Synthesis, Structural, Thermal, and Hirshfeld Surface Analysis of In(III) Tris (N-Methyl-N-Phenyl Dithiocarbamate)
Abstract
:1. Introduction
2. Results and Discussion
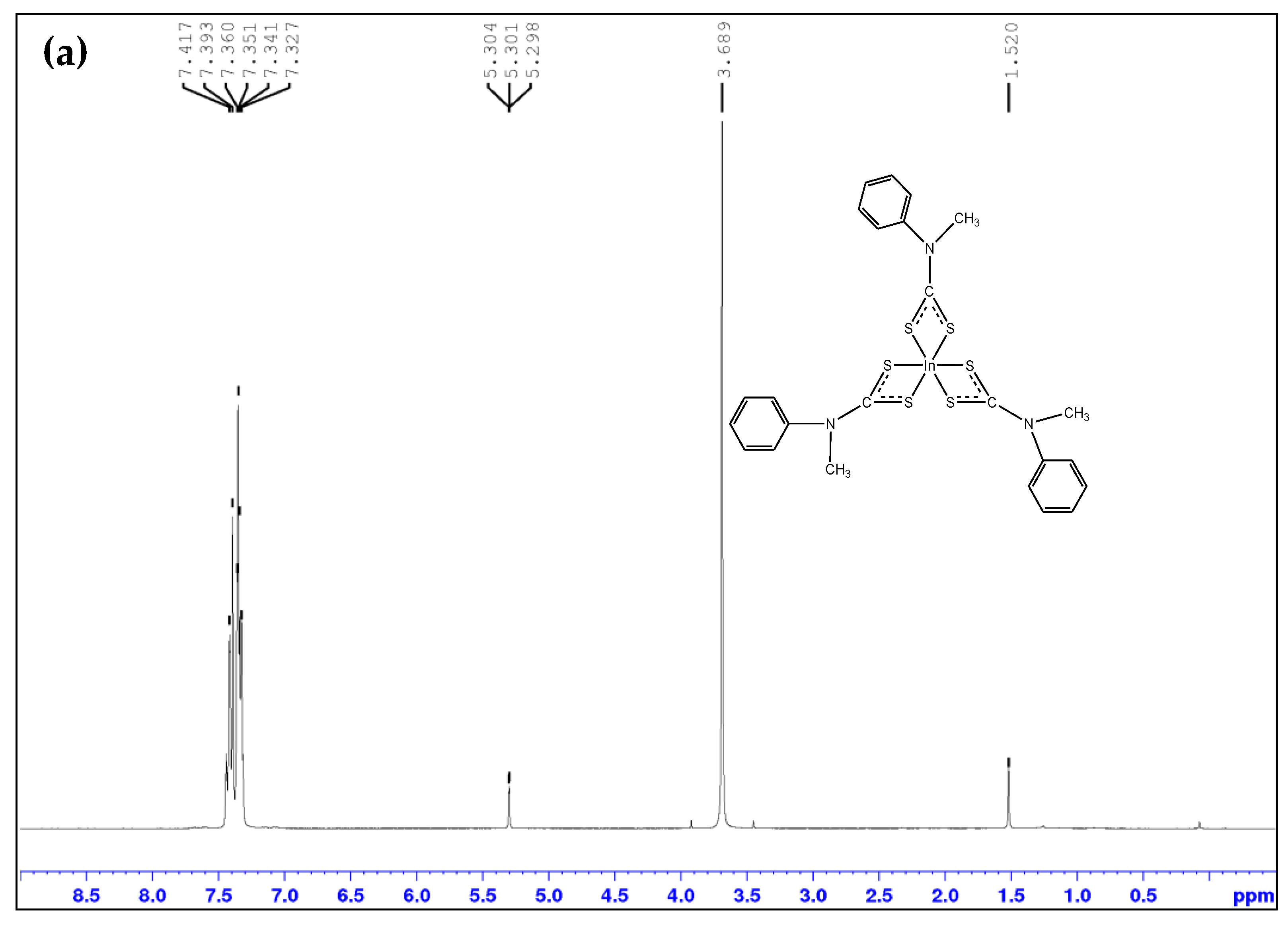
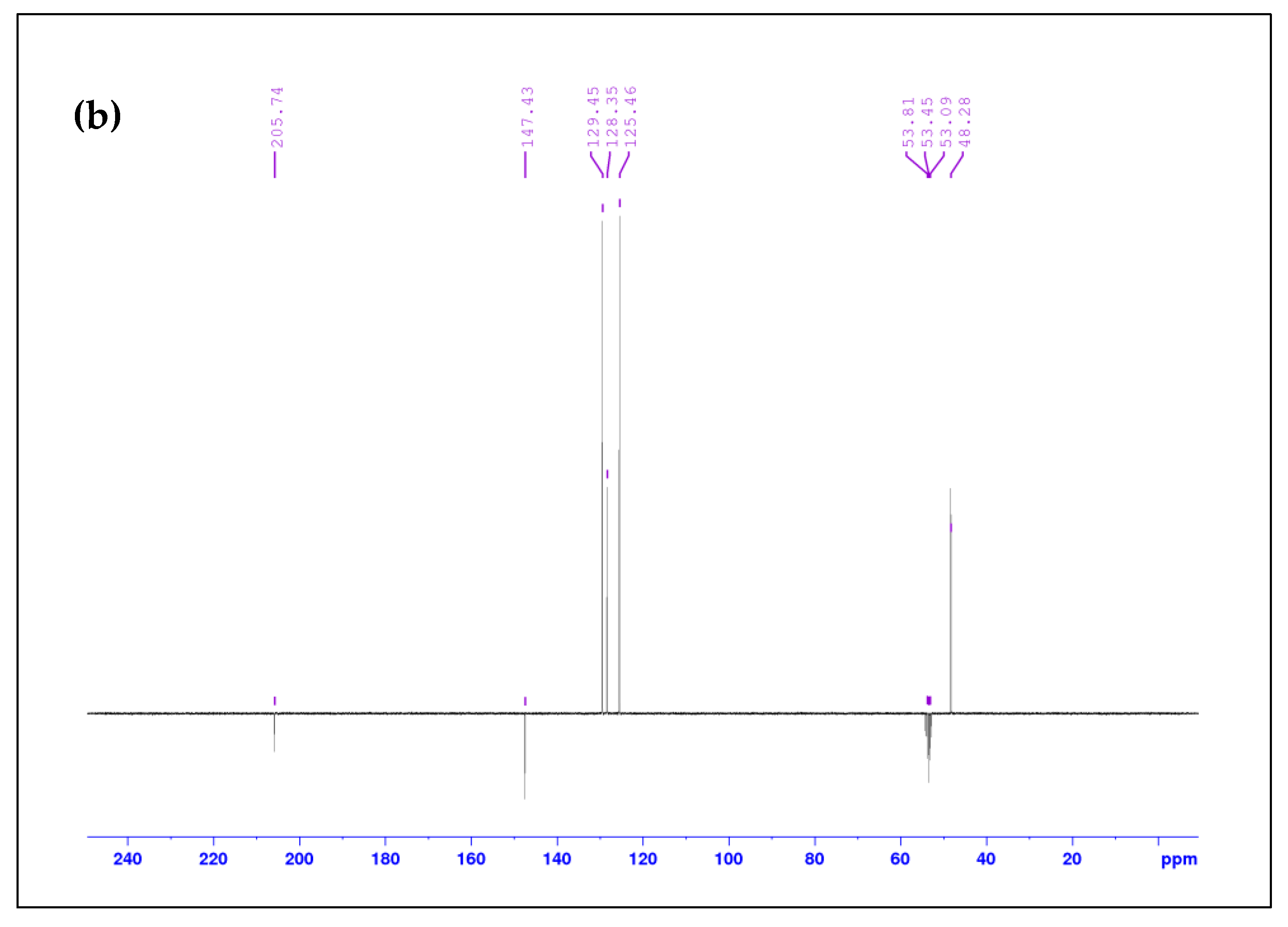
2.1. Spectroscopic Analysis
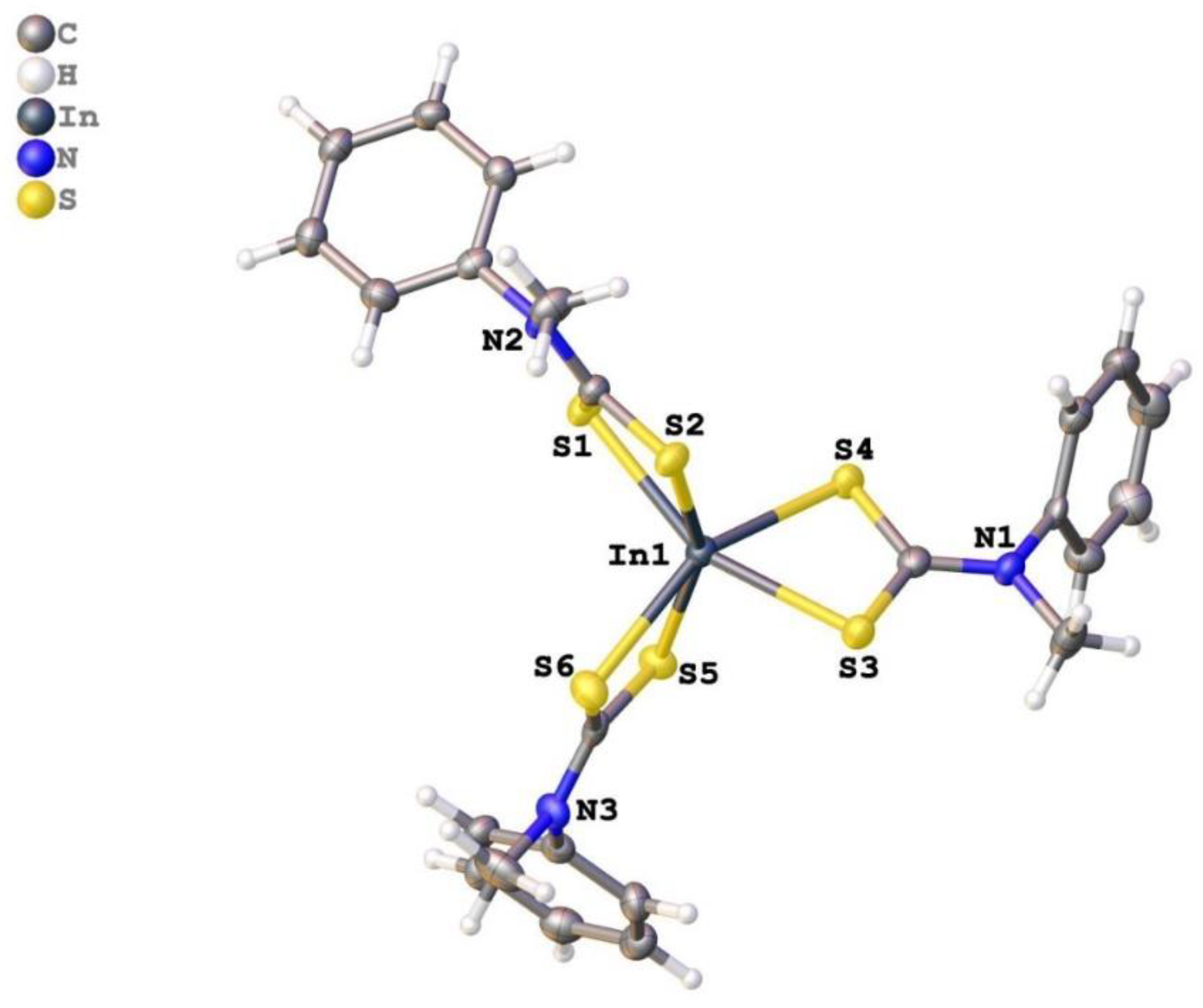
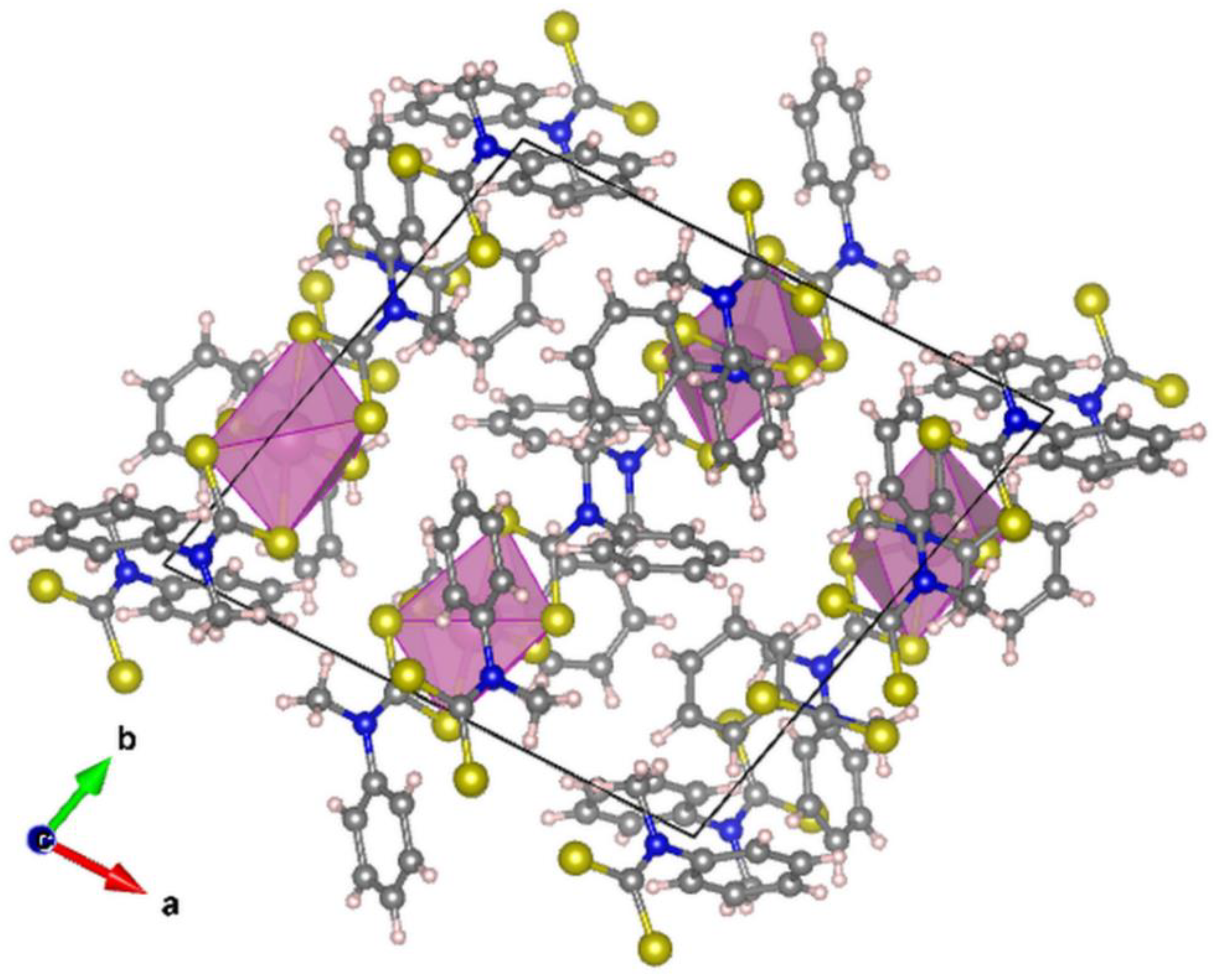
2.2. Crystal Structure Description
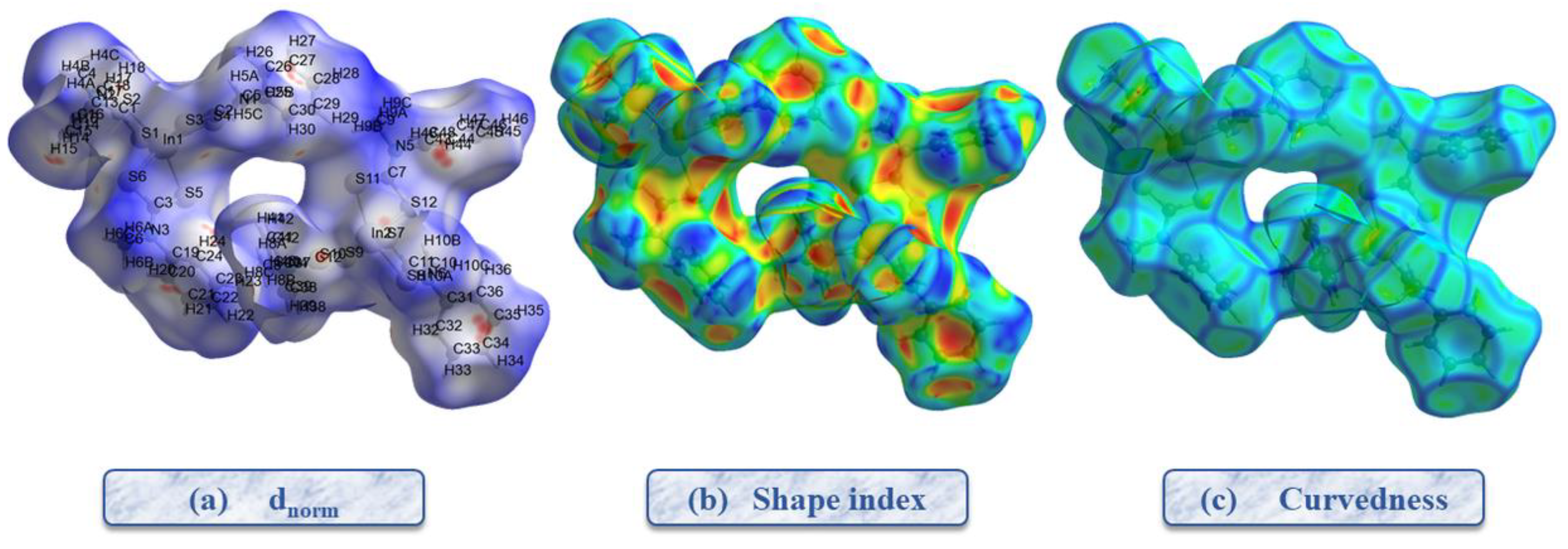
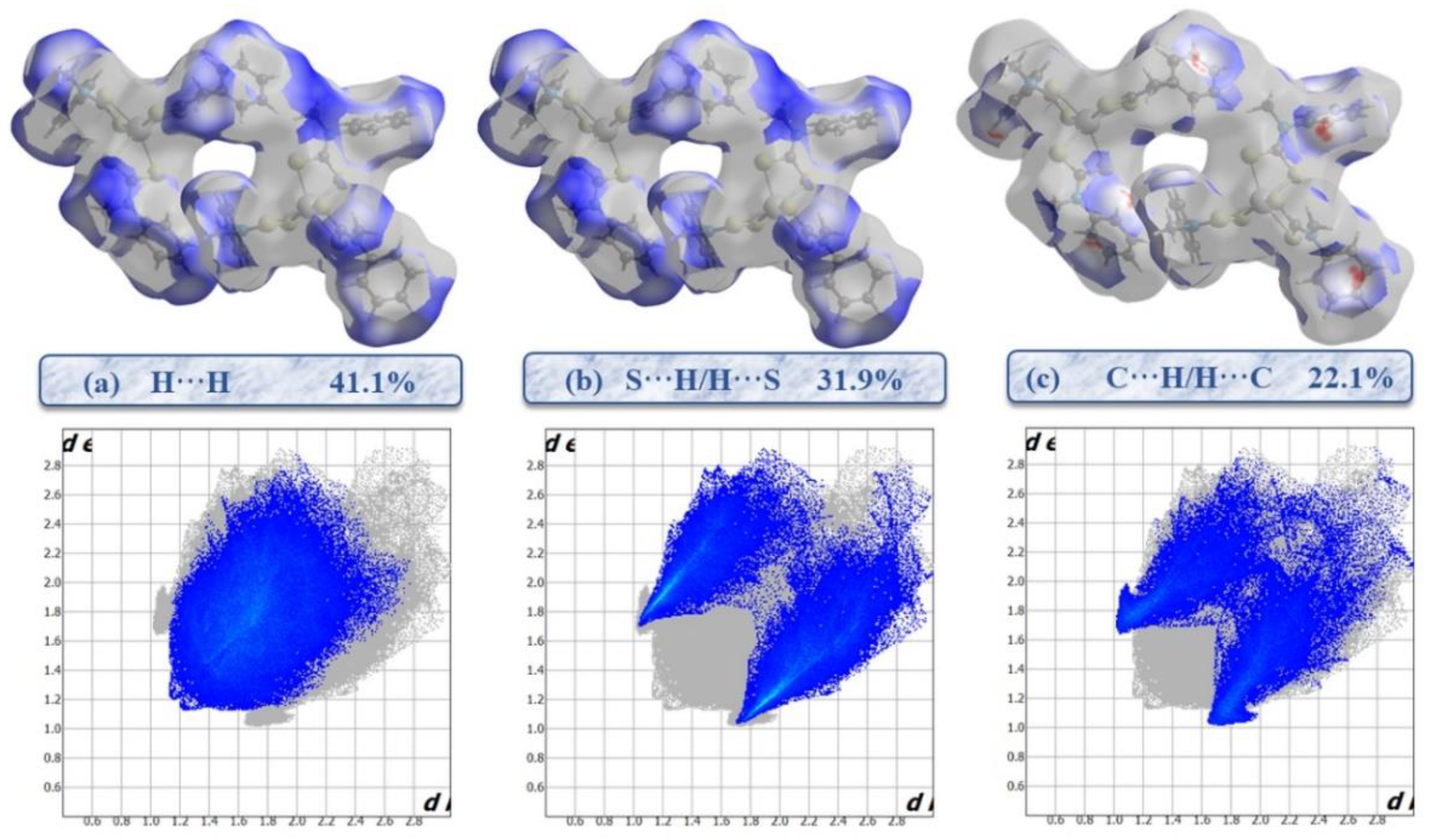
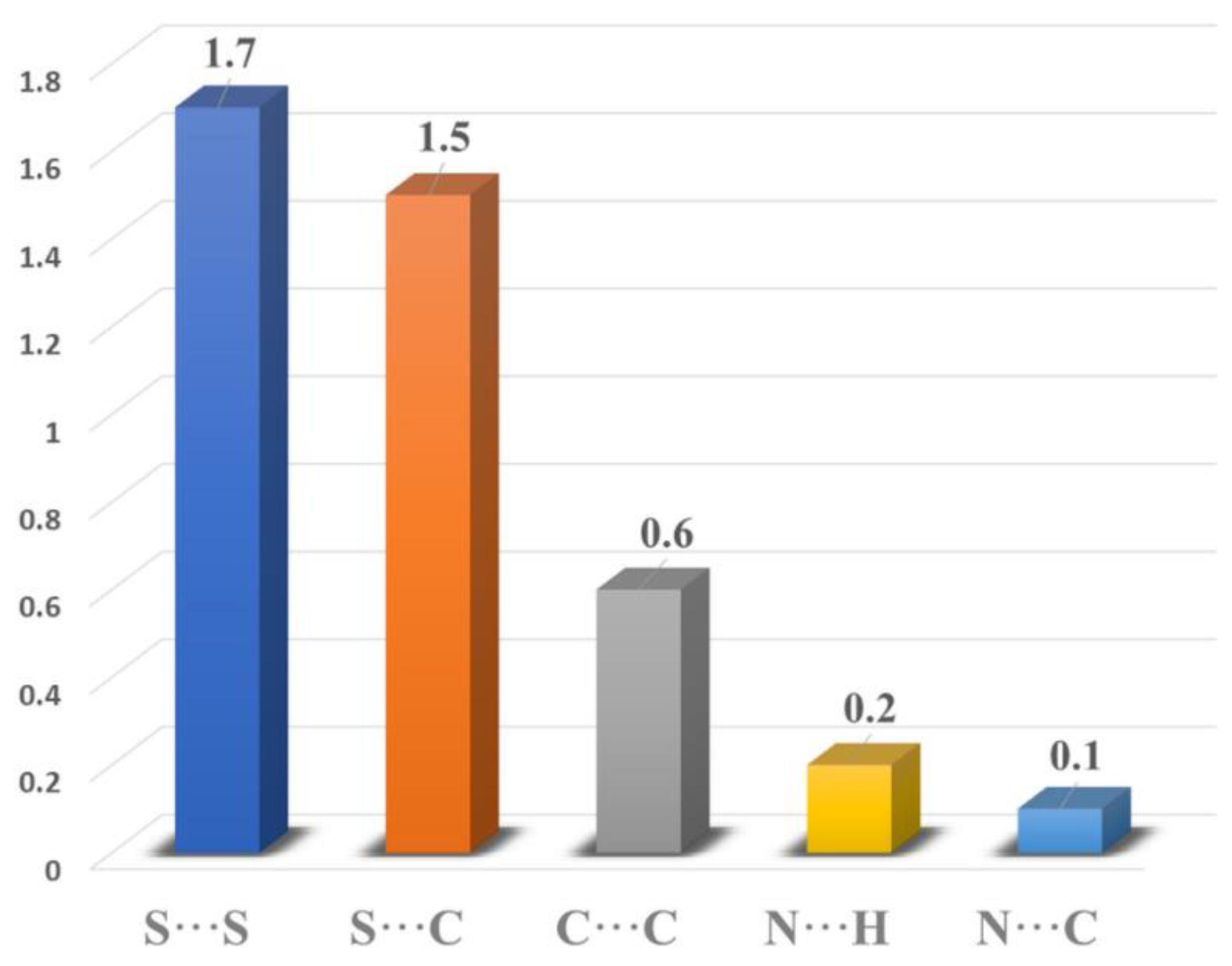
2.3. Hirshfeld Surface Analysis and 2D Fingerprints
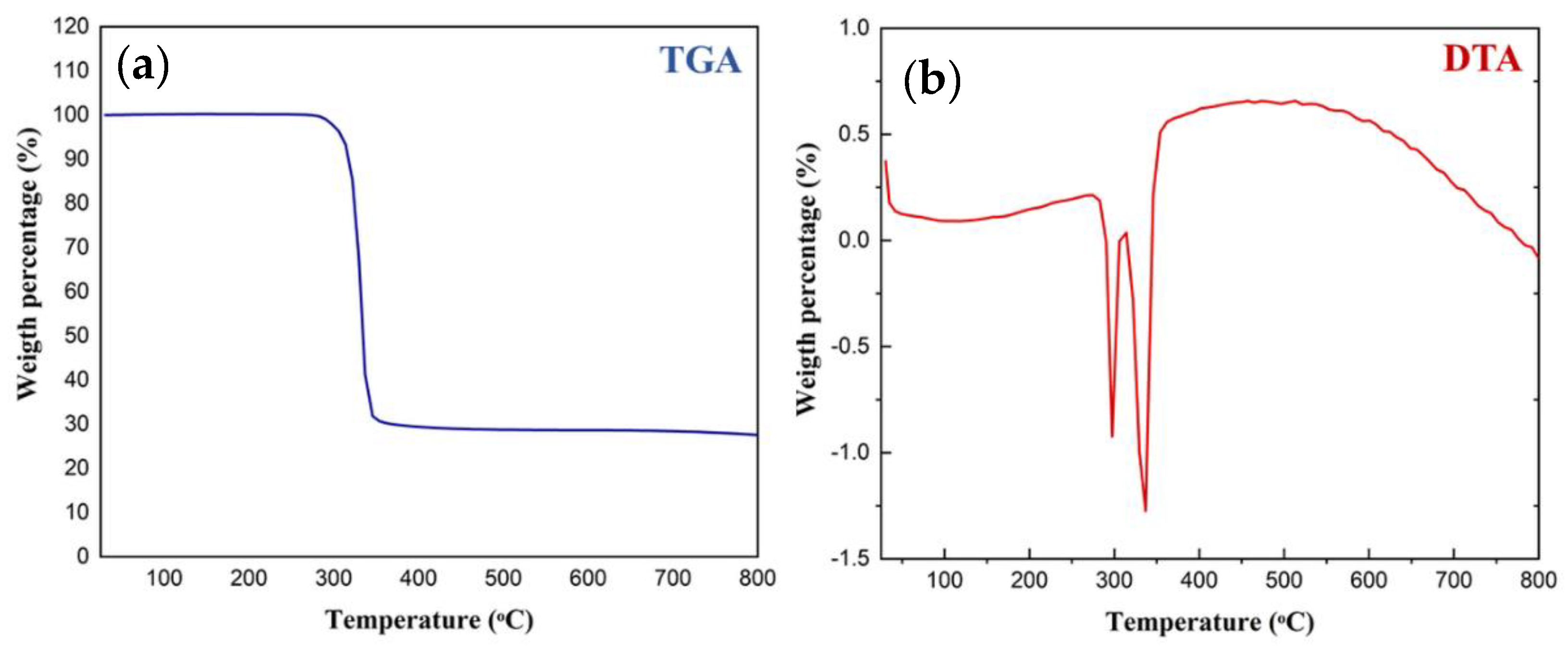
2.4. Thermal Studies
3. Materials and Methods
3.1. Preparation of Tris (N-Methyl-N-phenyldithiocarbamato) Indium(III)
3.2. Single-Crystal X-ray Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raţ, C.I.; Silvestru, C.; Breunig, H.J. Hypervalent organoantimony and-bismuth compounds with pendant arm ligands. Coord. Chem. Rev. 2013, 257, 818–879. [Google Scholar] [CrossRef]
- Braunschweig, H.; Cogswell, P.; Schwab, K. Synthesis, structure and reactivity of complexes containing a transition metal–bismuth bond. Coord. Chem. Rev. 2011, 255, 101–117. [Google Scholar] [CrossRef]
- Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T. Arylsulfonyl-N,N-diethyl-dithiocarbamates: A novel class of antitumor agents. Bioorg. Med. Chem. Lett. 2000, 10, 1887–1891. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Pérez, J.M.; Navarro, P.; Moreno, J.M.; Colacio, E.; Souza, P. Synthesis and characterization of novel palladium (II) complexes of bis (thiosemicarbazone). Structure, cytotoxic activity, and DNA binding of Pd (II)-benzyl bis (thiosemicarbazonate). J. Inorg. Biochem. 1999, 76, 29–37. [Google Scholar] [CrossRef]
- Ferreira, I.; De Lima, G.; Paniago, E.; Takahashi, J.; Pinheiro, C. Synthesis, characterization, and antifungal activity of new dithiocarbamate-based complexes of Ni (II), Pd (II) and Pt (II). Inorg. Chim. Acta 2014, 423, 443–449. [Google Scholar] [CrossRef]
- Nieuwenhuizen, P.J. Zinc accelerator complexes: Versatile homogeneous catalysts in sulfur vulcanization. Appl. Catal. A Gen. 2001, 207, 55–68. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Lu, S.-W.; Guo, H.-F.; Hu, N.-H. Synthesis, properties, and molecular structure of five-coordinate N, N-dibenzyldithiocarbamate complexes of titanocene, zirconocene and hafnocene. Polyhedron 1992, 11, 1131–1135. [Google Scholar] [CrossRef]
