Direct Formation of ZIF-8 Crystal Thin Films on the Surface of a Zinc Ion-Doped Polymer Substrate
Abstract
:1. Introduction
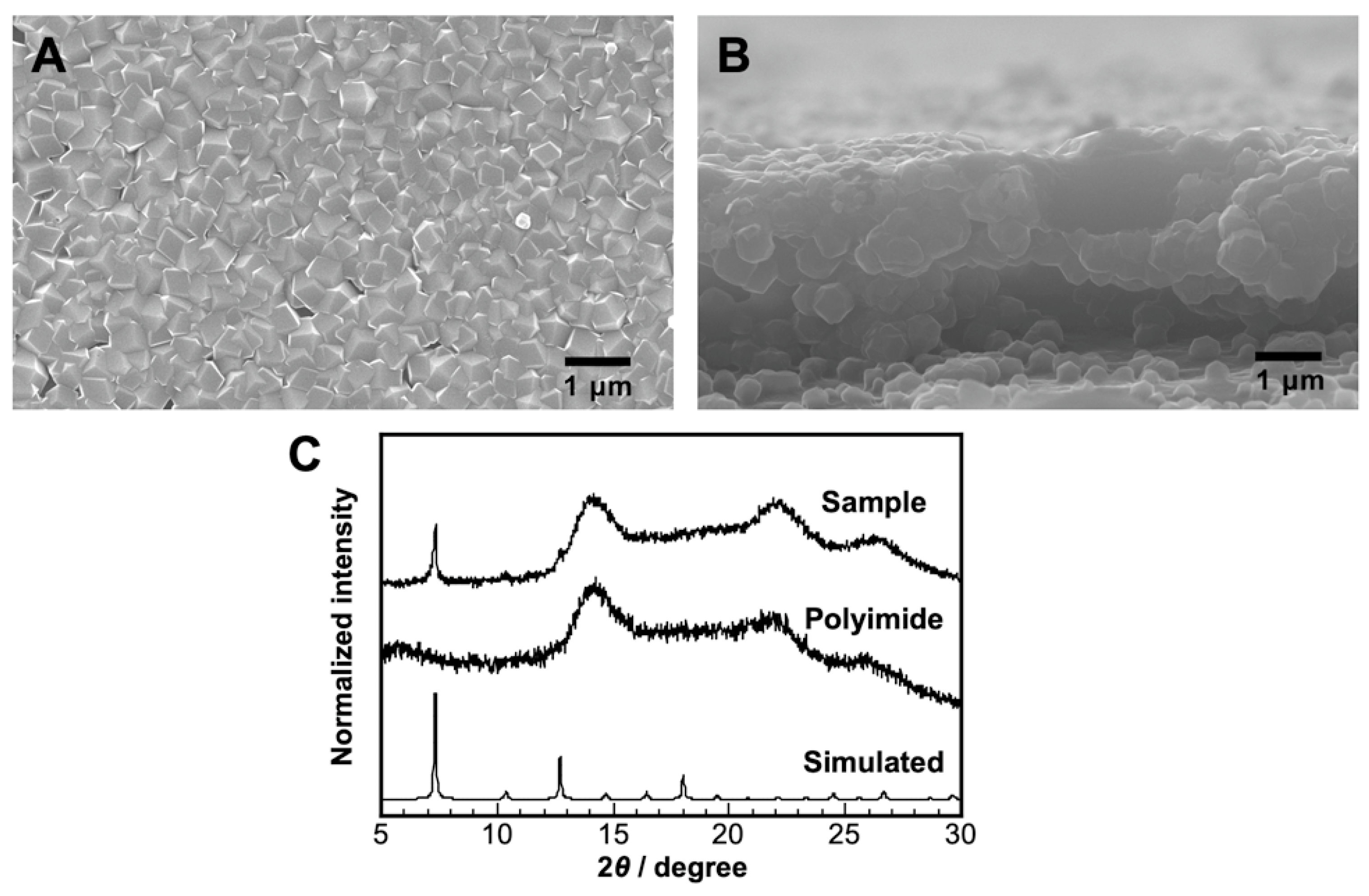
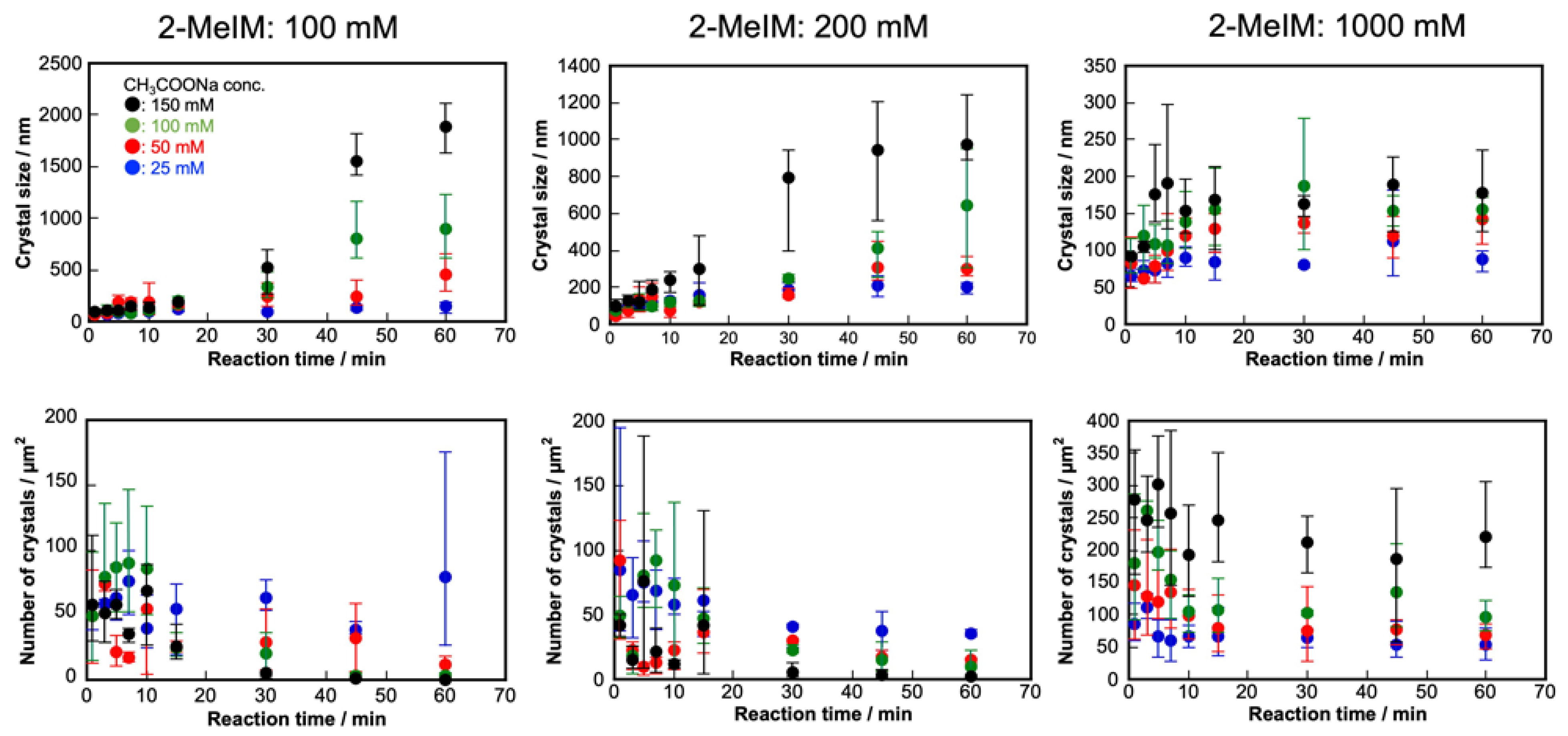
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Framework. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F. Crystallized Frameworks with Giant Pores: Are There Limits to the Possible? Acc. Chem. Res. 2005, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Jaheon, K. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent Advances in Gas Storage and Separation using Metal-Organic Frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Wei, Z.; Zhang, Z.; Xiang, S.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: Status and Challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Boyd, P.G.; Chidambaram, A.; García-Díez, E.; Ireland, C.P.; Daff, T.D.; Bounds, R.; Gładysiak, A.; Schouwink, P.; Moosavi, S.M.; Maroto-Valer, M.M.; et al. Data-Driven Design of Metal-Organic Frameworks for Wet Flue Gas CO2 Capture. Nature 2019, 576, 253–256. [Google Scholar] [CrossRef]
- Karim, A.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/Vapor Separation using Ultra-Microporous Metal-Organic Frameworks: Insights into the Structure/Separation Relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal-Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Lin, R.-B.; Xiang, S.; Wei, Z.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Liang, J.; Wang, X.-S.; Cao, R. Multifunctional Metal-Organic Framework Catalysts: Synergistic Catalysis and Tandem Reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. Sate of the Art and Prospects in Metal-Organic Framework (MOF)-based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-D.; Jiang, H.-L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, Z.; Yang, X.; Zhou, H.