Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation
Abstract
:1. Introduction
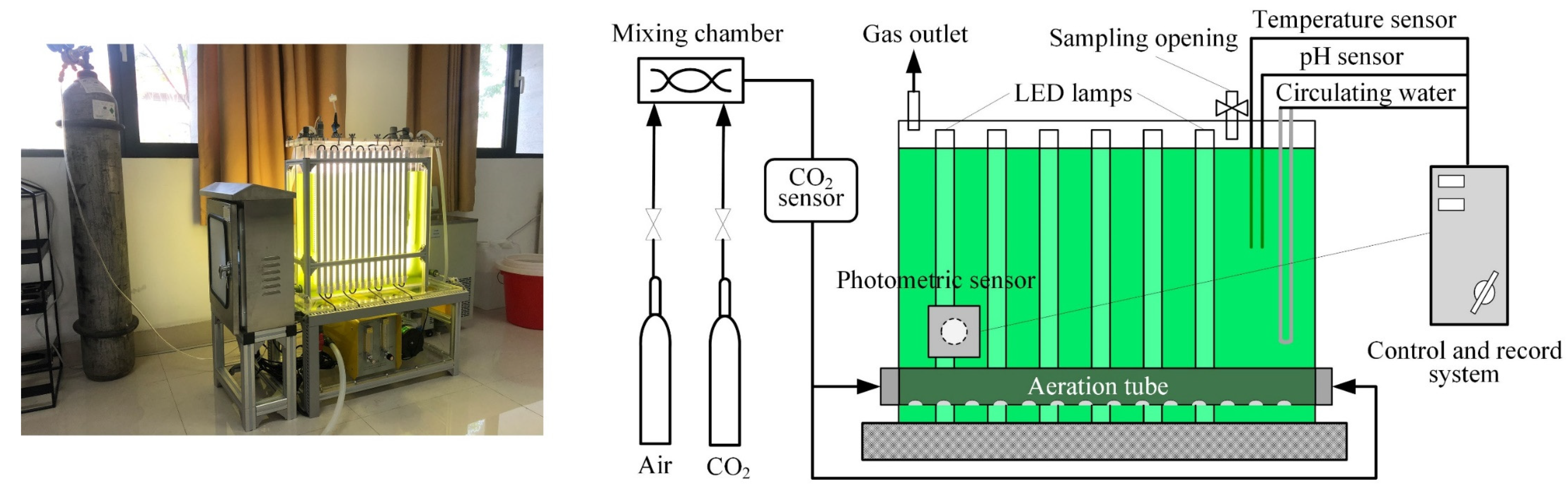
2. Experiments and Methodology
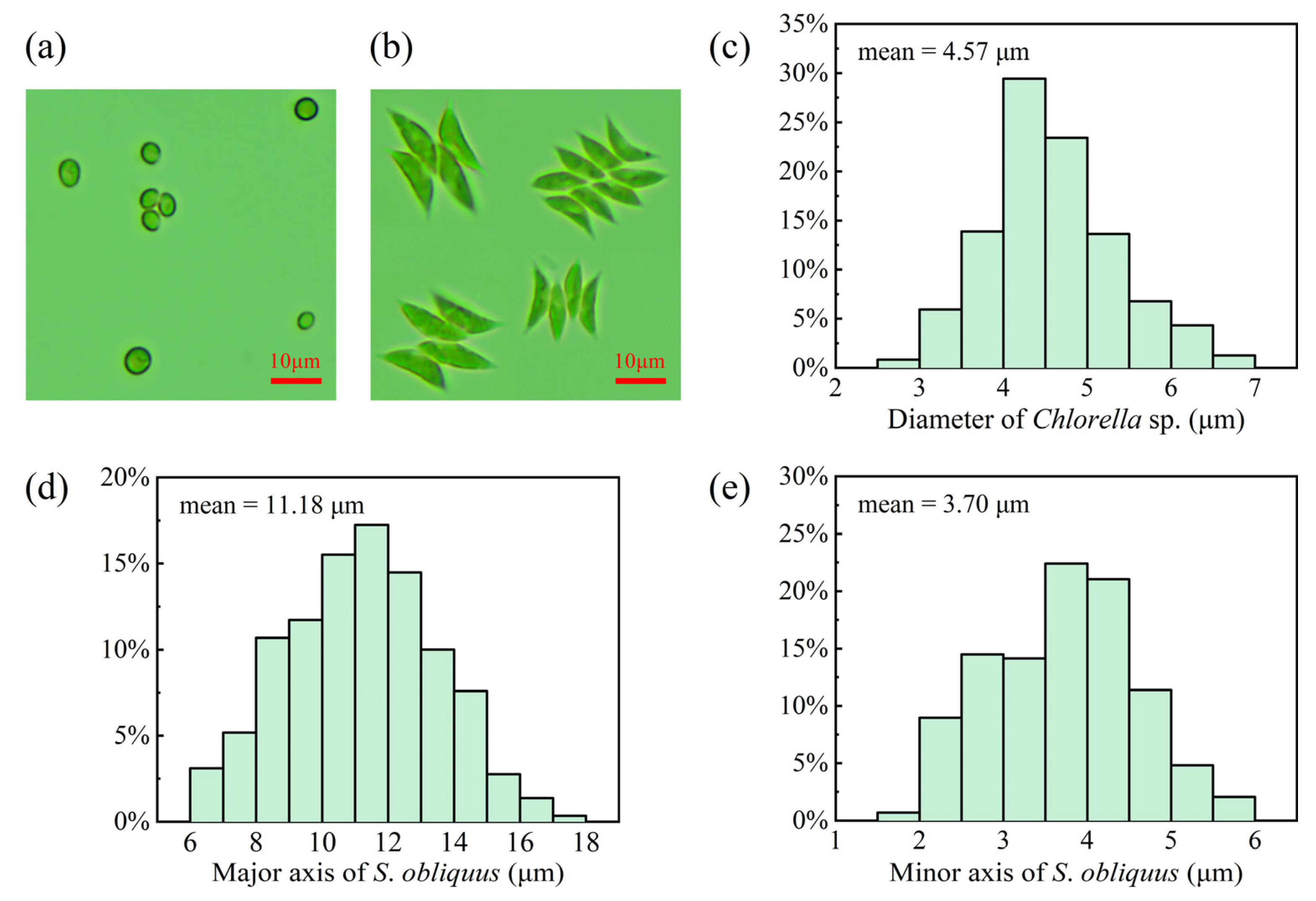
2.1. Cultivation and Sample Preparation
2.2. Radiation Characteristics Measurements
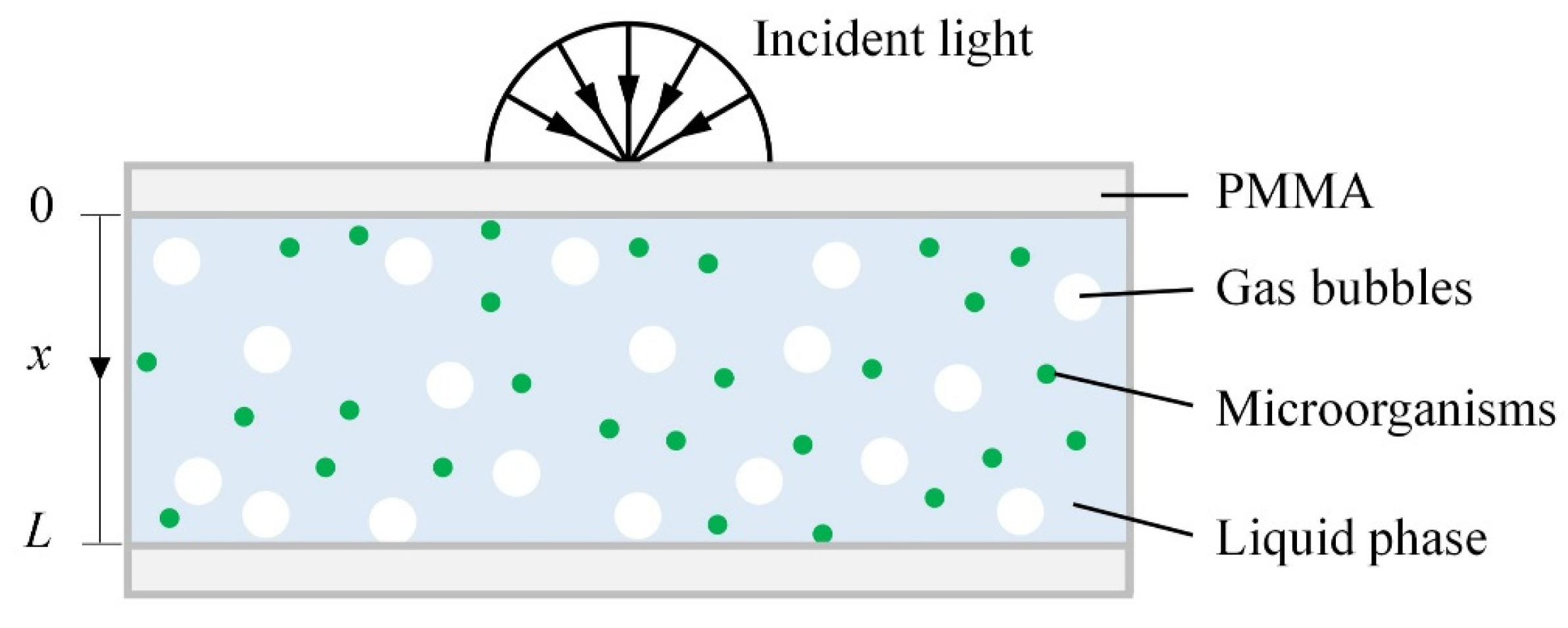
2.3. Radiation Transfer Modeling of the PBR
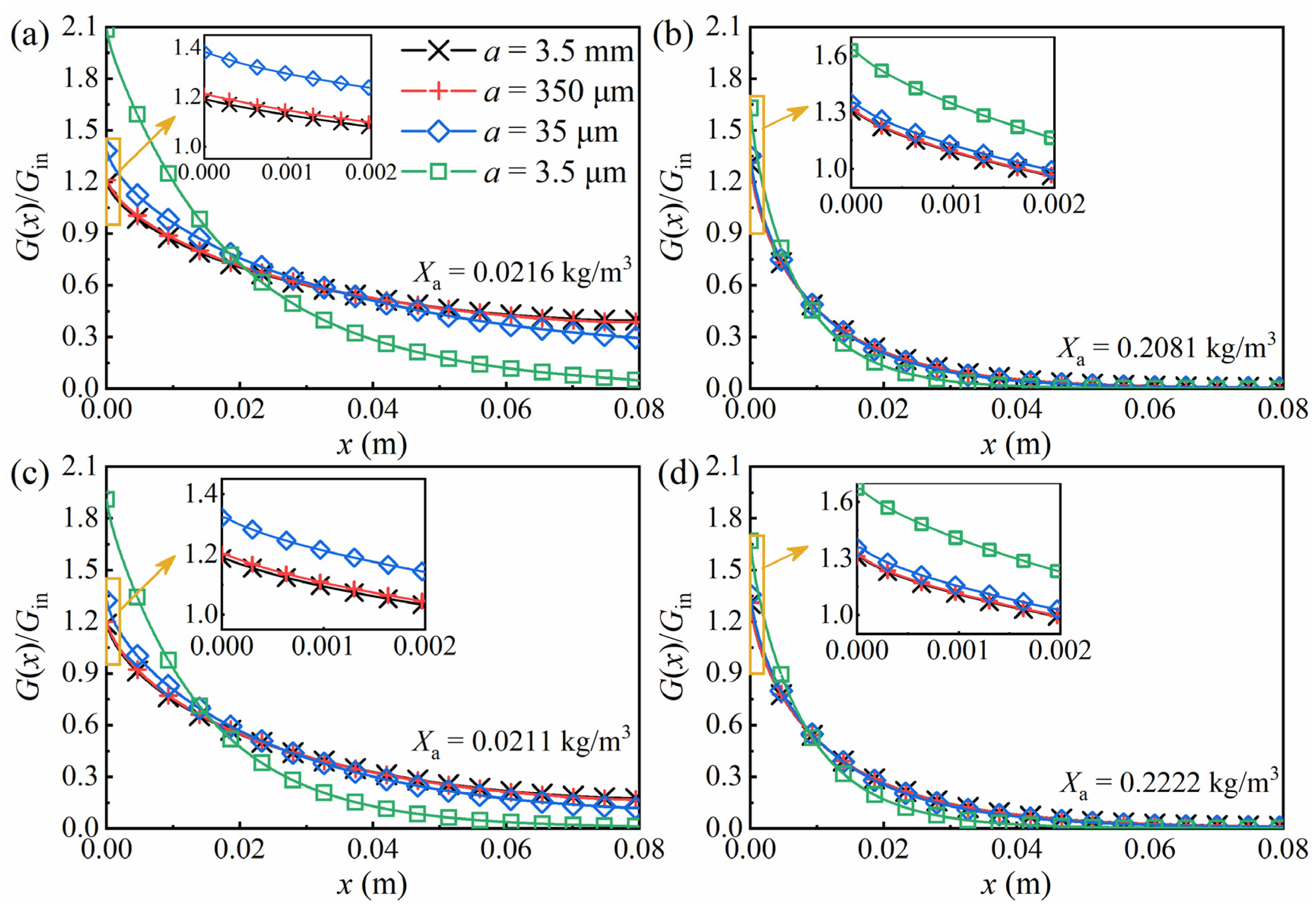
3. Results and Discussion
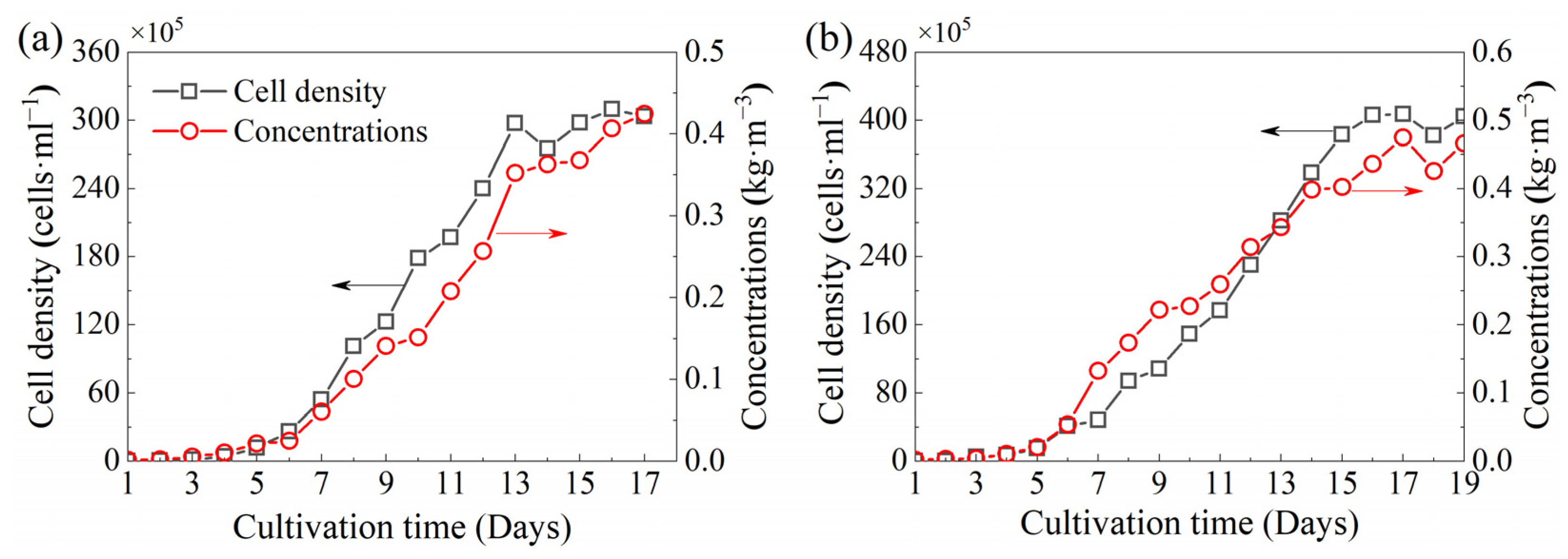
3.1. Cell Growth
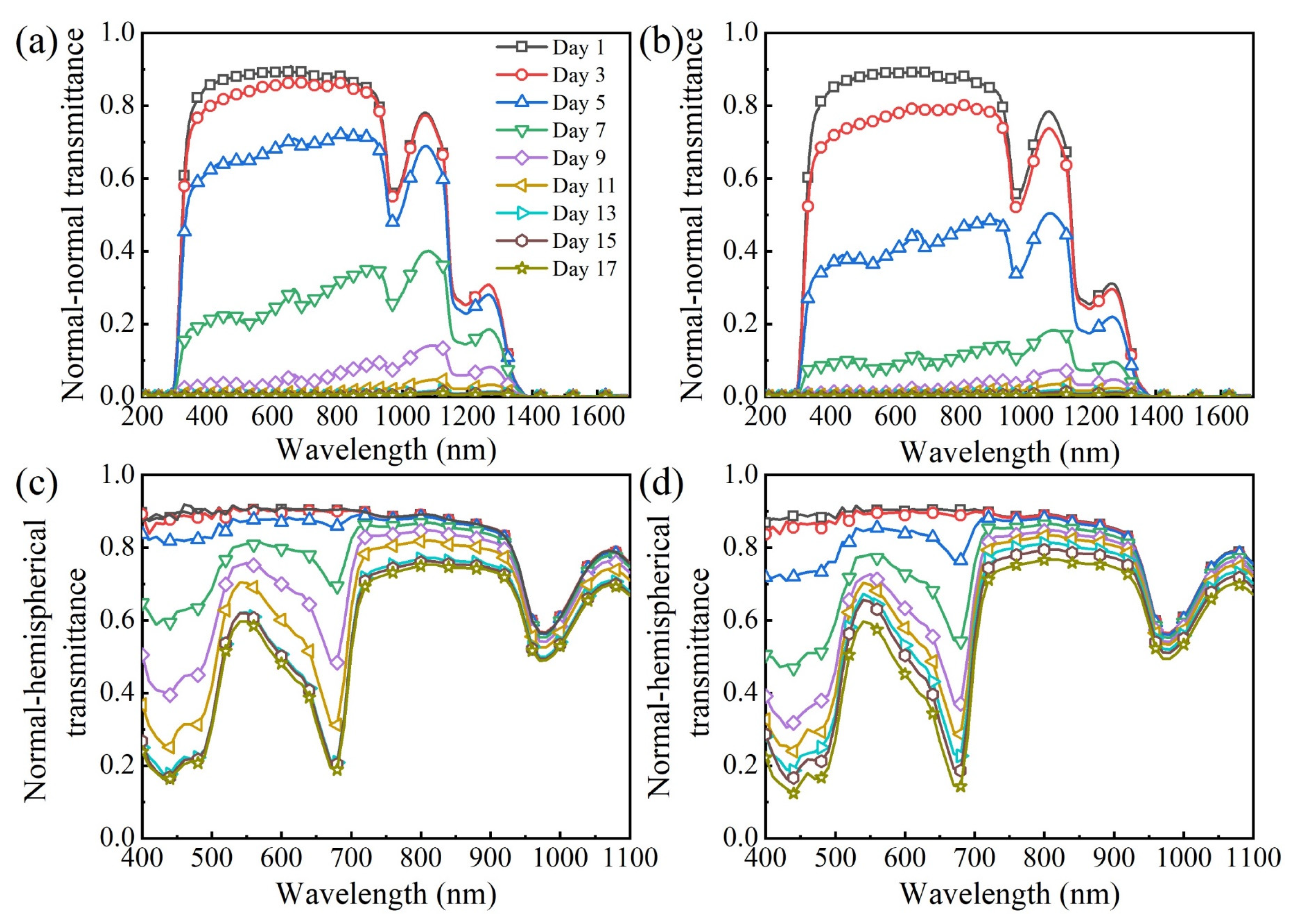
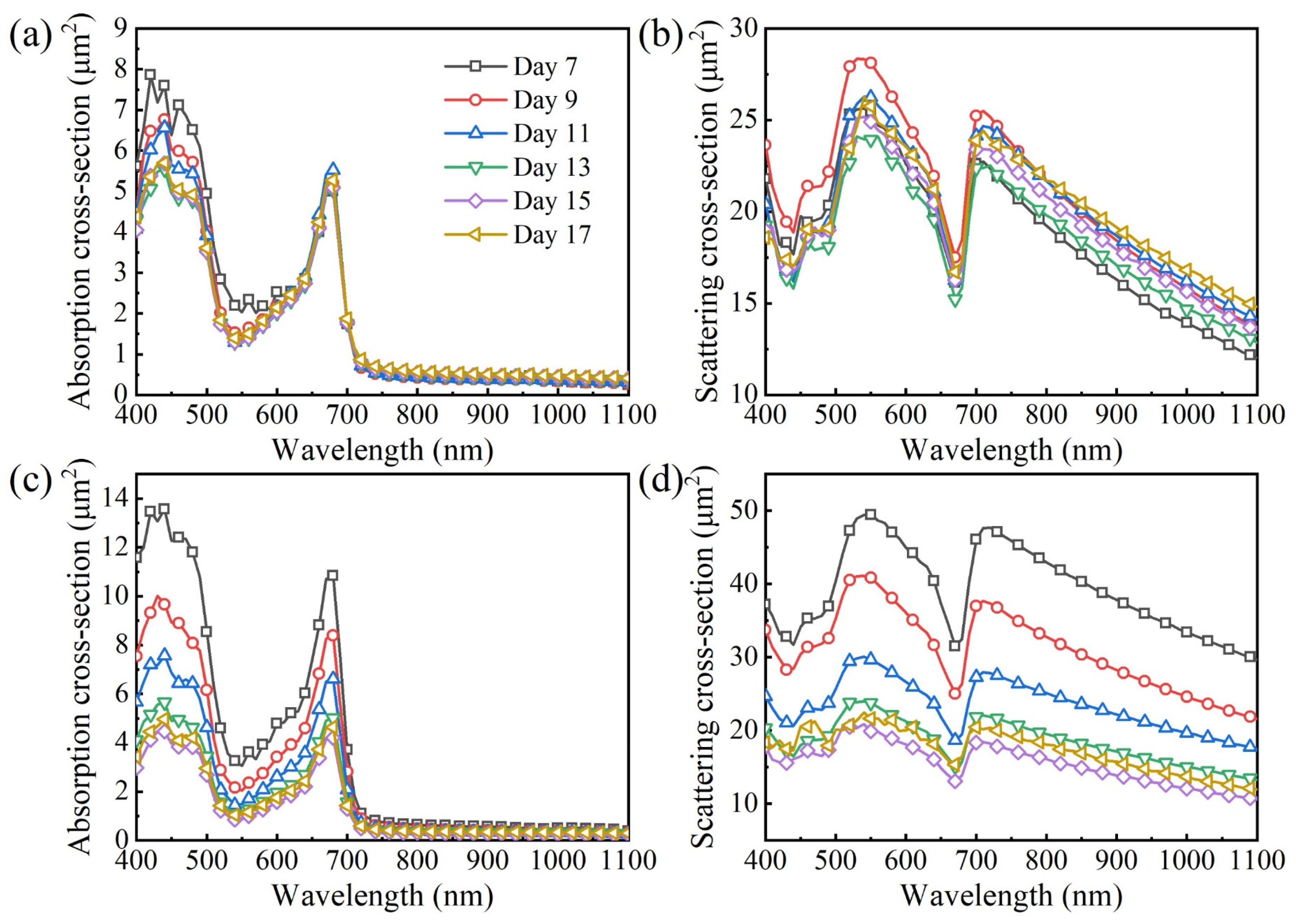
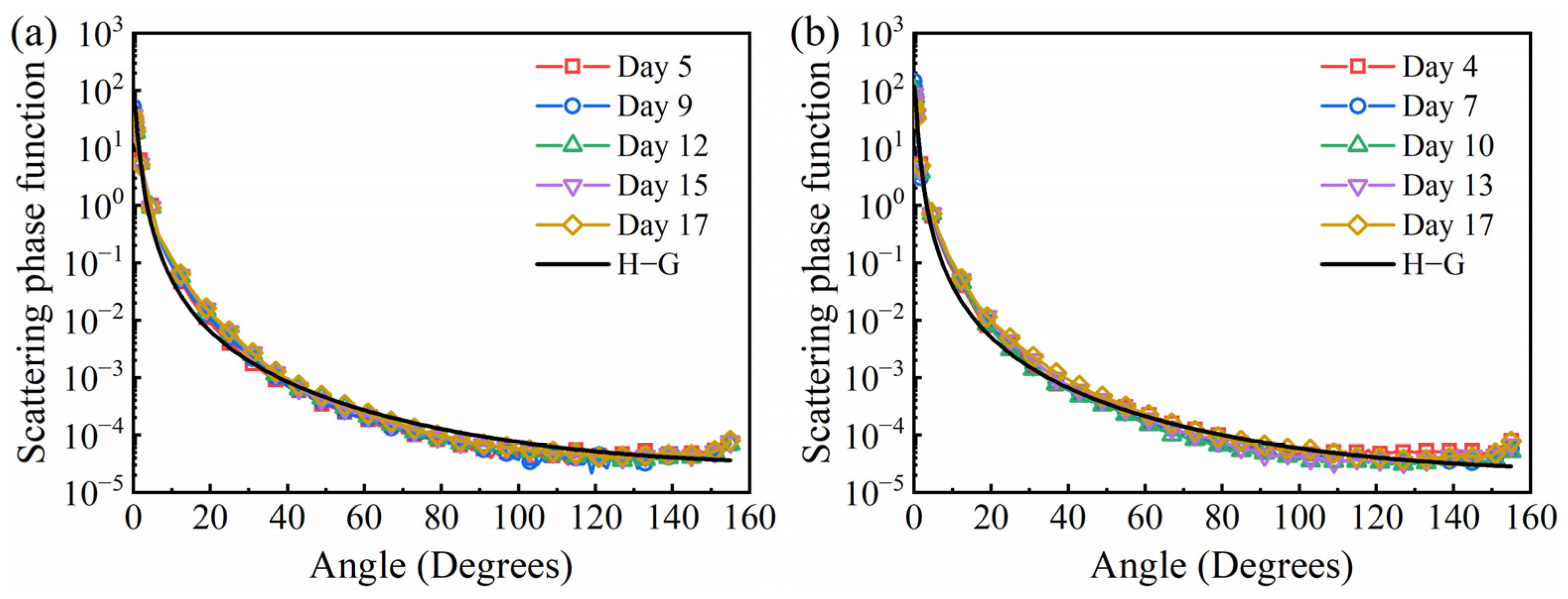
3.2. Time-Dependent Radiation Characteristics of Microalgae
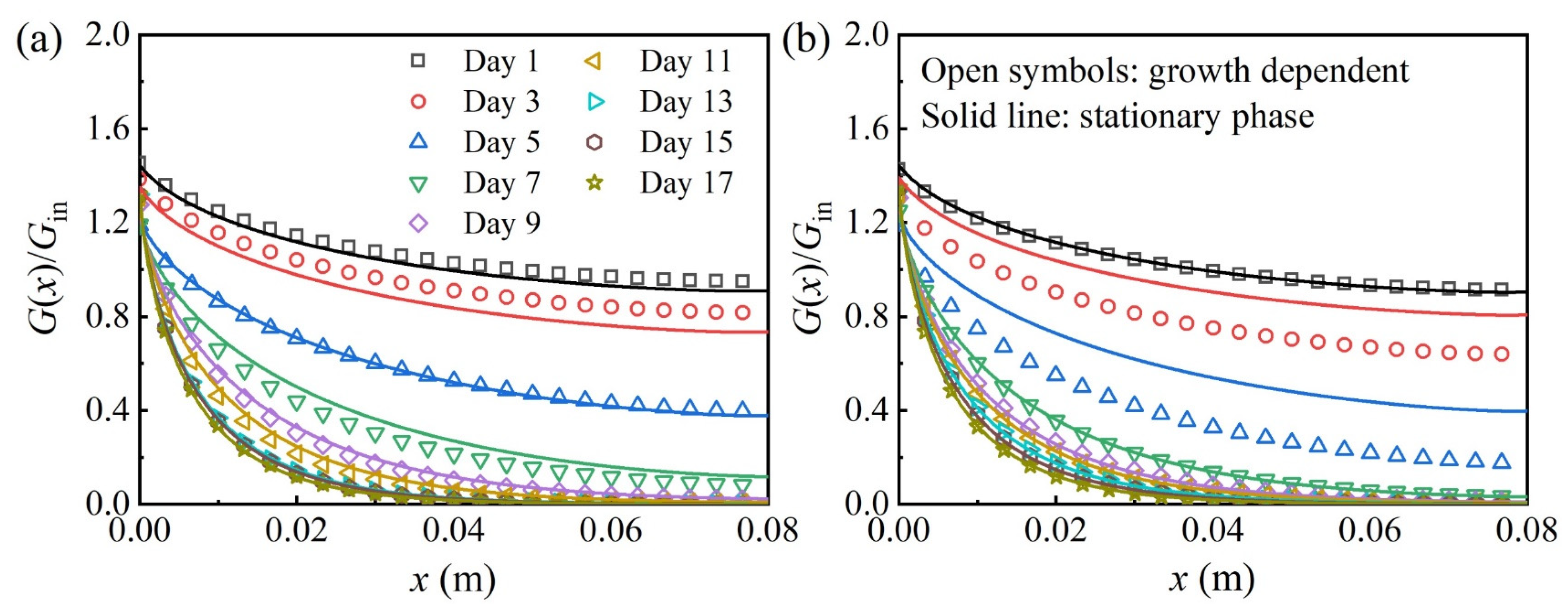
3.3. Time-Dependent Radiation Transfer in the PBR
3.4. Bubble Volume Fraction and Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreira, D.; Pires, J.C.M. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef]
- Brilman, W.; Alba, L.G.; Veneman, R. Capturing atmospheric CO2 using supported amine sorbents for microalgae cultivation. Biomass Bioenergy 2013, 53, 39–47. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Du, Z.; Hu, B.; Ma, X.; Cheng, Y.; Liu, Y.; Lin, X.; Wan, Y.; Lei, H.; Chen, P.; Ruan, R. Catalytic pyrolysis of microalgae and their three major components: Carbohydrates, proteins, and lipids. Bioresour. Technol. 2013, 130, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Tourlouki, K.; Tsavatopoulou, V.; Alexandropoulos, D.; Manariotis, I.D.; Mazzucato, S. A novel microalgae harvesting method using laser micromachined glass fiber reinforced polymers. Photonics 2020, 7, 42. [Google Scholar] [CrossRef]
- Legrand, J.; Artu, A.; Pruvost, J. A review on photobioreactor design and modelling for microalgae production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Powtongsook, S.; Pavasant, P. Enhanced productivity of Chaetoceros calcitrans in airlift photobioreactors. Bioresour. Technol. 2007, 98, 2123–2130. [Google Scholar] [CrossRef]
