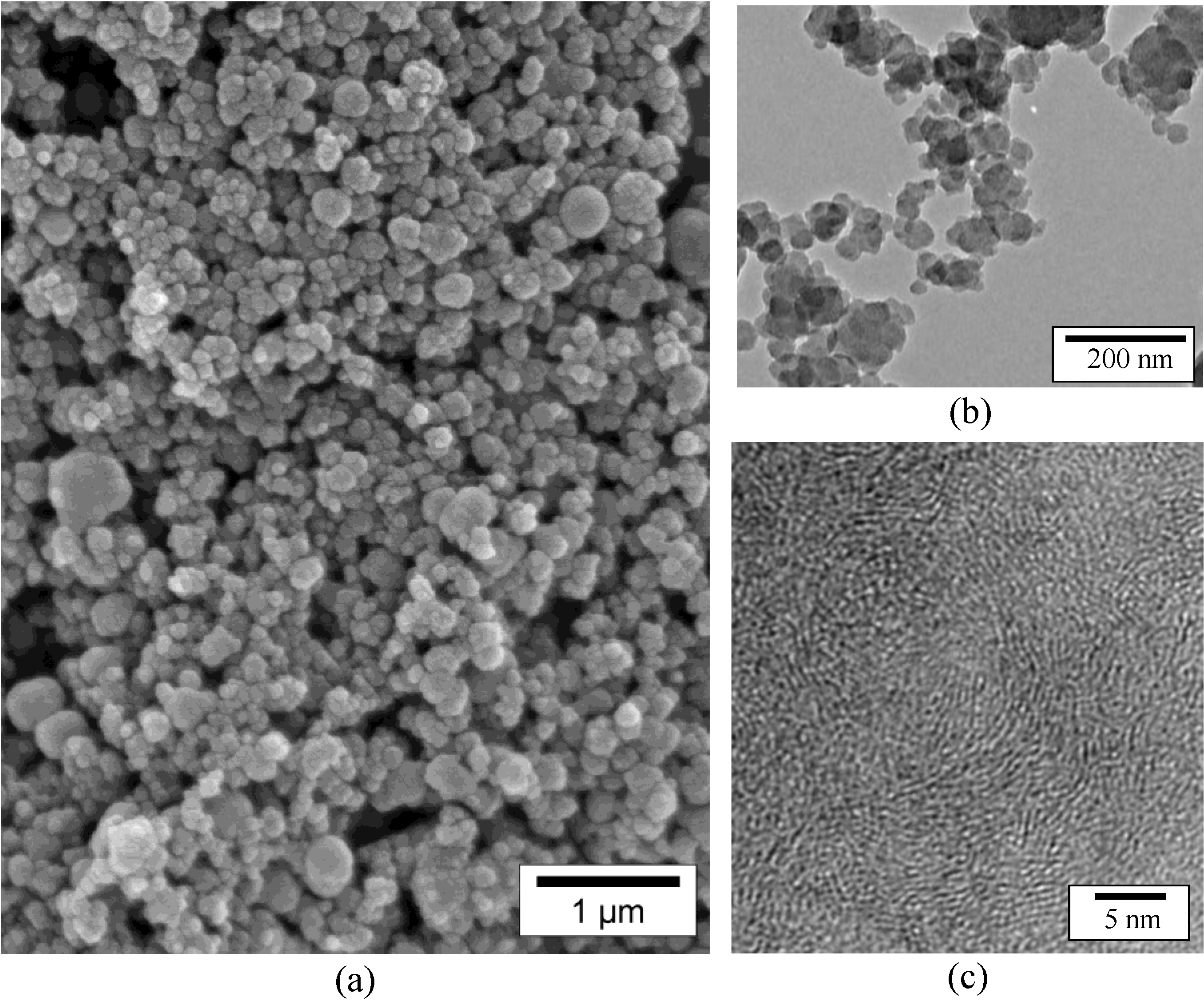
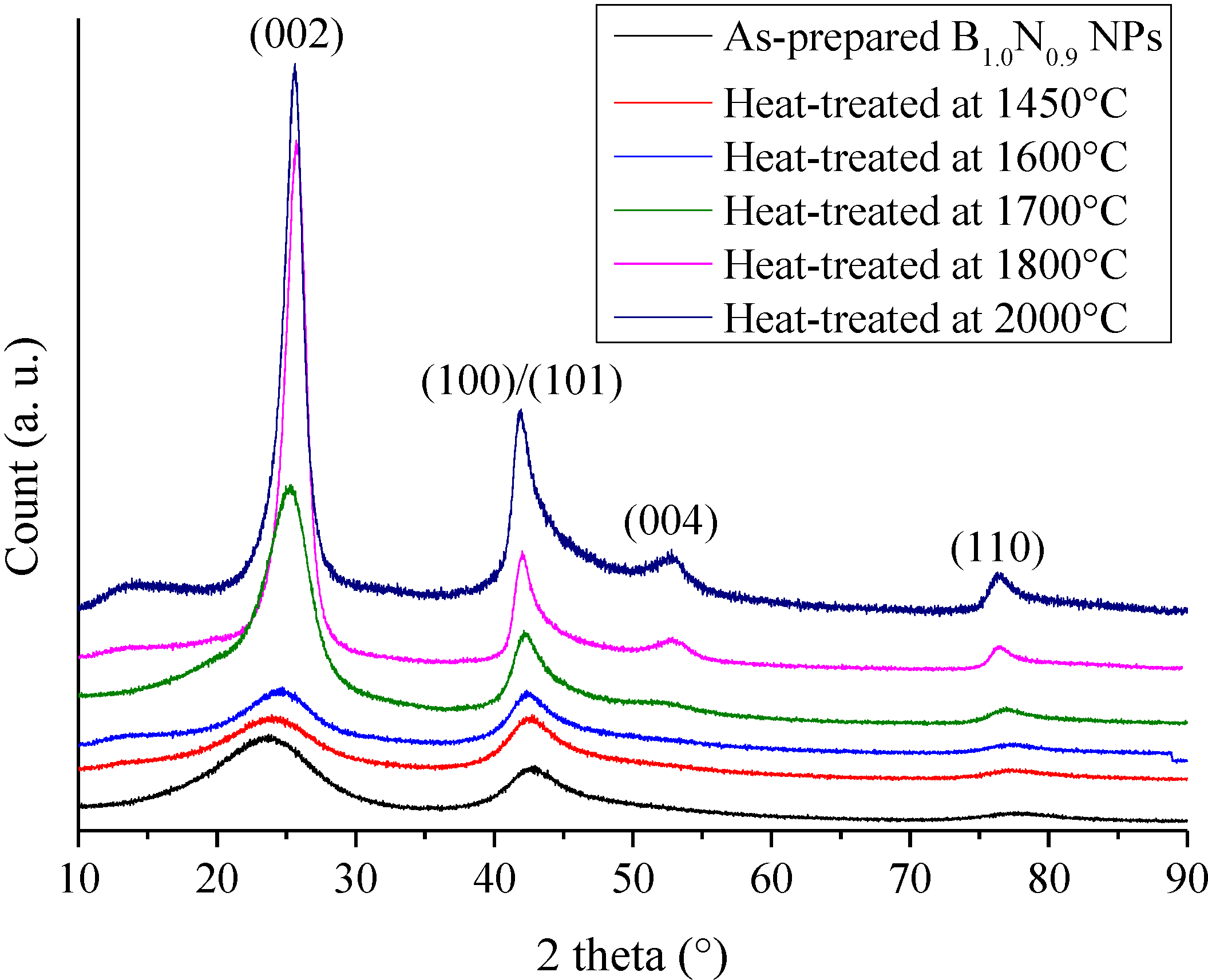
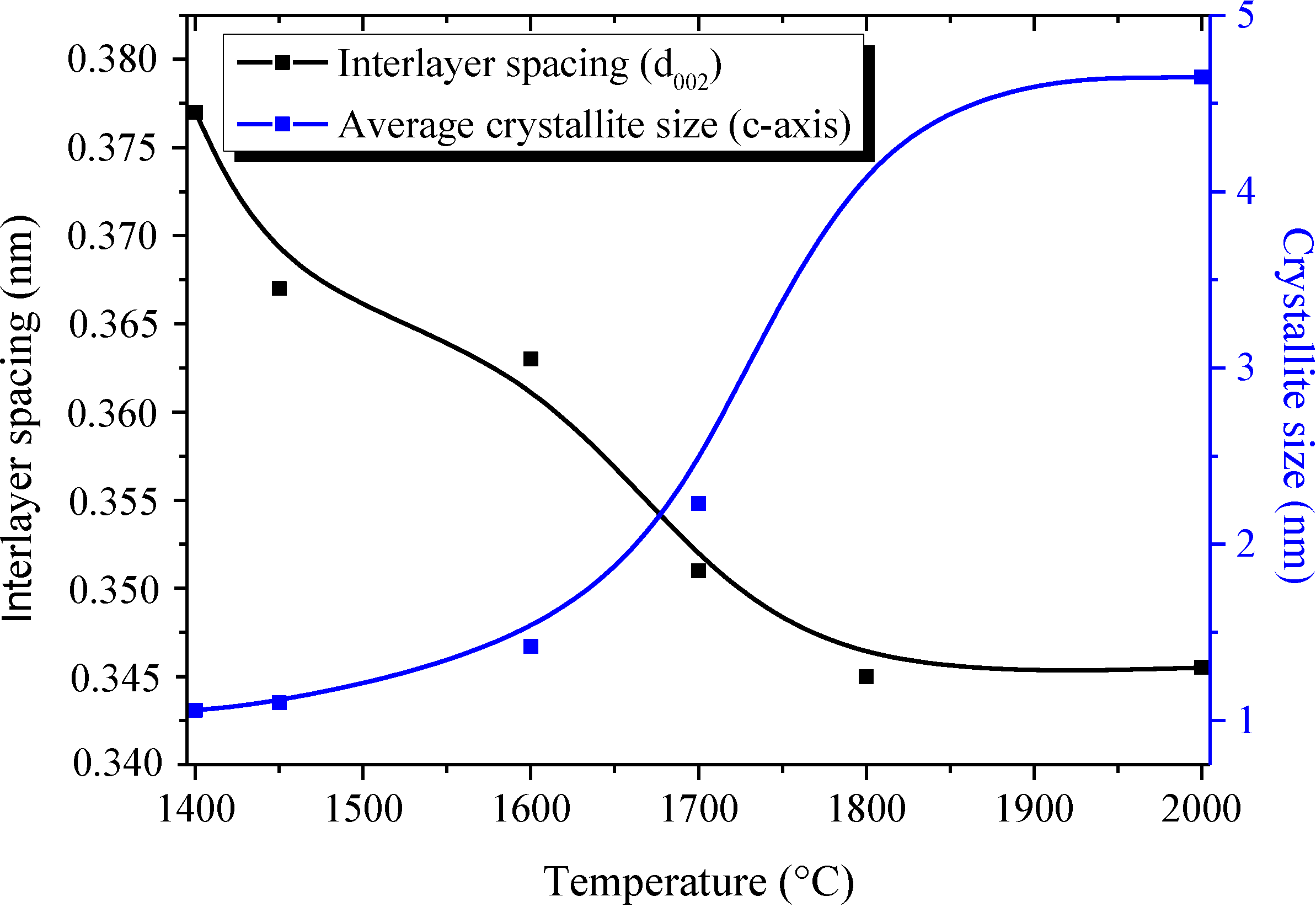
2.1. Borazine-Derived BN Nanoparticles
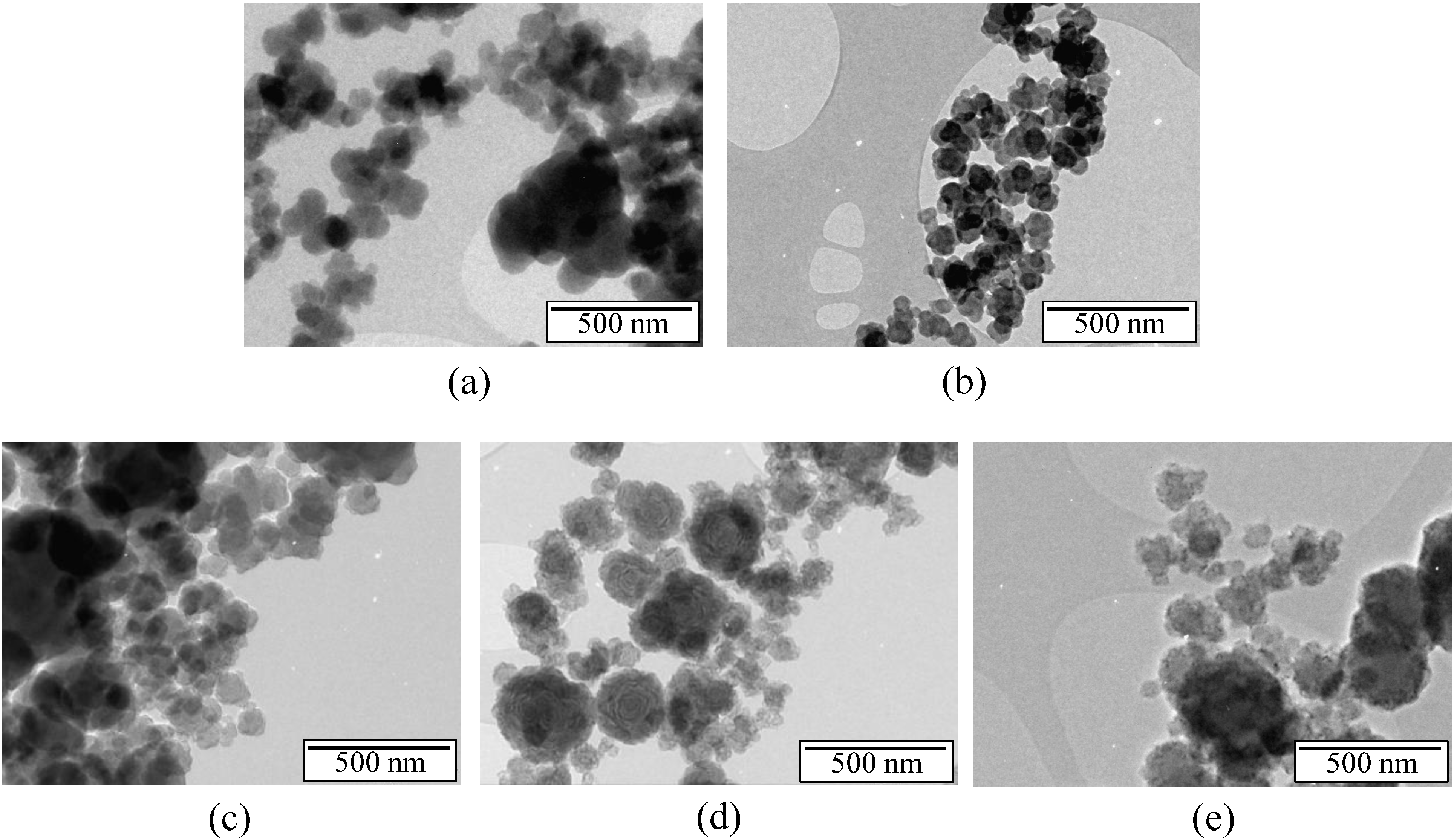
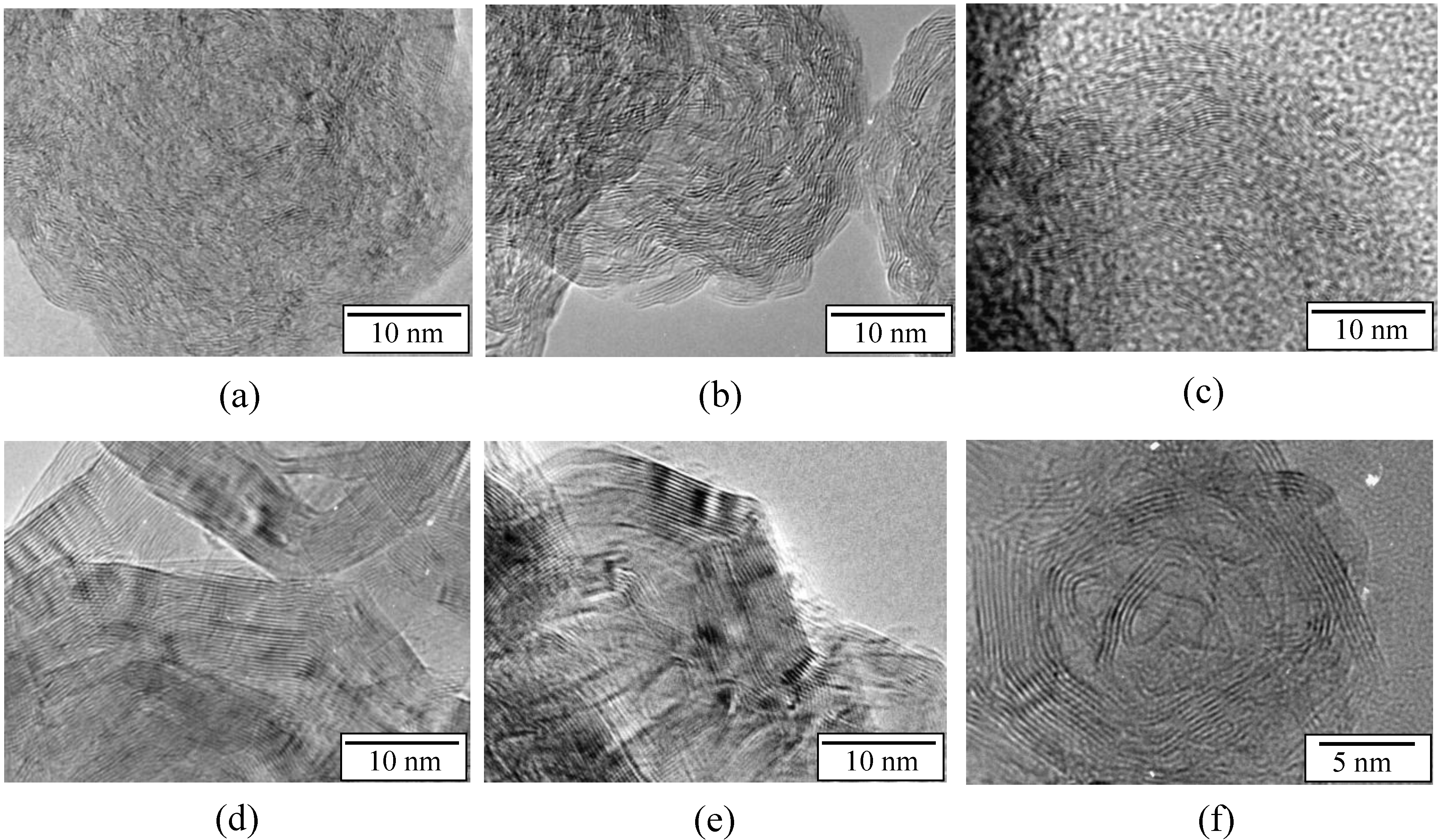
2.2. Hydrogen Storage Applications
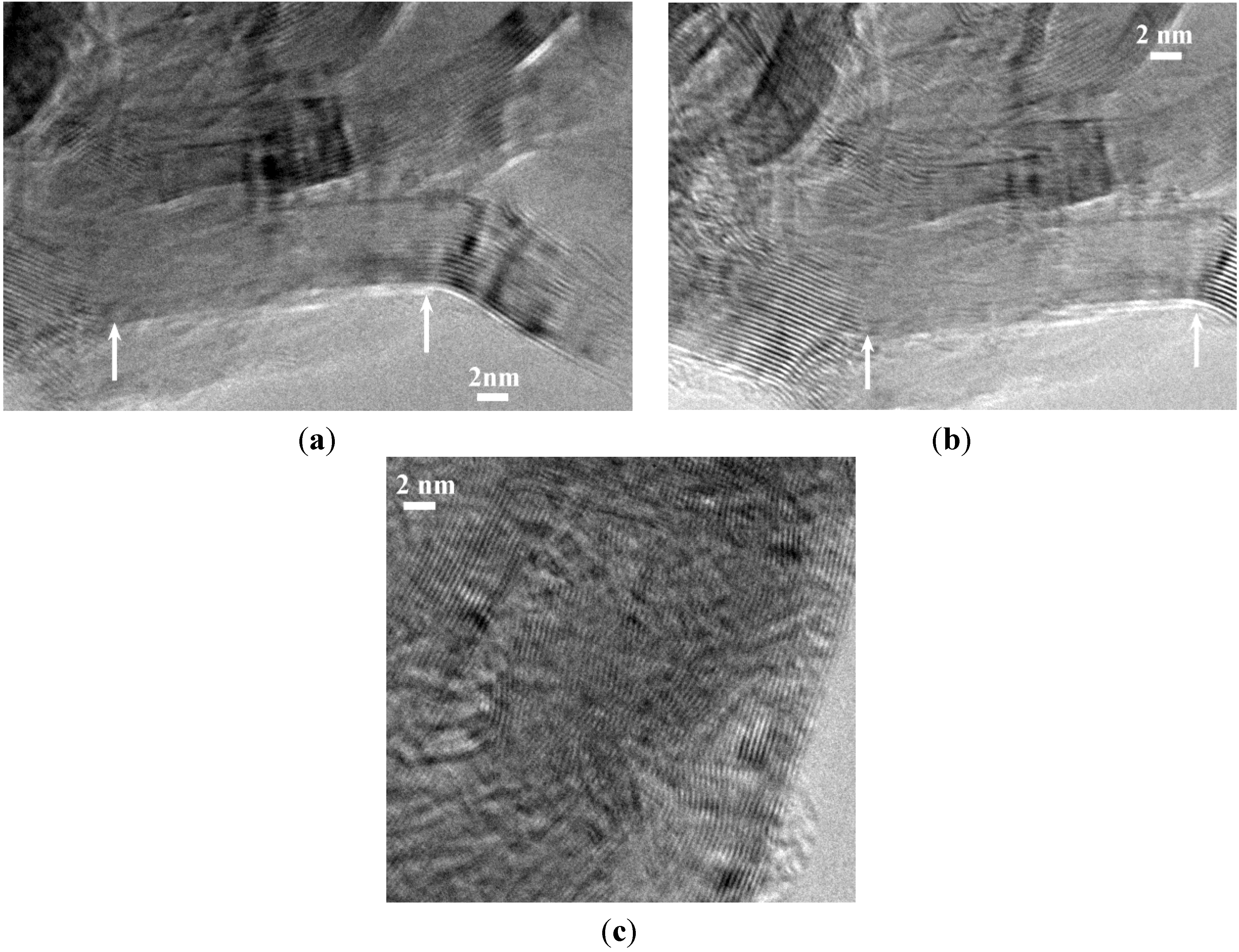
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paine, R.T.; Narula, C.K. Synthetic route to boron nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Wu, J.; Han, W.-Q.; Walukiewicz, W.; Ager, J.W., III; Shan, W.; Haller, E.E.; Zettl, A. Raman spectroscopy and time-resolved photoluminescence of BN and BxCyNz nanotubes. Nano Lett. 2004, 4, 647–650. [Google Scholar] [CrossRef]
- Watanabe, K.; Tanigushi, T.; Kanda, H. Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nat. Mater. 2004, 3, 404–409. [Google Scholar] [CrossRef]
- Kubota, Y.; Watanabe, K.; Tsuda, O.; Tanigushi, T. Deep ultraviolet light-emitting hexagonal boron nitride synthesized at atmospheric pressure. Science 2007, 317, 932–934. [Google Scholar] [CrossRef]