- Grainger, R.S.; Innocenti, P. New applications of dithiocarbamates in organic synthesis. Heteroat. Chem. Int. J. Main Group Elem. 2007, 18, 568–571. [Google Scholar]
- Ozturk, I.; Banti, C.N.; Kourkoumelis, N.; Manos, M.J.; Tasiopoulos, A.J.; Owczarzak, A.; Kubicki, M.; Hadjikakou, S.K. Synthesis, characterization and biological activity of antimony (III) or bismuth (III) chloride complexes with dithiocarbamate ligands derived from thiuram degradation. Polyhedron 2014, 67, 89–103. [Google Scholar] [CrossRef]
- Garje, S.S.; Jain, V.K. Chemistry of arsenic, antimony and bismuth compounds derived from xanthate, dithiocarbamate and phosphorus-based ligands. Coord. Chem. Rev. 2003, 236, 35–56. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Yadav, N.; Wadawale, A.; Jain, V.K.; Bohra, R. Monoorganobismuth (III) dithiocarboxylates: Synthesis, structures and their utility as molecular precursors for the preparation of Bi2S3 films and nanocrystals. Inorg. Chim. Acta 2010, 363, 375–380. [Google Scholar] [CrossRef]
- Sivasekar, S.; Ramalingam, K.; Rizzoli, C. Metal dithiocarbamate precursors for the preparation of a binary sulfide and a pyrochlore: Synthesis, structure, continuous shape measure and bond valence sum analysis of antimony (III) dithiocarbamates. Polyhedron 2015, 85, 598–606. [Google Scholar] [CrossRef]