- Benemann, J.R. Hydrogen production by microalgae. J. Appl. Phycol. 2000, 12, 291–300. [Google Scholar] [CrossRef]
- Cheng, J.; Song, Y.; Guo, W.; Miao, Y.; Chen, S.; Zhou, J. Developing microporous fibrous-diaphragm aerator to decrease bubble generation diameter for improving microalgal growth with CO2 fixation in a raceway pond. Bioresour. Technol. 2019, 276, 28–34. [Google Scholar] [CrossRef]
- Le Gouic, B.; Marec, H.; Pruvost, J.; Cornet, J.F. Investigation of growth limitation by CO2 mass transfer and inorganic carbon source for the microalga Chlorella vulgaris in a dedicated photobioreactor. Chem. Eng. Sci. 2021, 233, 116388. [Google Scholar] [CrossRef]
- Guo, W.B.; Cheng, J.; Liu, S.Z.; Feng, L.C.; Su, Y.N.; Li, Y.G. A novel porous nickel-foam filled CO2 absorptive photobioreactor system to promote CO2 conversion by microalgal biomass. Sci. Total Environ. 2020, 713, 136593. [Google Scholar] [CrossRef] [PubMed]
- Pruvost, J.; Legrand, J.; Legentilhomme, P.; Muller-Feuga, A. Simulation of microalgae growth in limiting light conditions: Flow effect. AICHE J. 2002, 48, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, F.G.A.; Camacho, F.G.; Perez, J.A.S.; Sevilla, J.M.F.; Grima, E.M. A model for light distribution and average solar irradiance inside outdoor tubular photobioreactors for the microalgal mass culture. Biotechnol. Bioeng. 1997, 55, 701–714. [Google Scholar] [CrossRef]
- Cornet, J.F.; Dussap, C.G.; Dubertret, G. A structured model for simulation of cultures of the cyanobacterium Spirulina platensis in photobioreactors: I. Coupling between light transfer and growth kinetics. Biotechnol. Bioeng. 1992, 40, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Cornet, J.F.; Dussap, C.G.; Gros, J.B.; Binois, C.; Lasseur, C. A simplified monodimensional approach for modeling coupling between radiant light transfer and growth kinetics in photobioreactors. Chem. Eng. Sci. 1995, 50, 1489–1500. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.F.; Goetz, V.; Legrand, J. Modeling dynamic functioning of rectangular photobioreactors in solar conditions. AICHE J. 2011, 57, 1947–1960. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, T.; Yang, J.; Yao, L.; Gao, L. Evaluation of radiative transfer using the finite volume method in cylindrical photoreactors. Chem. Eng. Sci. 2011, 66, 3930–3940. [Google Scholar] [CrossRef]
- Lee, E.; Pruvost, J.; He, X.; Munipalli, R.; Pilon, L. Design tool and guidelines for outdoor photobioreactors. Chem. Eng. Sci. 2014, 106, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Kandilian, R.; Pruvost, J.; Artu, A.; Lemasson, C.; Legrand, J.; Pilon, L. Comparison of experimentally and theoretically determined radiation characteristics of photosynthetic microorganisms. J. Quant. Spectros. Radiat. Transfer 2016, 175, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Ohi, N.; Ishiwata, Y.; Taguchi, S. Diel patterns in light absorption and absorption efficiency factors of Isochrysis galbana (Prymnesiophyceae). J. Phycol. 2002, 38, 730–737. [Google Scholar] [CrossRef]
- DuRand, M.D.; Green, R.E.; Sosik, H.M.; Olson, R.J. Diel variations in optical properties of Micromonas pusilla (Prasinophyceae). J. Phycol. 2002, 38, 1132–1142. [Google Scholar] [CrossRef] [Green Version]
- Heng, R.-L.; Pilon, L. Time-dependent radiation characteristics of Nannochloropsis oculata during batch culture. J. Quant. Spectros. Radiat. Transfer 2014, 144, 154–163. [Google Scholar] [CrossRef]