-C. Luminescent Sensors based on Metal-Organic Frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional Metal-Organic Frameworks as Effective Sensors of Gases and Volatile Compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Senthil, K.P.; Rouhi, J.; Baghayeri, M. A Novel Detection Method for Organophosphorus Insecticide Fenamiphos: Molecularly Imprinted Electrochemical Sensor Based on Core-Shell Co3O4@MOF-74 Nanocomposite. J. Colloid. Int. Sci. 2021, 592, 175–185. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, W.; Jiao, Y.; Xu, Y.; Lin, H. Metal-Phenolic Network as Precursor for Fabrication of Metal-Organic Framework (MOF) Nanofiltration Membrane for Efficient Desalination. J. Membr. Sci. 2021, 624, 119101. [Google Scholar] [CrossRef]
- He, S.; Zhu, B.; Jiang, X.; Han, G.; Li, S.; Lau, C.H.; Wu, Y.; Zhang, Y.; Shao, L. Symbiosis-inspired De Novo Synthesis of Ultrahigh MOF Growth Mixed Matrix Membranes For Sustainable Carbon Capture. Proc. Natl. Acad. Sci. USA 2022, 119, e2114964119. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhao, W.; Dou, H.; Zhao, X.; Liu, Y.; Zhang, B.; Yang, X. Defect-Free Metal-Organic Framework Membrane for Precise Ion/Solvent Separation toward Highly Stable Magnesium Metal Anode. Adv. Mater. 2022, 34, 2108114. [Google Scholar] [CrossRef]
- Xu, L.-H.; Li, S.-H.; Mao, H.; Li, Y.; Zhang, A.-S.; Wang, S.; Liu, W.-M.; Lv, J.; Wang, T.; Cai, W.-W.; et al. Highly Flexible and Superhydrophobic MOF Nanosheet Membrane for Ultrafast Alcohol-Water Separation. Science 2022, 378, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Peng, Y.; Yao, R.; Song, H.; Zhu, C.; Yang, W. Flexible Soft-Solid Metal-Organic Framework Composite Membranes for H2/CO2 Separation. Angew. Chem. Int. Ed. 2022, 61, e202117577. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, F.; Wei, Q.; Wang, N.; Qin, X.; Zhang, S.; Wang, B.; Nie, Z.; Ji, S.; Yan, H.; et al. Oriented Nano-Microstructure-Assisted Controllable Fabrication of Metal-Organic Framework Membranes on Nickel Foam. Adv. Mater. 2016, 28, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, M.; Strauss, I.; Mundstock, A.; Meng, H.; Caro, J. MOF-in-COF Molecular Sieving Membrane for Selective Hydrogen Separation. Nat. Commun. 2021, 12, 38. [Google Scholar] [CrossRef]