- Dutta, D.P.; Jain, V.; Chaudhury, S.; Tiekink, E. Indium tris N-methylpiperazinylcarbodithioate: Synthesis, structure and its transformation into indium sulfide. Main Group Met. Chem. 2001, 24, 405–408. [Google Scholar] [CrossRef]
- Ronconi, L.; Giovagnini, L.; Marzano, C.; Bettìo, F.; Graziani, R.; Pilloni, G.; Fregona, D. Gold dithiocarbamate derivatives as potential antineoplastic agents: Design, spectroscopic properties, and in vitro antitumor activity. Inorg. Chem. 2005, 44, 1867–1881. [Google Scholar] [CrossRef]
- Gurumoorthy, G.; Rani, P.J.; Thirumaran, S.; Ciattini, S. Cobalt (III) dithiocarbamates for anion sensing and preparation of cobalt sulfide and cobalt-iron sulfide nanoparticles: Photocatalytic degradation of dyes with as-prepared nanoparticles. Inorg. Chim. Acta 2017, 455, 132–139. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Gurumoorthy, G.; Thirumaran, S.; Ciattini, S. Synthesis, characterization, cytotoxicity and antimicrobial studies on Bi (III) dithiocarbamate complexes containing furfuryl group and their use for the preparation of Bi2O3 nanoparticles. Polyhedron 2017, 121, 70–79. [Google Scholar] [CrossRef]
