Micropatterning MoS2/Polyamide Electrospun Nanofibrous Membranes Using Femtosecond Laser Pulses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PA6-MoS2 Nanofibers
2.3. Femtosecond Laser Micromachining and Characterization
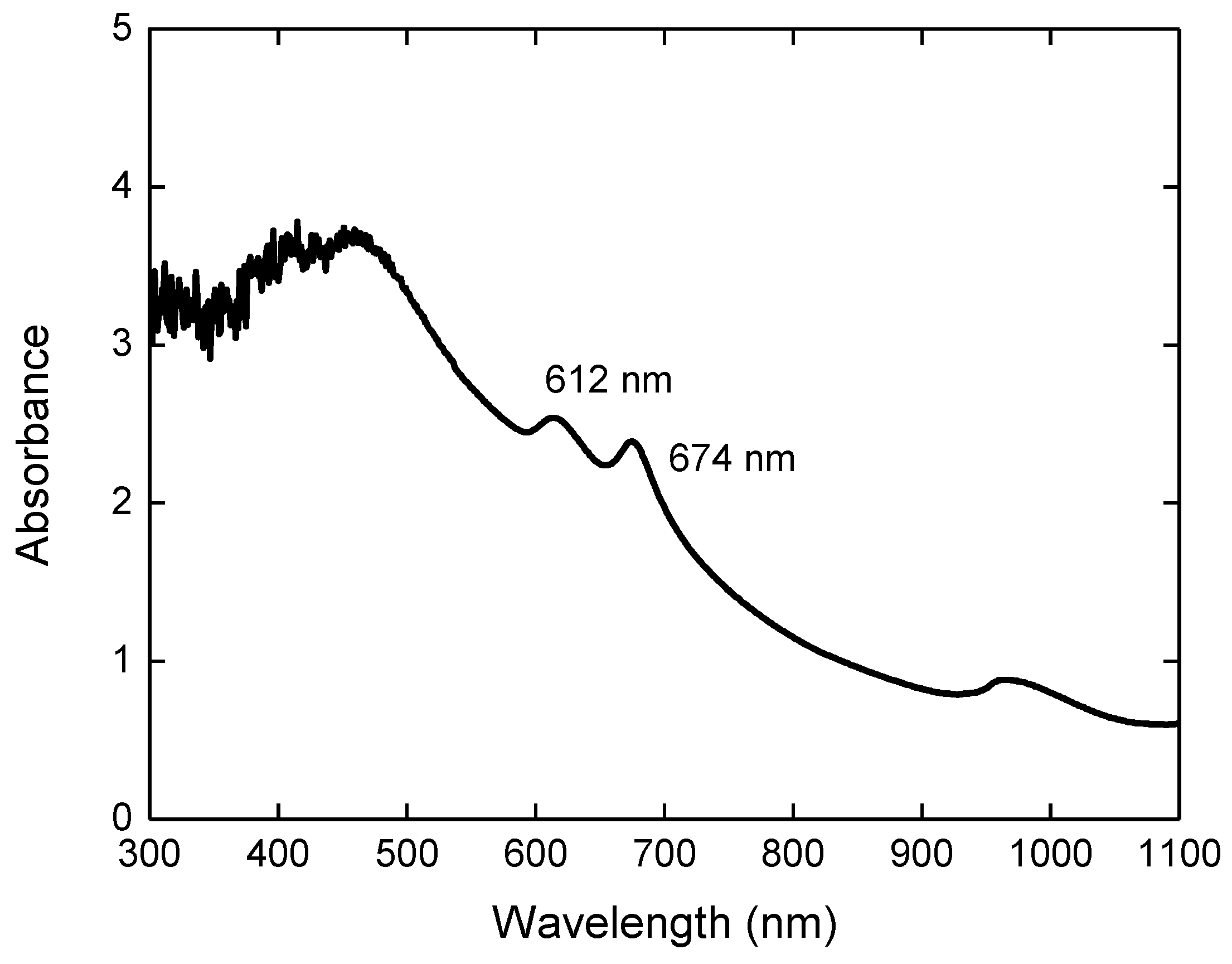
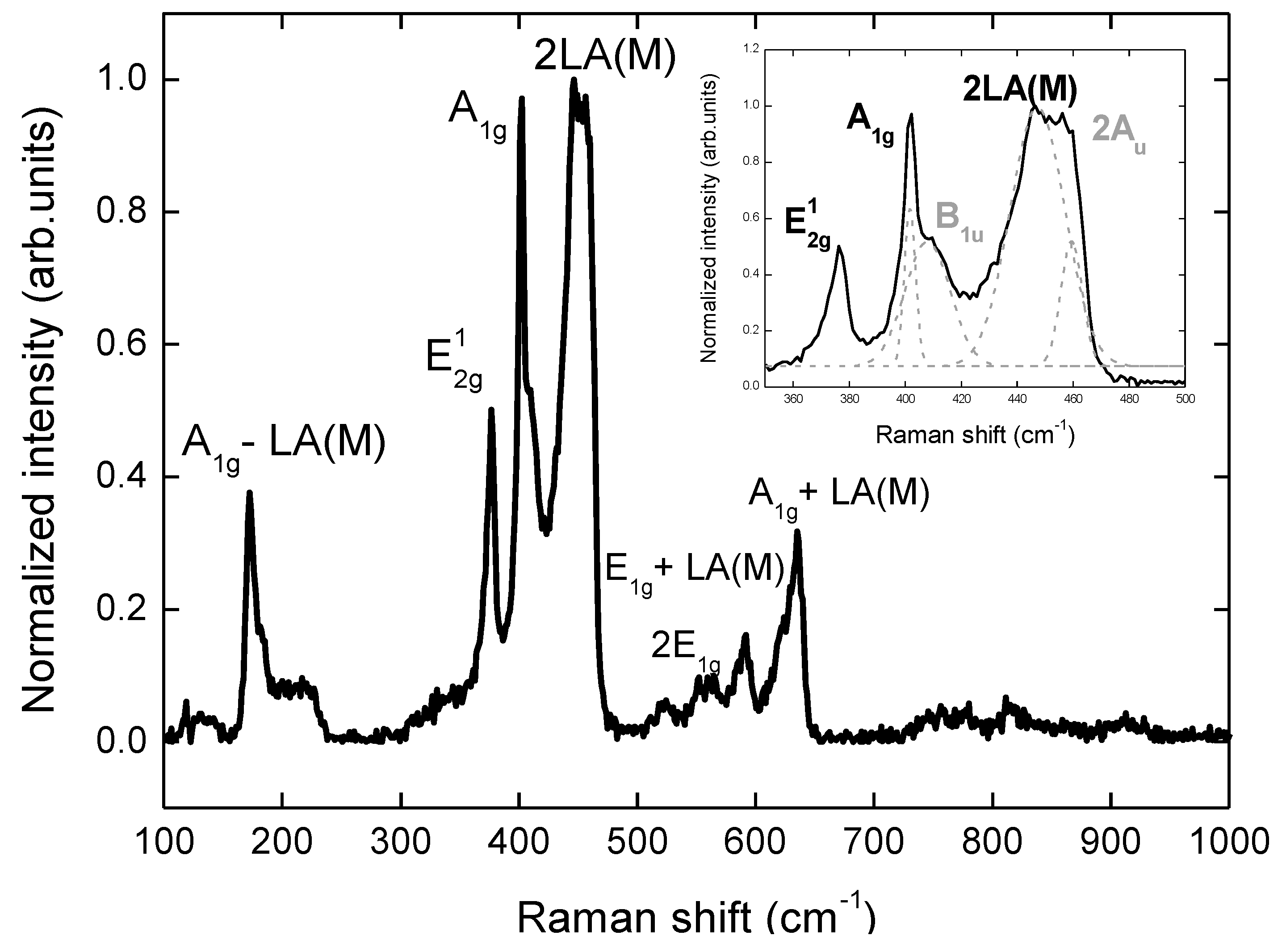
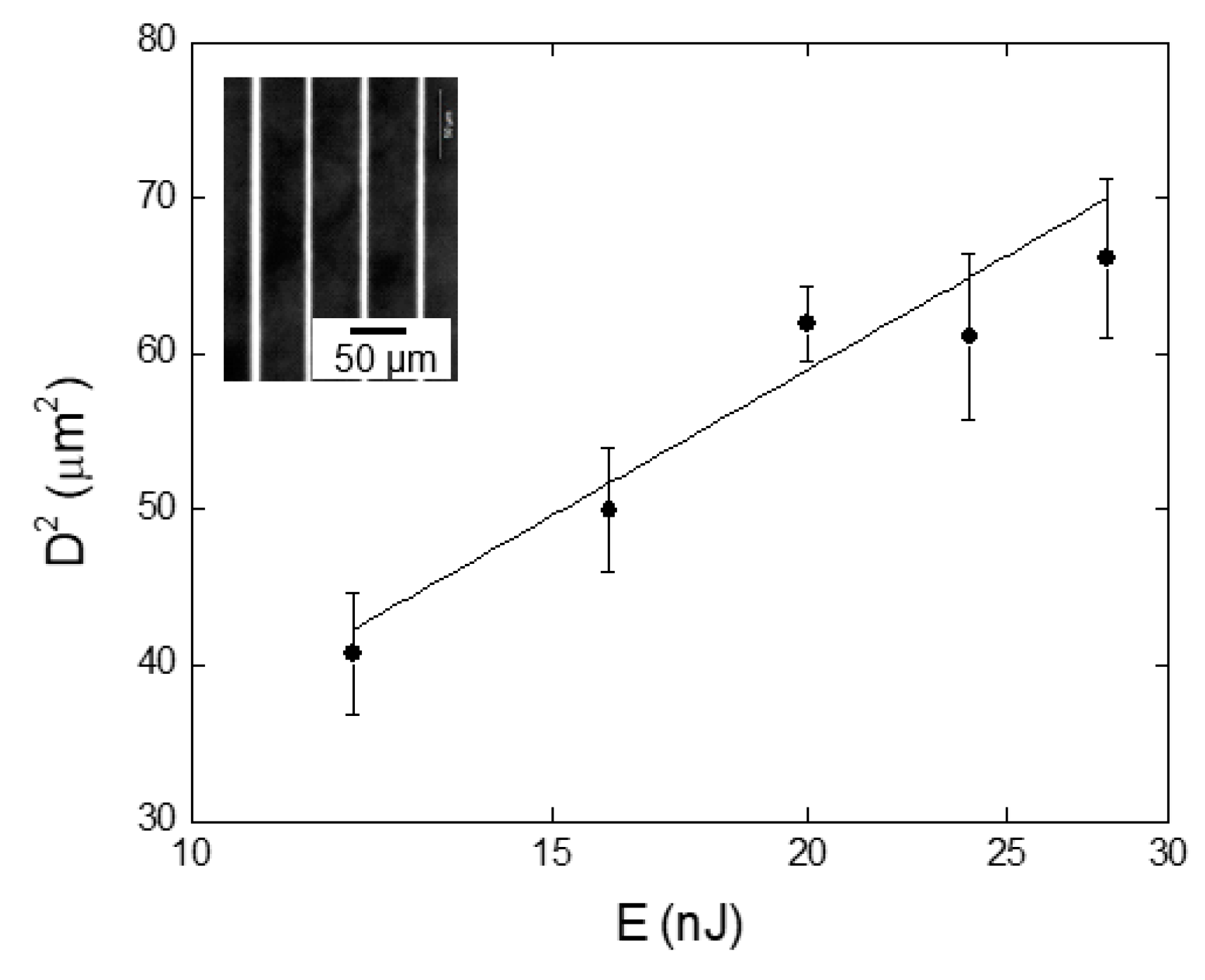
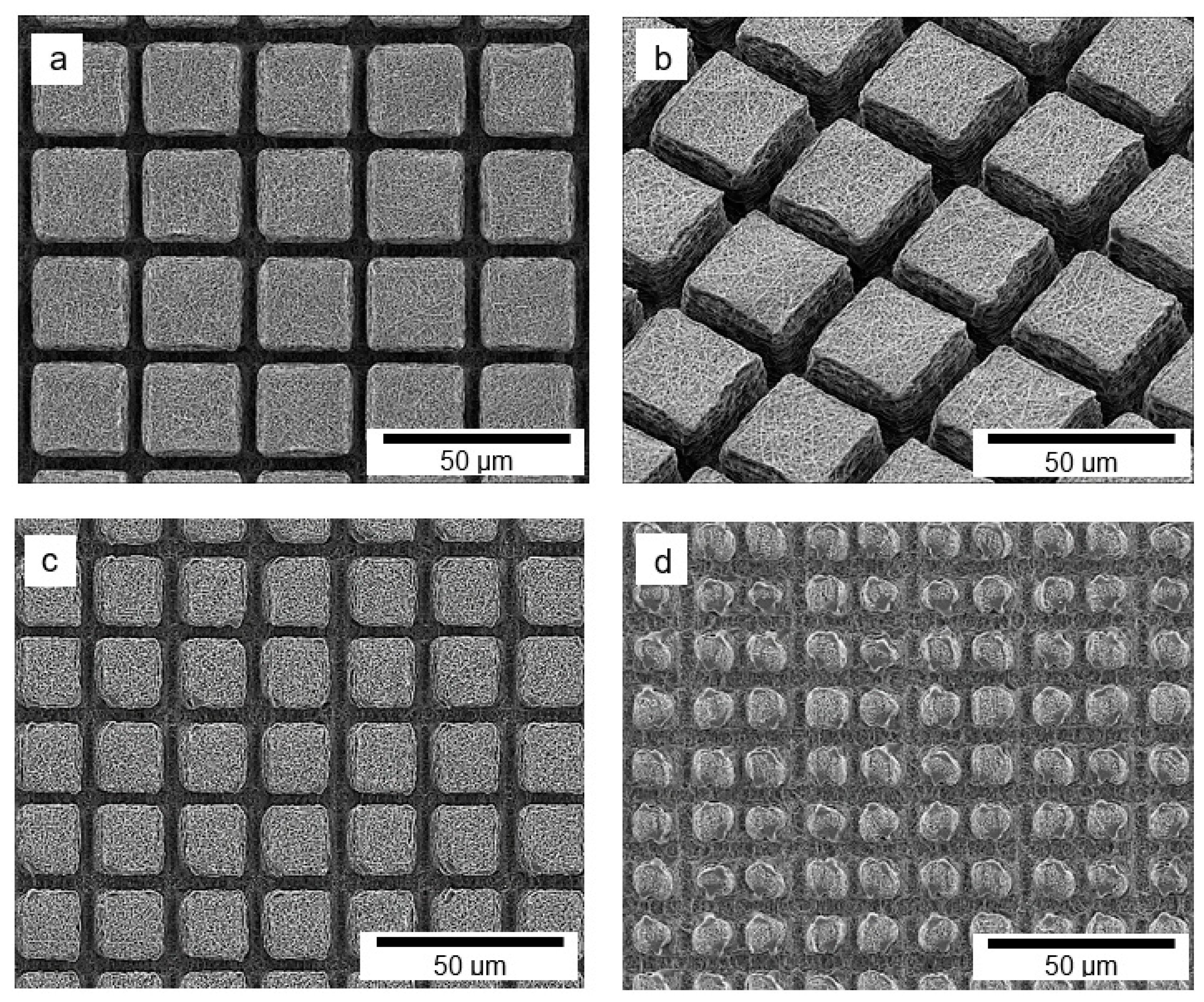
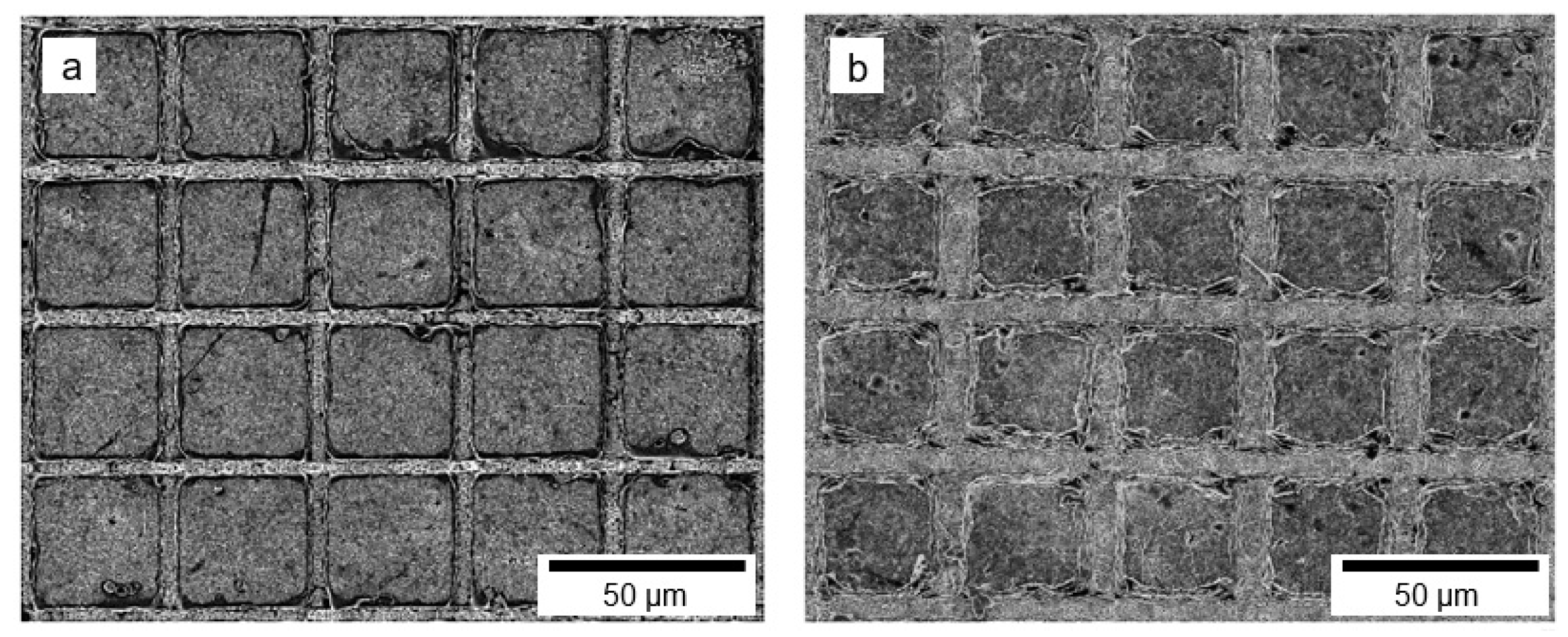
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Wong, S.L. Functionalization of 2D transition metal dichalcogenides for biomedical applications. Mater. Sci. Eng. C 2017, 70, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Chatterjee, K. Recent advances in the field of transition metal dichalcogenides for biomedical applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shan, J.; Zhang, W.; Su, S.; Yuwen, L.; Wang, L. Recent Advances in Synthesis and Biomedical Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets. Small 2017, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, W.; Feng, X.; Song, L.; Hu, Y. MoS2/Polymer Nanocomposites: Preparation, Properties, and Applications. Polym. Rev. 2017, 57, 440–466. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z.; Daeneke, T.; Strano, M.S.; Pumera, M.; Gras, S.L. Two-Dimensional Transition Metal Dichalcogenides in Biosystems. Adv. Funct. Mater. 2015, 25, 5086–5099. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Dutta, H.S.; Gogoi, S.; Devi, R.; Khan, R. Nanostructured MoS2 Based Advanced Biosensors: A Review. ACS Appl. Nano Mater. 2017. [Google Scholar] [CrossRef]
- Wu, S.; Wang, J.; Jin, L.; Li, Y.; Wang, Z. Effects of Polyacrylonitrile/MoS2 Composite Nanofibers on the Growth Behavior of Bone Marrow Mesenchymal Stem Cells. ACS Appl. Nano Mater. 2018. [Google Scholar] [CrossRef]
- Kurapati, R.; Kostarelos, K.; Prato, M.; Bianco, A. Biomedical Uses for 2D Materials Beyond Graphene: Current Advances and Challenges Ahead. Adv. Mater. 2016, 6052–6074. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Tourlomousis, F.; Chang, R.C.; Yang, E. Influence of Transition Metal Dichalcogenide Surfaces on Cellular Morphology and Adhesion. ACS Appl. Biol. Mater. 2018. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Hu, B.; Li, Z.; Li, J.; Gao, Y.; Wang, Z.; Hao, J. Synergistic Effects of Electrical Stimulation and Aligned Nanofibrous Microenvironment on Growth Behavior of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 18543–18550. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Xue, R.; Lannutti, J.J.; Farson, D.F. Ablation characteristics of electrospun core-shell nanofiber by femtosecond laser. Mater. Sci. Eng. C 2016, 65, 232–239. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Paula, K.T.; Gaál, G.; Almeida, G.F.B.; Andrade, M.B.; Facure, M.H.M.; Correa, D.S.; Riul, A.; Rodrigues, V.; Mendonça, C.R. Femtosecond laser micromachining of polylactic acid/graphene composites for designing interdigitated microelectrodes for sensor applications. Opt. Laser Technol. 2018, 101, 74–79. [Google Scholar] [CrossRef]
- Correa, D.S.; Cardoso, M.R.; Tribuzi, V.; Misoguti, L.; Mendonca, C.R. Femtosecond laser in polymeric materials: Microfabrication of doped structures and micromachining. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 176–186. [Google Scholar] [CrossRef]
- Tanvir Ahmmed, K.M.; Grambow, C.; Kietzig, A.M. Fabrication of micro/nano structures on metals by femtosecond laser micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Alubaidy, M.; Venkatakrishnan, K.; Tan, B. Fabrication of a reinforced polymer microstructure using femtosecond laser material processing. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- Park, C.; Xue, R.; Lannutti, J.J.; Farson, D.F. A comparison of chinese and western civilisation: By an early Sociologist. Sociol. Rev. 1927, 19, 89–105. [Google Scholar] [CrossRef]
- Jenness, N.J.; Wu, Y.; Clark, R.L. Fabrication of three-dimensional electrospun microstructures using phase modulated femtosecond laser pulses. Mater. Lett. 2012, 66, 360–363. [Google Scholar] [CrossRef]