- Heng, R.-L.; Lee, E.; Pilon, L. Radiation characteristics and optical properties of filamentous cyanobacterium Anabaena cylindrica. J. Opt. Soc. Am. A 2014, 31, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.M.; Ma, C.Y.; Liu, L.H. Temporal scaling of the growth dependent optical properties of microalgae. J. Quant. Spectros. Radiat. Transfer. 2018, 214, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.Y.; Zhao, J.M.; Liu, L.H.; Zhang, L. Growth-dependent radiative properties of Chlorella vulgaris and its influence on prediction of light fluence rate in photobioreactor. J. Appl. Phycol. 2019, 31, 235–247. [Google Scholar] [CrossRef]
- Araujo, S.D.; Garcia, V.M.T. Growth and biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 2005, 246, 405–412. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, S.; Guo, W.; Song, Y.; Kumar, S.; Kubar, A.A.; Su, Y.; Li, Y. Developing staggered woven mesh aerator with three variable-micropore layers in recycling water pipeline to enhance CO2 conversion for improving Arthrospira growth. Sci. Total Environ. 2021, 760, 143941. [Google Scholar] [CrossRef]
- Cheng, J.; Miao, Y.; Guo, W.; Song, Y.; Tian, J.; Zhou, J. Reduced generation time and size of carbon dioxide bubbles in a volute aerator for improving Spirulina sp. growth. Bioresour. Technol. 2018, 270, 352–358. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, S.; Ding, Y.-d.; Liao, Q.; Huang, Y.; Zhu, X. Optimizing the gas distributor based on CO2 bubble dynamic behaviors to improve microalgal biomass production in an air-lift photo-bioreactor. Bioresour. Technol. 2017, 233, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berberoglu, H.; Yin, J.; Pilon, L. Light transfer in bubble sparged photobioreactors for H2 production and CO2 mitigation. Int. J. Hydrog. Energy 2007, 32, 2273–2285. [Google Scholar] [CrossRef] [Green Version]
- Berberoglu, H.; Pilon, L.; Melis, A. Radiation characteristics of Chlamydomonas reinhardtii CC125 and its truncated chlorophyll antenna transformants tla1, tlaX and tla1-CW+. Int. J. Hydrog. Energy 2008, 33, 6467–6483. [Google Scholar] [CrossRef]