- Bonati, F.; Ugo, R. Organotin (iv) n, n-disubstituted dithiocarbamates. J. Organomet. Chem. 1967, 10, 257–268. [Google Scholar] [CrossRef]
- Van Gaal, H.; Diesveld, J.; Pijpers, F.; Van der Linden, J. Carbon-13 NMR spectra of dithiocarbamates. Chemical shifts, carbon-nitrogen stretching vibration frequencies and. pi.-bonding in the NCS2 fragment. Inorg. Chem. 1979, 18, 3251–3260. [Google Scholar] [CrossRef]
- Tan, Y.S.; Yeo, C.I.; Tiekink, E.R.; Heard, P.J. Dithiocarbamate complexes of platinum group metals: Structural aspects and applications. Inorganics 2021, 9, 60. [Google Scholar] [CrossRef]
- Gowda, V.; Sarma, B.; Larsson, A.C.; Lantto, P.; Antzutkin, O.N. Bi (III) Complexes Containing Dithiocarbamate Ligands: Synthesis, Structure Elucidation by X-ray Diffraction, Solid-State 13C/15N NMR, and DFT Calculations. ChemistrySelect 2020, 5, 8882–8891. [Google Scholar] [CrossRef]
- Dutta, D.P.; Jain, V.K.; Knoedler, A.; Kaim, W. Dithiocarbamates of gallium (III) and indium (III): Syntheses, spectroscopy, and structures. Polyhedron 2002, 21, 239–246. [Google Scholar] [CrossRef]