- Chen, L.-W.; Hao, Y.-C.; Guo, Y.; Zhang, Q.; Li, J.; Gao, W.-Y.; Ren, L.; Su, X.; Hu, L.; Zhang, N.; et al. Metal-Organic Framework Membranes Encapsulating Gold Nanoparticles for Direct Plasmonic Photocatalytic Nitrogen Fixation. J. Am. Chem. Soc. 2021, 143, 5727–5736. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, F.; Chen, F.; Luan, T.-X.; Li, P.; Xu, S.; Gao, J. A Zr-MOF Nanoflower Sensor and Its Mixed-Matric Membrane for the Highly Sensitive Detection of Nitroaromatics. J. Mater. Chem. C 2022, 10, 7469–7475. [Google Scholar] [CrossRef]
- Mohammed, Y.A.Y.A.; Abdel-Mohsen, A.M.; Zhang, Q.-J.; Younas, M.; Zhong, L.-B.; Yang, J.-C.E.; Zheng, Y.-M. Facile Synthesis of ZIF-8 Incorporated Electrospun PAN/PEI Nanofibrous Composite Membrane for Efficient Cr(VI) Adsorption from Water. Chem Eng. J. 2023, 461, 141972. [Google Scholar] [CrossRef]
- Lin, Y.; Li, W.-H.; Wen, Y.; Wang, G.-E.; Ye, X.-L.; Xu, G. Layer-by-Layer Growth of Preferred-Oriented MOF Thin Film on Nanowire Array for High-Performance Chemiresistive Sensing. Angew. Chem. Int. Ed. 2021, 60, 25758–25761. [Google Scholar] [CrossRef]
- Goswami, S.; Rimoldi, M.; Anderson, R.; Lee, C.; Li, X.; Li, A.; Deria, P.; Chen, L.X.; Schaller, R.D.; Gómez-Gualdrón, D.A.; et al. Toward Ideal Metal-Organic Framework Thin-Film Growth via Automated Layer-by-Layer Deposition: Examples Based on Perylene Diimide Linkers. Chem. Mater. 2022, 34, 9446–9454. [Google Scholar] [CrossRef]
- Zheng, R.; Fu, Z.-H.; Deng, W.-H.; Wen, Y.; Wu, A.-Q.; Ye, X.-L.; Xu, G. The Growth Mechanism of a Conductive MOF Thin Film in Spray-based Layer-by-Layer Liquid Phase Epitaxy. Angew. Chem. Int. Ed. 2022, 61, e202212797. [Google Scholar] [CrossRef]
- Yao, J.; Dong, D.; Li, D.; He, L.; Xu, G.; Wang, H. Contra-Diffusion Synthesis of ZIF-8 Films on a Polymer Substrate. Chem. Commun. 2011, 47, 2559–2561. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Okada, K.; Hara, T.; Ikigaki, K.; Tokudome, Y.; Thornton, A.W.; Hill, A.J.; Williams, T.; Doonan, C.; Takahashi, M. Centimetre scale micropore alignment in oriented polycrystalline Metal-Organic Framework films via heteroepitaxial growth. Nat. Mater. 