- Woon Choi, H.; Johnson, J.K.; Nam, J.; Farson, D.F.; Lannutti, J. Structuring electrospun polycaprolactone nanofiber tissue scaffolds by femtosecond laser ablation. J. Laser Appl. 2007, 19, 225–231. [Google Scholar] [CrossRef]
- Jun, I.; Kim, K.; Chung, Y.W.; Shin, H.J.; Han, H.S.; Edwards, J.R.; Ok, M.R.; Kim, Y.C.; Seok, H.K.; Shin, H.; et al. Effect of spatial arrangement and structure of hierarchically patterned fibrous scaffolds generated by a femtosecond laser on cardiomyoblast behavior. J. Biomed. Mater. Res. Part A 2018, 106, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, J.; Farson, D.F.; Lannutti, J.J.; Rokhlin, S.I. Wettability modification of electrospun poly(ε-caprolactone) fibers by femtosecond laser irradiation in different gas atmospheres. Appl. Surf. Sci. 2011, 257, 3547–3553. [Google Scholar] [CrossRef]
- Lim, Y.C.; Johnson, J.; Fei, Z.; Wu, Y.; Farson, D.F.; Lannutti, J.J.; Choi, H.W.; Lee, L.J. Micropatterning and characterization of electrospun poly(ε-caprolactone)/gelatin nanofiber tissue scaffolds by femtosecond laser ablation for tissue engineering applications. Biotechnol. Bioeng. 2011, 108, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lin, H.; Jia, B. Two-dimensional material functional devices enabled by direct laser fabrication. Front. Optoelectron. 2018, 11, 2–22. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, E.; Hwang, D.J. Femtosecond laser patterning, synthesis, defect formation, and structural modification of atomic layered materials. MRS Bull. 2016, 41, 1002–1007. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS2with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van Der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of few-layer MoS2nanosheet-coated TiO2nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Hydrophilic nylon 6,6 nanofibers supported thin film composite membranes for engineered osmosis. J. Memb. Sci. 2014, 457, 162–169. [Google Scholar] [CrossRef]
- Chow, P.K.; Singh, E.; Viana, B.C.; Gao, J.; Luo, J.; Li, J.; Lin, Z.; Elías, A.L.; Shi, Y.; Wang, Z.; Terrones, M.; et al. Wetting of Mono and Few-Layered WS2 and MoS2 Films Supported on Si/SiO2 Substrates. ACS Nano 2015, 9, 3023–3031. [Google Scholar] [CrossRef]
- Kozbial, A.; Gong, X.; Liu, H.; Li, L. Understanding the Intrinsic Water Wettability of Molybdenum Disulfide (MoS2). Langmuir 2015, 31, 8429–8435. [Google Scholar] [CrossRef]
- Annamalai, M.; Gopinadhan, K.; Han, S.A.; Saha, S.; Park, H.J.; Cho, E.B.; Kumar, B.; Patra, A.; Kim, S.W.; Venkatesan, T. Surface energy and wettability of van der Waals structures. Nanoscale 2016, 8, 5764–5770. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.; Palummo, M.; Grossman, J.C. Extraordinary sunlight absorption and one nanometer thick photovoltaics using two-dimensional monolayer materials. Nano Lett. 2013, 13, 3664–3670. [Google Scholar] [CrossRef]