- Jain, V.K.; Wadawale, A.; Kushwah, N.P.; Pal, M.K. Organo-gallium and indium complexes with dithiolate and oxo ligands: Synthesis, structures and applications. J. Chem. Sci. 2011, 123, 107–112. [Google Scholar] [CrossRef]
- Saiyed, T.A.; Adeyemi, J.O.; Onwudiwe, D.C. The structural chemistry of zinc (ii) and nickel (ii) dithiocarbamate complexes. Open Chem. 2021, 19, 974–986. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Ferjani, H.; Ben Smida, Y.; Onwudiwe, D.C.; Elamin, N.Y.; Ezzine, S.; Almotlaq, N.S. An Experimental and Theoretical Study of the Optical Properties of (C2H7N4O)2BiCl5 for an Optoelectronic Application. Inorganics 2022, 10, 48. [Google Scholar] [CrossRef]
- Setifi, Z.; Ferjani, H.; Jelsch, C.; Glidewell, C.; Dege, N.; Setifi, F. Synthesis, Structure and Hirshfeld Surface Analysis of a New Iron Complex [Fe(N4Py)(tcnspr)](tcnspr). J. Inorg. Organomet. Polym. Mater. 2021, 31, 3054–3061. [Google Scholar] [CrossRef]
- Bessergenev, V.G.; Bessergenev, A.V.; Ivanova, E.N.; Kovalevskaya, Y.A. Study of In2S3 Thin Films by Diffraction of Synchrotron Radiation. J. Solid-State Chem. 1998, 137, 6–11. [Google Scholar] [CrossRef]
