Diode Laser Lithotription Technique Based on Optothermal Converter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retrospective Analysis
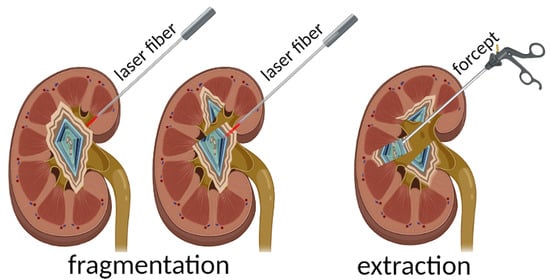
2.2. “Hot-Spot” Fragmentation Technique
2.3. Stones
- Group 1—stone fragmentation at a wavelength of 0.81 μm in liquid (n-27);
- Group 2—stone fragmentation at a wavelength of 0.97 μm in liquid (n-36);
- Group 3—stone fragmentation at a wavelength of 1.47 μm in liquid (n-15).
2.4. Statistical Analysis
3. Results
3.1. Evaluation of the Frequency of Infectious and Inflammatory Complications after Endoscopic/Percutaneous Operations for Urolithiasis
3.2. Development of the Stone Fragmentation Methodology through the “Hot Spot” Method
3.3. Development of the Clinical Algorithm of the Urinary Stone Fragmentation
- (1)
- Insertion of sterile optical fiber (diameter 550 µm) into a ureteral catheter No. 6 Ch (the distal end is cut to move the optical fiber, in particular, into the bladder cavity);
- (2)
- Install a rubber stopper at the proximal end of the optical fiber equal to the thickness of the stone (according to ultrasound and/or MSCT data), to prevent its distal end from being pulled out of the hollow guide tube more than the thickness of the stone;
- (3)
- Install a catheterization cystoscope No. 25 Ch into the bladder, through the working channel of which the structure is introduced into its cavity;
- (4)
- Press the optical fiber against the stone perpendicularly to the surface along the line of the planned fragmentation;
- (5)
- Start the laser emission to create several channels-perforations along the fragmentation line;
- (6)
- Extract the stone fragments with endoscopic forceps;
- (7)
- Control examination of the bladder cavity and install a Foley catheter for one day.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Türk, C.; Neisius, A.; Petrik, A.; Seitz, C.; Skolarikos, A.; Tepeler, A.; Thomas, K. EAU Guidelines on Urolithiasis. Uroweb. 2017. Available online: http://uroweb.org/wp-content/uploads/20-Urolithiasis_2017_web.pdf (accessed on 24 August 2021).
- Herrmann, T.R.W.; Liatsikos, E.N.; Nagele, U.; Traxer, O.; Merseburger, A.S. EAU Guidelines on Laser Technologies. Eur. Urol. 2012, 61, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Leijte, J.A.; Oddens, J.R.; Lock, T.M. Holmium laser lithotripsy for ureteric calculi: Predictive factors for complications and success. J. Endourol. 2008, 22, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Knudsen, B.E. Optimizing use of the holmium: YAG laser for surgical management of urinary lithiasis. Curr. Urol. Rep. 2014, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.E.; Hollander, A.B.; Soni, S.D.; Link, R.E.; Mayer, W.A. To dust or not to dust: A systematic review of ureteroscopic laser lithotripsy techniques. Curr. Urol. Rep. 2017, 18, 32. [Google Scholar] [CrossRef]
- Weiss, B.; Shah, O. Evaluation of dusting versus basketing–can new technologies improve stone-free rates? Nat. Rev. Urol. 2016, 13, 726–733. [Google Scholar] [CrossRef]
- Matlaga, B.R.; Chew, B.; Eisner, B.; Humphreys, M.; Knudsen, B.; Krambeck, A.; Lange, D.; Lipkin, M.; Miller, N.L.; Monga, M.; et al. Ureteroscopic laser lithotripsy: A review of dusting vs fragmentation with extraction. J. Endourol. 2018, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.N.; Chang, M.F.; Chang, A.; Lenoir, J.; Bagley, D.H. Effectiveness of highfrequency holmium: YAG laser stone fragmentation: The “popcorn effect”. J. Endourol. 2018, 22, 645–650. [Google Scholar] [CrossRef]
- Yang, T.; Liu, S.; Hu, J.; Wang, L.; Jiang, H. The evaluation of risk factors for postoperative infectious complications after percutaneous nephrolithotomy. Biomed Res. Int. 2017, 2017, 4832051. [Google Scholar] [CrossRef]
- Bredikhin, V.I. Optothermal properties of a converter of laser radiation to the radiation of a high-temperature point source. Mater. Res. Express 2019, 6, 065412. [Google Scholar] [CrossRef]
- Verdaasdonk, R.M.; Borst, C.; Boulanger, L.H.M.A.; van Gemert, M.J.C. Laser angioplasty with a metal laser probe (‘hot tip’): Probe temperature in blood. Lasers Med. Sci. 1987, 2, 153–158. [Google Scholar] [CrossRef]
- Belikov, A.V.; Skrypnik, A.V. Soft Tissue Cutting Efficiency by 980 nm Laser with Carbon-,Erbium-, and Titanium-Doped Optothermal Fiber Converters. Lasers Surg. Med. 2018, 51, 185–200. [Google Scholar] [CrossRef]
- Bredikhin, V.I.; Bityurin, N.M.; Kamensky, V.A.; Smirnova, L.A.; Salomatina, E.V.; Streltsova, O.S.; Pochtin, D.P. 06.02.2015 Method of Contact Lithotripsy Patent RU2 604 800C2 Date of Commencement 06.02.2015, Date of Publication of the Application 27.08.2016. Available online: https://www.fips.ru/iiss/document.xhtml?faces-redirect=true&id=7bc9b3aad1d8a1f5fa4ee97b1f3e1629 (accessed on 15 October 2021).