- Macnaughton, J.B.; Moewes, A.; Wilks, R.G.; Zhou, X.T.; Sham, T.K.; Tanigushi, T.; Watanabe, K.; Chan, C.Y.; Zhang, W.J.; Bello, I.; et al. Electronic structure of boron nitride single crystals and films. Phys. Rev. B 2005, 72, 195113:1–195113:8. [Google Scholar]
- Zhong, W.; Wang, S.; Li, J.; Bechelany, M.C.; Ghisleni, R.; Rossignol, F.; Balan, C.; Chartier, T.; Bernard, S.; Miele, P.; et al. Design of carbon fibre reinforced boron nitride matrix composites by vacuum-assisted polyborazylene transfer moulding and pyrolysis. J. Eur. Ceram. Soc. 2013, 33, 2979–2992. [Google Scholar] [CrossRef]
- Balmain, W.H. Bemerkungen über die Bildung von Verbindungen des Bors und Siliciums mit Stickstoff und gewissen Metallen. J. Prakt. Chem. 1842, 27, 422–430. (In German) [Google Scholar] [CrossRef]
- Lipp, A.; Schwetz, K.A.; Hunold, K. Hexagonal boron nitride: Fabrication, properties and applications. J. Eur. Ceram. Soc. 1989, 5, 3–9. [Google Scholar] [CrossRef]