- Dumcenco, D.; Ovchinnikov, D.; Marinov, K.; Lazić, P.; Gibertini, M.; Marzari, N.; Sanchez, O.L.; Kung, Y.C.; Krasnozhon, D.; Chen, M.W.; Bertolazzi, S.; et al. Large-area epitaxial monolayer MOS2. ACS Nano 2015, 9, 4611–4620. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Felder, P.; Yang, X.; Baum, G.; Huber, R. Photofragment Translational Spectroscopy of Fluorinated Halomethanes. Isr. J. Chem. 1994, 34, 33–42. [Google Scholar] [CrossRef]
- Gołasa, K.; Grzeszczyk, M.; Bozek, R.; Leszczyński, P.; Wysmołek, A.; Potemski, M.; Babiński, A. Resonant Raman scattering in MoS2- From bulk to monolayer. Solid State Commun. 2014, 197, 53–56. [Google Scholar] [CrossRef]
- Windom, B.C.; Sawyer, W.G.; Hahn, D.W. A raman spectroscopic study of MoS2 and MoO3: Applications to tribological systems. Tribol. Lett. 2011, 42, 301–310. [Google Scholar] [CrossRef]
- Jeong, S.; Shin, H.-Y.; Shin, R.H.; Jo, W.; Yoon, S.; Rübhausen, M. Raman scattering studies of the lattice dynamics in layered MoS2. J. Korean Phys. Soc. 2015, 66, 1575–1580. [Google Scholar] [CrossRef]
- Chakraborty, B.; Matte, H.S.S.R.; Sood, A.K.; Rao, C.N.R. Layer-dependent resonant Raman scattering of a few layer MoS2. J. Raman Spectrosc. 2013, 44, 92–96. [Google Scholar] [CrossRef]