- Berberoglu, H.; Gomez, P.S.; Pilon, L. Radiation characteristics of Botryococcus braunii, Chlorococcum littorale, and Chlorella sp. used for CO2 fixation and biofuel production. J. Quant. Spectros. Radiat. Transfer 2009, 110, 1879–1893. [Google Scholar] [CrossRef]
- Wheaton, Z.C.; Krishnamoorthy, G. Modeling radiative transfer in photobioreactors for algal growth. Comput. Electron. Agric. 2012, 87, 64–73. [Google Scholar] [CrossRef]
- McHardy, C.; Luzi, G.; Lindenberger, C.; Agudo, J.R.; Delgado, A.; Rauh, C. Numerical analysis of the effects of air on light distribution in a bubble column photobioreactor. Algal Res. 2018, 31, 311–325. [Google Scholar] [CrossRef]
- Luzi, G.; McHardy, C.; Lindenberger, C.; Rauh, C.; Delgado, A. Comparison between different strategies for the realization of flashing-light effects—Pneumatic mixing and flashing illumination. Algal Res. 2019, 38, 101404. [Google Scholar] [CrossRef]
- Watanabe, K.; Imase, M.; Sasaki, K.; Ohmura, N.; Saiki, H.; Tanaka, H. Composition of the sheath produced by the green alga Chlorella sorokiniana. Lett. Appl. Microbiol. 2006, 42, 538–543. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.Y.; Lee, J.Y.; Ahn, C.Y.; Oh, H.M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef]
- Ansari, F.A.; Shriwastav, A.; Gupta, S.K.; Rawat, I.; Bux, F. Exploration of Microalgae Biorefinery by Optimizing Sequential Extraction of Major Metabolites from Scenedesmus obliquus. Ind. Eng. Chem. Res. 2017, 56, 3407–3412. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C.; Zhao, J.M.; Wang, C.C.; Liu, L.H. Improved transmission method for measuring the optical extinction coefficient of micro/nano particle suspensions. Appl. Opt. 2016, 55, 8171–8179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, C.; Zhao, J.; Liu, L. A new method for determining the optical constants of highly transparent solids. Appl. Spectrosc. 