- Bajpai, A.; Tiwari, S. Application of thermogravimetric analysis for characterisation of bisdithiocarbamate of urea and its copper (II) complex. Thermochim. Acta 2004, 411, 139–148. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis and characterization of metal complexes of N-alkyl-N-phenyl dithiocarbamates. Polyhedron 2010, 29, 1431–1436. [Google Scholar] [CrossRef]
- Bruker AXS Inc. APEX2, Version 2014.11-0; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
| Complex | Indium(III) Tris (N-Methyl-N-phenyldithiocarbamate) |
|---|---|
| Empirical formula | C24H24InN3S6 |
| Formula weight (g/mol) | 661.64 |
| Crystal system, space group | Triclinic, P-1 |
| a (Å) | 14.6017 (18) |
| b (Å) | 14.9653 (18) |
| c (Å) | 15.3953 (18) |
| α (°) | 62.209 (2) |
| β (°) | 76.315 (2) |
| ɣ (°) | 72.310 (2) |
| V (Å3) | 2817.4 (6) |
| Z | 4 |
| µ (mm−1) | 1.30 |
| Dx (Mg m−3) | 1.560 |
| F (000) | 1336 |
| Crystal size (mm) | 0.20 × 0.16 × 0.13 |
| Crystal habit | Block, colourless |
| θmin/θmax (deg) | 2.4/25.5 |
| Observed refl. with I > 2σ(I) | 8571 |
| Measured reflections | 57,388 |
| Independent reflections | 10,615 |
| Rint | 0.069 |
| Data/restraints/parameters | 10,615/0/607 |
| wR(F2) | 0.160 |
| R[F2 > 2σ(F2)] | 0.053 |
| GooF = S | 1.11 |
| Δρmax/Δρmin (e.Å−3) | 3.22/−0.70 |
| CCDC number | 2184738 |
| D-H···A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| C17-H17···S10viii | 0.95 | 2.87 | 3.596(6) | 135 |