- Lei, W.; Portehault, D.; Liu, D.; Qin, S.; Chen, Y. Porous boron nitride nanosheets for effective water cleaning. Nat. Commun. 2013, 4, 1777–1783. [Google Scholar] [CrossRef]
- Rousseas, M.; Goldstein, A.P.; Mickelson, W.; Worsley, M.A.; Woo, L.; Zettl, A. Synthesis of highly crystalline sp2-bonded boron nitride aerogels. ACS Nano 2013, 7, 8540–8546. [Google Scholar] [CrossRef]
- Li, J.; Xiao, X.; Xu, X.; Lin, J.; Huang, Y.; Xue, Y.; Jin, P.; Zou, J.; Tang, C. Activated boron nitride as an effective adsorbent for metal ions and organic polluants. Nature 2013, 3, 3208–3214. [Google Scholar]
- Weng, Q.; Wang, X.; Zhi, C.; Bando, Y.; Golberg, D. Boron nitride porous microbelts for hydrogen storage. ACS Nano 2013, 7, 1558–1565. [Google Scholar] [CrossRef]
- Siria, A.; Poncharal, P.; Biance, A.-L.; Fulcrand, R.; Blasé, X.; Purcell, S.T.; Bocquet, L. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 2013, 494, 455–458. [Google Scholar] [CrossRef]