2017, 71, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Zhao, J.; Tan, J. Optical properties of sodium chloride solution within the spectral range from 300 to 2500 nm at room temperature. Appl. Spectrosc. 2015, 69, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Palik, E.D. Handbook of Optical Constants of Solids; Academic Press: San Diego, CA, USA, 1985. [Google Scholar]
- Modest, M.F. Radiative Heat Transfer, 3rd ed.; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Raithby, G.D.; Chui, E.H. A finite-volume method for predicting a radiant-heat transfer in enclosures with participating media. J. Heat Transf. -Trans. Asme 1990, 112, 415–423. [Google Scholar] [CrossRef]
- Chai, J.C. One-dimensional transient radiation heat transfer modeling using a finite-volume method. Numer. HeatTransf. Part B Fundam. 2003, 44, 187–208. [Google Scholar] [CrossRef]
- Henyey, L.G.; Greenstein, J.L. Diffuse radiation in the galaxy. Astrophys. J. 1941, 93, 70–83. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Chen, H.; Gao, C. Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Pilon, L.; Berberoglu, H.; Kandilian, R. Radiation transfer in photobiological carbon dioxide fixation and fuel production by microalgae. J. Quant. Spectros. Radiat. Transfer 2011, 112, 2639–2660. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhao, J.M.; Liu, L.H.; Zhang, L. The growth dependent radiative properties of microalgae and light field distribution within photobioreactors. In Proceedings of the Progress in Electromagnetics Research Symposium-Fall (PIERS-FALL), Singapore, 19–22 November 2017. [Google Scholar]
- Pilon, L.; Kandilian, R. Interaction between light and photosynthetic microorganisms. In Advances in Chemical Engineering; Legrand, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 107–149. [Google Scholar]