| C46-H46···S5vi | 0.95 | 2.97 | 3.521(6) | 134 |
| X-H···Cg | H···Cg | X···Cg | X-H···Cg |
|---|---|---|---|
| C(14)-H(14)···Cg(5) | 2.58 | 3.517(7) | 170 |
| C(24)-H(24)···Cg(6) | 2.54 | 3.472(6) | 168 |
| C(26)-H(26)···Cg(4) | 2.64 | 3.580(6) | 173 |
| C(32)-H(32)···Cg(11) | 2.53 | 3.464(7) | 167 |
| C(39)-H(39)···Cg(4) | 2.82 | 3.516(7) | 131 |
| C(42)-H(42)···Cg(12) | 2.58 | 3.486(7) | 160 |
| C(44)-H(44)···Cg(10) | 2.51 | 3.392(6) | 155 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferjani, H.; Onwudiwe, D.C. Synthesis, Structural, Thermal, and Hirshfeld Surface Analysis of In(III) Tris (N-Methyl-N-Phenyl Dithiocarbamate). Inorganics 2022, 10, 146. https://doi.org/10.3390/inorganics10100146
Ferjani H, Onwudiwe DC. Synthesis, Structural, Thermal, and Hirshfeld Surface Analysis of In(III) Tris (N-Methyl-N-Phenyl Dithiocarbamate). Inorganics. 2022; 10(10):146. https://doi.org/10.3390/inorganics10100146
Chicago/Turabian StyleFerjani, Hela, and Damian C. Onwudiwe. 2022. "Synthesis, Structural, Thermal, and Hirshfeld Surface Analysis of In(III) Tris (N-Methyl-N-Phenyl Dithiocarbamate)" Inorganics 10, no. 10: 146. https://doi.org/10.3390/inorganics10100146