- Bernard, S. Design, Processing and Properties of Ceramic Materials from Preceramic Precursors in Materials Science and Technologies; Nova Publishers: New York, NY, USA, 2012. [Google Scholar]
- Alauzun, J.G.; Ungureanu, S.; Brun, N.; Bernard, S.; Miele, P.; Backov, R.; Sanchez, C. Novel Monolith-type Boron Nitride Hierarchical Foams Obtained through Integrative Chemistry. J. Mater. Chem. 2011, 21, 14025–14030. [Google Scholar] [CrossRef]
- Termoss, H.; Toury, B.; Payan, S.; Brioude, A.; Bernard, S.; Cornu, D.; Vallette, S.; Benayoun, S.; Miele, P. Preparation of boron nitride-based coatings on metallic substrates via infrared irradiation of dip-coated polyborazylene. J. Mater. Chem. 2009, 19, 2671–2674. [Google Scholar] [CrossRef]
- Stock, A.; Pohland, E. Borwasserstoffe, IX.: B3N3H6. Ber. Dtsch. Chem. Ges. 1926, 59(B), 2215–2223. (In German) [Google Scholar] [CrossRef]
- Wideman, T.; Sneddon, L.G. Convenient procedures for the laboratory preparation of borazine. Inorg. Chem. 1995, 34, 1002–1003. [Google Scholar] [CrossRef]