- Kandilian, R.; Lee, E.; Pilon, L. Radiation and optical properties of Nannochloropsis oculata grown under different irradiances and spectra. Bioresour. Technol. 2013, 137, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Pico-Marco, E.; Navarro, J.L.; Bruno-Barcena, J.M. A closed loop exponential feeding law: Invariance and global stability analysis. J. Process Control 2006, 16, 395–402. [Google Scholar] [CrossRef]
- Pandey, S.S.; Kumar, D.; Tiwari, B.S. Chloroplast metabolic engineering for sustainable agriculture. In Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production; Dubey, S.K., Pandey, A., Sangwan, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 149–162. [Google Scholar]
- Stenzel, O. The Physics of Thin Film Optical Spectra; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhang, Z.M. Nano/Microscale Heat Transfer; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Gershun, A. Fresnel reflection of diffusely incident light. J. Opt. Soc. Am. 1945, 35, 162–163. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, J.; Li, X.; Zhao, J.; Liu, L. Complex refractive indices measurements of polymers in visible and near-infrared bands. Appl. Opt. 2020, 59, 2337–2344. [Google Scholar] [CrossRef]
- Sorokin, C.; Krauss, R.W. The effects of light intensity on the growth rates of green algae. Plant Physiol. 1958, 33, 109–113. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1998. [Google Scholar]



| ωeff | [G(0) − Gin]/Gin, % | |||||||
|---|---|---|---|---|---|---|---|---|
| fb = 0 | fb = 0.003 | fb = 0.03 | fb = 0.3 | fb = 0 | fb = 0.003 | fb = 0.03 | fb = 0.3 | |
| Chlorella sp. (Xa = 0.0216 kg/m3) | 0.745 | 0.751 | 0.793 | 0.930 | 18.6 | 18.9 | 21.2 | 42.2 |
| Chlorella sp. (Xa = 0.2081 kg/m3) | 0.879 | 0.879 | 0.881 | 0.896 | 30.5 | 30.6 | 31.3 | 38.7 |
| S. obliquus (Xa = 0.0211 kg/m3) | 0.835 | 0.836 | 0.848 | 0.917 | 18.4 | 18.6 | 20.2 | 36.5 |