- Liu, J.M. Simple technique for measurements of pulsed Gaussian-beam spot sizes. Opt. Lett. 1982, 7, 196–198. [Google Scholar] [CrossRef]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; Michaelis de Vasconcellos, S.; Bratschitsch, R. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2. Opt. Express 2013, 21, 4908. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Wu, J.; Lin, M.; Wang, L.; Zhang, T. Photoluminescence of MoS2 Prepared by Effective Grinding-Assisted Sonication Exfoliation. J. Nanomater. 2014, 2014, 107. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paula, K.T.; Mercante, L.A.; Schneider, R.; Correa, D.S.; Mendonca, C.R. Micropatterning MoS2/Polyamide Electrospun Nanofibrous Membranes Using Femtosecond Laser Pulses. Photonics 2019, 6, 3. https://doi.org/10.3390/photonics6010003
Paula KT, Mercante LA, Schneider R, Correa DS, Mendonca CR. Micropatterning MoS2/Polyamide Electrospun Nanofibrous Membranes Using Femtosecond Laser Pulses. Photonics. 2019; 6(1):3. https://doi.org/10.3390/photonics6010003
Chicago/Turabian StylePaula, Kelly T., Luiza A. Mercante, Rodrigo Schneider, Daniel S. Correa, and Cleber R. Mendonca. 2019. "Micropatterning MoS2/Polyamide Electrospun Nanofibrous Membranes Using Femtosecond Laser Pulses" Photonics 6, no. 1: 3. https://doi.org/10.3390/photonics6010003