- Salles, V.; Bernard, S.; Li, J.; Brioude, A.; Chehaidi, S.; Foucaud, S.; Miele, P. Design of Highly Dense Boron Nitride by the Combination of Spray-Pyrolysis of Borazine and Additive-free Sintering of Derived Ultrafine Powders. Chem. Mater. 2009, 21, 2920–2929. [Google Scholar] [CrossRef]
- Bernard, S.; Salles, V.; Li, J.; Brioude, A.; Bechelany, M.; Demirci, U.B.; Miele, P. High-Yield Synthesis of Hollow Boron Nitride Nano-Polyhedrons. J. Mater. Chem. 2011, 21, 8694–8699. [Google Scholar]
- Salles, V.; Bernard, S.; Chiriac, R.; Miele, P. Structural and Thermal Properties of Boron Nitride Nanoparticles. J. Eur. Ceram. Soc. 2012, 32, 1867–1871. [Google Scholar] [CrossRef]
- Moussa, G.; Demirci, U.B.; Malo, S.; Bernard, S.; Miele, P. Boron Nitride Nanopolyhedrons with hollow core@Mesoporous Shell structure: From Design to solid-state hydrogen storage application. J. Mater. Chem. A 2014, 2, 7717–7722. [Google Scholar] [CrossRef]
- Shore, S.G.; Parry, R.W. The crystalline compound ammonia boraone H3NBH3. J. Am. Chem. Soc. 1955, 77, 6084–6085. [Google Scholar] [CrossRef]