| S. obliquus (Xa = 0.2222 kg/m3) | 0.879 | 0.879 | 0.881 | 0.899 | 30.5 | 30.6 | 31.4 | 40.2 |
| ωeff | [G(0) − Gin]/Gin, % | |||||||
|---|---|---|---|---|---|---|---|---|
| a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | |
| Chlorella sp. (Xa = 0.0216 kg/m3) | 0.751 | 0.790 | 0.919 | 0.989 | 18.9 | 21.1 | 38.0 | 108.5 |
| Chlorella sp. (Xa = 0.2081 kg/m3) | 0.879 | 0.880 | 0.894 | 0.950 | 30.6 | 31.0 | 35.0 | 62.9 |
| S. obliquus (Xa = 0.0211 kg/m3) | 0.836 | 0.847 | 0.909 | 0.982 | 18.6 | 20.0 | 32.2 | 90.9 |
| S. obliquus (Xa = 0.2222 kg/m3) | 0.879 | 0.881 | 0.896 | 0.955 | 30.6 | 31.1 | 35.8 | 66.9 |
| fb = 0.003 | fb = 0.03 | fb = 0.3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | |
| Chlorella sp. (Xa = 0.0216 kg/m3) | 1.6492 | 1.6468 | 1.6107 | 1.2296 | 1.6507 | 1.6139 | 1.2284 | 0.5322 | 1.6614 | 1.2714 | 0.5480 | 0.2325 |
| Chlorella sp. (Xa = 0.2081 kg/m3) | 0.6412 | 0.6386 | 0.6144 | 0.4729 | 0.6457 | 0.6208 | 0.4760 | 0.2307 | 0.7010 | 0.5302 | 0.2522 | 0.1207 |
| S. obliquus (Xa = 0.0211 kg/m3) | 0.5978 | 0.5978 | 0.5872 | 0.4483 | 0.6013 | 0.5905 | 0.4490 | 0.2056 | 0.6349 | 0.4789 | 0.2163 | 0.0905 |
| S. obliquus (Xa = 0.2222 kg/m3) | 0.1841 | 0.1837 | 0.1793 | 0.1504 | 0.1858 | 0.1813 | 0.1516 | 0.0850 | 0.2057 | 0.1696 | 0.0929 | 0.0441 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, T.; Lin, L.; Li, X.; Yang, J.-Y.; Zhao, J.; Liu, L. Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation. Photonics 2022, 9, 864. https://doi.org/10.3390/photonics9110864
Fei T, Lin L, Li X, Yang J-Y, Zhao J, Liu L. Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation. Photonics. 2022; 9(11):864. https://doi.org/10.3390/photonics9110864
Chicago/Turabian StyleFei, Tianhao, Li Lin, Xingcan Li, Jia-Yue Yang, Junming Zhao, and Linhua Liu. 2022. "Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation" Photonics 9, no. 11: 864. https://doi.org/10.3390/photonics9110864