- Bredikhin, V.; Kamensky, V.; Sapogova, N.; Elagin, V.; Shakhoval, M.; Snopova, L.; Bityurin, N. Indirect laser surgery. Appl. Phys. A 2016, 122, 181. [Google Scholar] [CrossRef]
- Belikov, A.V.; Skrypnik, A.V.; Kurnyshev, V.Y. Thermal and optical modeling of ‘blackened’ tips for diode laser surgery. In Biophotonics: Photonic Solutions for Better Health Care V; SPIE: Bellingham, WA, USA, 2016; Volume 9887, p. 98873C. [Google Scholar] [CrossRef]
- Elagin, V.; Smirnov, A.; Yusupov, V.; Kirillov, A.; Ignatova, N.; Streltsova, O.; Grebenkin, E.; Kamensky, V. The bactericidal effect of continuous wave laser with strongly absorbing coating at the fiber tip. J. Innov. Opt. Health Sci. 2018, 11, 1850029. [Google Scholar] [CrossRef] [Green Version]
- Streltsova, O.S.; Grebenkin, E.V.; Pochtin, D.P.; Bredikhin, V.I.; Kamensky, V.A. Contact laser lithotripsy using strongly heated distal tip of optic fiber. Mod. Tehnol. Med. 2017, 9, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Kwiecinska, B.; Murchison, D.; Scott, E. Optical properties of graphite. J. Microsc. 1977, 109, 289–302. [Google Scholar] [CrossRef]
- Li, X.-H.; Zhao, R.; Liu, B.; Yu, Y.-Q. Determination of urinary stone composition using dual-energy spectral CT: Initial in vitro analysis. Clin. Radiol. 2013, 68, e370–e377. [Google Scholar] [CrossRef] [PubMed]
- Sapogova, N.; Bredikhin, V.; Bityurin, N.; Kamensky, V.; Zhigarcov, V.; Yusupov, V. Model for indirect laser surgery. Biomed Opt. Express 2017, 8, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Streltsova, O.S.; Grebenkin, E.V.; Bredikhin, V.I.; Yunusova, K.E.; Elagin, V.V.; Kamensky, V.A. Diode laser lithotripsy of urinary calculi using controlled fragmentation technique. Sovrem. Tehnol. Med. 2019, 11, 103–109. [Google Scholar] [CrossRef]
- Kawahara, T.; Ito, H.; Terao, H.; Ogawa, T.; Uemura, H.; Kubota, Y.; Matsuzaki, J. Stone area and volume are correlated with operative time for cystolithotripsy for bladder calculi using a holmium: Yttrium garnet laser. Scand. J. Urol. Nephrol. 2012, 46, 298–303. [Google Scholar] [CrossRef]
- Romanova, Y.; Mulabaev, N.S.; Tolordava, E.R.; Seregin, A.V.; Seregin, I.V.; Alexeeva, N.V.; Stepanova, T.V.; Levina, G.A.; Barhatova, O.I.; Gamova, N.A.; et al. Microbial communities on kidney stones. Mol. Gen. Mikrobiol. Virusol. 2015, 30, 78–84. [Google Scholar] [CrossRef]
- Eswara, J.R.; Shariftabrizi, A.; Sacco, D. Positive stone culture is associated with a higher rate of sepsis after endourological procedures. Urolithiasis 2013, 41, 411–414. [Google Scholar] [CrossRef] [PubMed]




| Operation | NLE | ULT | ULE |
|---|---|---|---|
| Nephrolithotripsy (NLT) | <0.01 | <0.001 | <0.001 |
| Nephrolithoextraction (NLE) | - | <0.001 | <0.001 |
| Ureterolithotripsy (ULT) | <0.001 | - | <0.001 |
| Stone Parameters | 0.81 μm | 0.97 μm | 1.47 μm |
|---|---|---|---|
| n | 27/9 * | 36/9 * | 15/0 * |
| Size, mm | 10.26 ± 5.99 | 12.5 ± 4.89 | 8.4 ± 1.89 |
| Mean fragmentation time, s | 16 ± 7.82 | 12.06 ± 4.42/31.27 ± 20.4 ** | 4.5 ± 1.32/19.14 ± 9.4 ** |
| Mean X-ray density, HU | 813.3 ± 479.88 | 1030 ± 426.8 | 932 ± 345.6 |
| X-ray density of non-fragmented stone, HU | >1000 (n = 9) | >1400 (n = 9) | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streltsova, O.S.; Grebenkin, E.V.; Bityurin, N.M.; Bredikhin, V.I.; Elagin, V.V.; Vlasov, V.V.; Kamensky, V.A. Diode Laser Lithotription Technique Based on Optothermal Converter. Photonics 2021, 8, 452. https://doi.org/10.3390/photonics8100452
Streltsova OS, Grebenkin EV, Bityurin NM, Bredikhin VI, Elagin VV, Vlasov VV, Kamensky VA. Diode Laser Lithotription Technique Based on Optothermal Converter. Photonics. 2021; 8(10):452. https://doi.org/10.3390/photonics8100452
Chicago/Turabian StyleStreltsova, Olga S., Evgeny V. Grebenkin, Nikita M. Bityurin, Vladimir I. Bredikhin, Vadim V. Elagin, Vasily V. Vlasov, and Vladislav A. Kamensky. 2021. "Diode Laser Lithotription Technique Based on Optothermal Converter" Photonics 8, no. 10: 452. https://doi.org/10.3390/photonics8100452