- Demirci, U.B.; Bernard, S.; Chiriac, R.; Toche, F.; Miele, P. Hydrogen release by thermolysis of ammonia borane NH3BH3 and then hydrolysis of its by-product [BNHx]. J. Power Sources 2011, 196, 279–286. [Google Scholar] [CrossRef]
- Moussa, G.; Bernard, S.; Demirci, U.B.; Chiriac, R.; Miele, P. Room-temperature hydrogen release from activated carbon-confined ammonia borane. Int. J. Hydr. Energy 2012, 37, 13437–13445. [Google Scholar] [CrossRef]
- Stephens, F.H.; Pons, V.; Baket, R.T. Ammonia-Borane: The Hydrogen Source par excellence. Dalton Trans. 2007, 2613–2626. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B–N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar]
- Sanyal, U.; Demirci, U.B.; Jagirdar, B.R.; Miele, P. Hydrolysis of ammonia borane as hydrogen source: Fundamental issues and potential solutions towards implementation. ChemSusChem. 2011, 4, 1731–1739. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 1079–1124. [Google Scholar]
- De Jong, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem. 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Gutowska, A.; Li, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold Mediates Hydrogen Release and the Reactivity of Ammonia Borane. Angew. Chem. Int. Ed. 2005, 44, 3578–3582. [Google Scholar]
- Gadipelli, S.; Ford, J.; Zhou, W.; Wu, H.; Udovic, T.J.; Yildirim, T. Nanoconfinement and catalytic dehydrogenation of ammonia borane by magnesium-metal-organic-framework-74. Chem. Eur. J. 2011, 17, 6043–6047. [Google Scholar]
- Srinivas, G.; Travis, W.; Ford, J.; Wu, H.; Guo, Z.-X.; Yildirim, T. Nanoconfined ammonia borane in a flexible metal–organic framework Fe–MIL-53: Clean hydrogen release with fast kinetics. J. Mater. Chem. A 2013, 1, 4167–4172. [Google Scholar] [CrossRef]
- Peng, Y.; Ben, T.; Jia, Y.; Yang, D.; Zhao, H.; Qiu, S.; Yao, X. Dehydrogenation of Ammonia Borane Confined by Low-Density Porous Aromatic Framework. J. Phys. Chem. C 2012, 116, 25694. [Google Scholar]