2017, 16, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ikigaki, K.; Okada, K.; Tokudome, Y.; Toyao, T.; Falcaro, P.; Doonan, C.; Takahashi, M. Oriented growth of multiple layered thin films of metal-organic frameworks (MOF-on-MOF). Angew. Chem. Int. Ed. 2019, 58, 6886–6890. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, T.; Kumano, M.; Mantani, K.; Matsuyama, T.; Miyanaga, A.; Ohhashi, T.; Takashima, Y.; Minami, H.; Suzuki, T.; Imagawa, K.; et al. Interfacial Synthetic Approach for Constructing Metal-Organic Framework Crystals Using Metal Ion-Doped Polymer Substrate. Cryst. Growth Des. 2016, 16, 2472–2476. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Mantani, K.; Miyanaga, A.; Matsuyama, T.; Ohhashi, T.; Takashima, Y.; Akamatsu, K. Morphology Control of Metal-Organic Frameworks Based on Paddle-Wheel Units on Ion-Doped Polymer Substrate Using An Interfacial Growth Approach. Langmuir 2016, 32, 6068–6073. [Google Scholar] [CrossRef] [PubMed]
- Ohhashi, T.; Tsuruoka, T.; Fujimoto, S.; Takashima, Y.; Akamatsu, K. Controlling the Orientation of Metal-Organic Framework Crystals by an Interfacial Growth Approach Using a Metal Ion-Doped Polymer Substrate. Cryst. Growth Des. 2018, 18, 402–408. [Google Scholar] [CrossRef]
- Ohhashi, T.; Tsuruoka, T.; Takashima, Y.; Akamatsu, K. Control of the Nucleation and Growth Processes of Metal-Organic Frameworks Using a Metal Ion-Doped Polymer Substrate for the Construction of Continuous Films. CrystEngComm 2019, 21, 4851–4854. [Google Scholar] [CrossRef]
- Reboul, J.; Furukawa, S.; Horike, N.; Tsotsalas, M.; Hirai, K.; Uehara, H.; Kondo, M.; Louvain, N.; Sakata, O.; Kitagawa, S. Mesoscopic Architectures of Porous Coordination Polymers Fabricated by Pseudomorphic Replication. Nat. Mater. 2012, 11, 717–723. [Google Scholar] [CrossRef]
- Okada, K.; Ricco, R.; Tokudome, Y.; Styles, M.J.; Hill, A.J.; Takahashi, M.; Falcaro, P. Copper Conversion into Cu(OH)2 Nanotubes for Positioning Cu3(BTC)2 MOF Crystals; Controlling the Growth on Flat Plates, 3D Architectures, and as Patterns. Adv. Funct. Mater. 2014, 24, 1969–1977. [Google Scholar] [CrossRef]