- Sutton, A.D.; Burrell, A.K.; Dixon, D.A.; Garner, E.B., III; Gordon, J.C.; Nakagawa, T.; Ott, K.C.; Robinson, J.P.; Vasiliu, M. Regeneration of ammonia borane spent fuel by direct reaction with hydrazine and liquid ammonia. Science 2011, 331, 1426–1429. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moussa, G.; Salameh, C.; Bruma, A.; Malo, S.; Demirci, U.B.; Bernard, S.; Miele, P. Nanostructured Boron Nitride: From Molecular Design to Hydrogen Storage Application. Inorganics 2014, 2, 396-409. https://doi.org/10.3390/inorganics2030396
Moussa G, Salameh C, Bruma A, Malo S, Demirci UB, Bernard S, Miele P. Nanostructured Boron Nitride: From Molecular Design to Hydrogen Storage Application. Inorganics. 2014; 2(3):396-409. https://doi.org/10.3390/inorganics2030396
Chicago/Turabian StyleMoussa, Georges, Chrystelle Salameh, Alina Bruma, Sylvie Malo, Umit B. Demirci, Samuel Bernard, and Philippe Miele. 2014. "Nanostructured Boron Nitride: From Molecular Design to Hydrogen Storage Application" Inorganics 2, no. 3: 396-409. https://doi.org/10.3390/inorganics2030396