- Ma, K.; Wang, Y.; Chen, Z.; Islamoglu, T.; Lai, C.; Wang, X.; Fei, B.; Farha, O.K.; Xin, J.H. Facile and Scalable Coating of Metal-Organic Frameworks on Fibrous Substrates by a Coordination Replication Method at Room Temperature. ACS Appl. Mater. Interfaces 2019, 11, 22714–22721. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jia, X.; Jin, Y.; Li, Y.; Wang, S.; Song, H. One-Pot Synthesis of Core–Shell ZIF-8@ZnO Porous Nanospheres with Improved Ethanol Gas Sensing. J. Mater. Sci. Mater. Electron 2020, 31, 22534–22545. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Liu, Z.; An, Y.; Zhong, Y.; Hu, Z.; Li, S.; Chen, Z.; Wang, S.; Sheng, X.; et al. Eu-Doped Zeolitic Imidazolate Framework-8 Modified Mixed-Crystal TiO2 for Efficient Removal of Basic Fuchsin from Effluent. Materials 2021, 14, 7265. [Google Scholar] [CrossRef]
- Jnido, G.; Ohms, G.; Viöl, W. Deposition of Zinc Oxide Coatings on Wood Surfaces Using the Solution Precursor Plasma Spraying Process. Coatings 2021, 11, 183. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, L.; Kim, E.J.; Hahn, S.H. Electronic Structure and Optical Properties of Zn(OH)2: LDA+U Calculations and Intense Yellow Luminescence. RSC Adv. 2015, 5, 87496–87503. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuruoka, T.; Araki, K.; Kawauchi, K.; Takashima, Y.; Akamatsu, K. Direct Formation of ZIF-8 Crystal Thin Films on the Surface of a Zinc Ion-Doped Polymer Substrate. Inorganics 2024, 12, 21. https://doi.org/10.3390/inorganics12010021
Tsuruoka T, Araki K, Kawauchi K, Takashima Y, Akamatsu K. Direct Formation of ZIF-8 Crystal Thin Films on the Surface of a Zinc Ion-Doped Polymer Substrate. Inorganics. 2024; 12(1):21. https://doi.org/10.3390/inorganics12010021
Chicago/Turabian StyleTsuruoka, Takaaki, Kaito Araki, Kouga Kawauchi, Yohei Takashima, and Kensuke Akamatsu. 2024. "Direct Formation of ZIF-8 Crystal Thin Films on the Surface of a Zinc Ion-Doped Polymer Substrate" Inorganics 12, no. 1: 21. https://doi.org/10.3390/inorganics12010021





