Efficient Disinfection of Tap and Surface Water with Single High Power 285 nm LED and Square Quartz Tube
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Setup
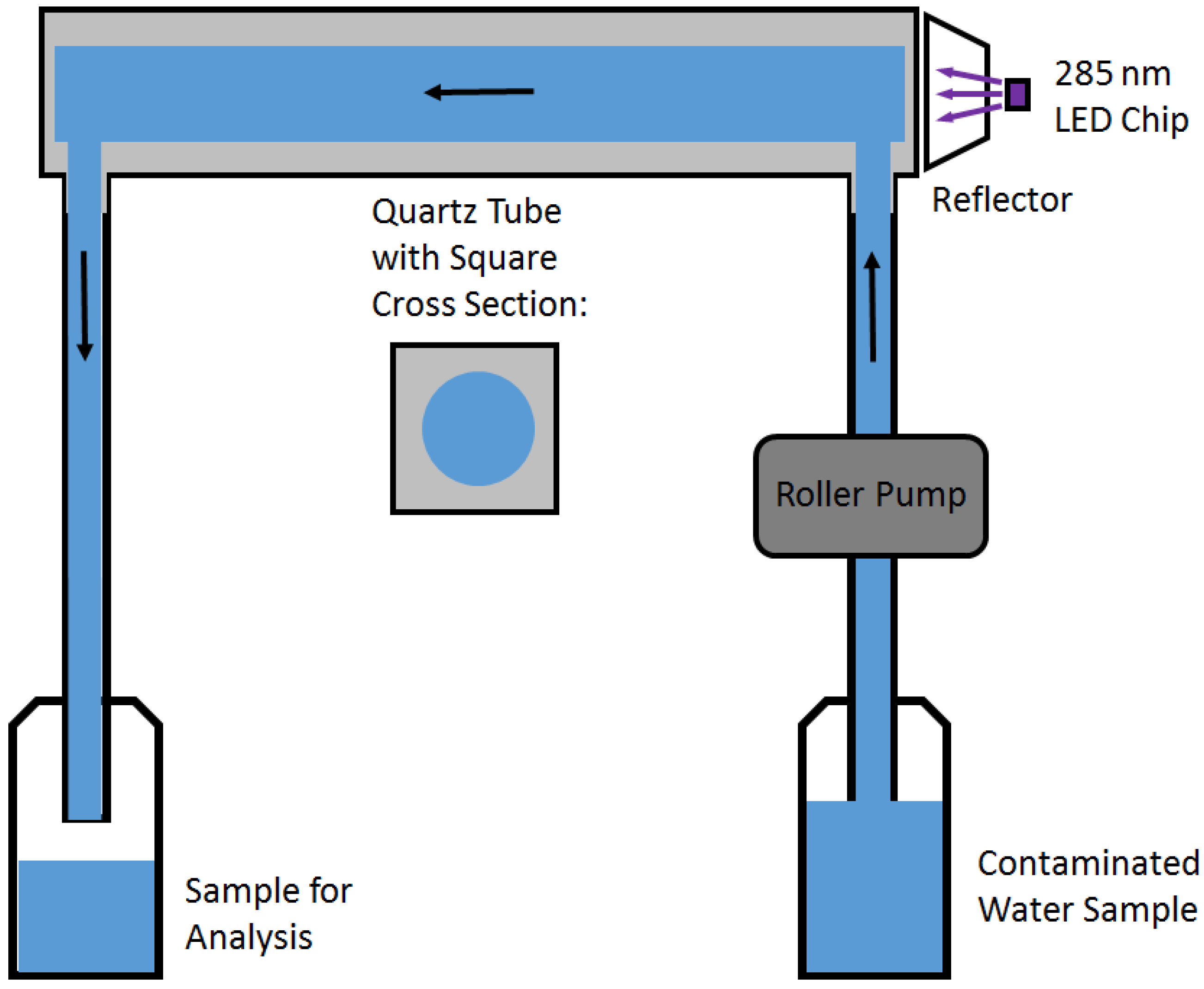
2.2. Measurement Procedure and Analysis
3. Results and Discussion

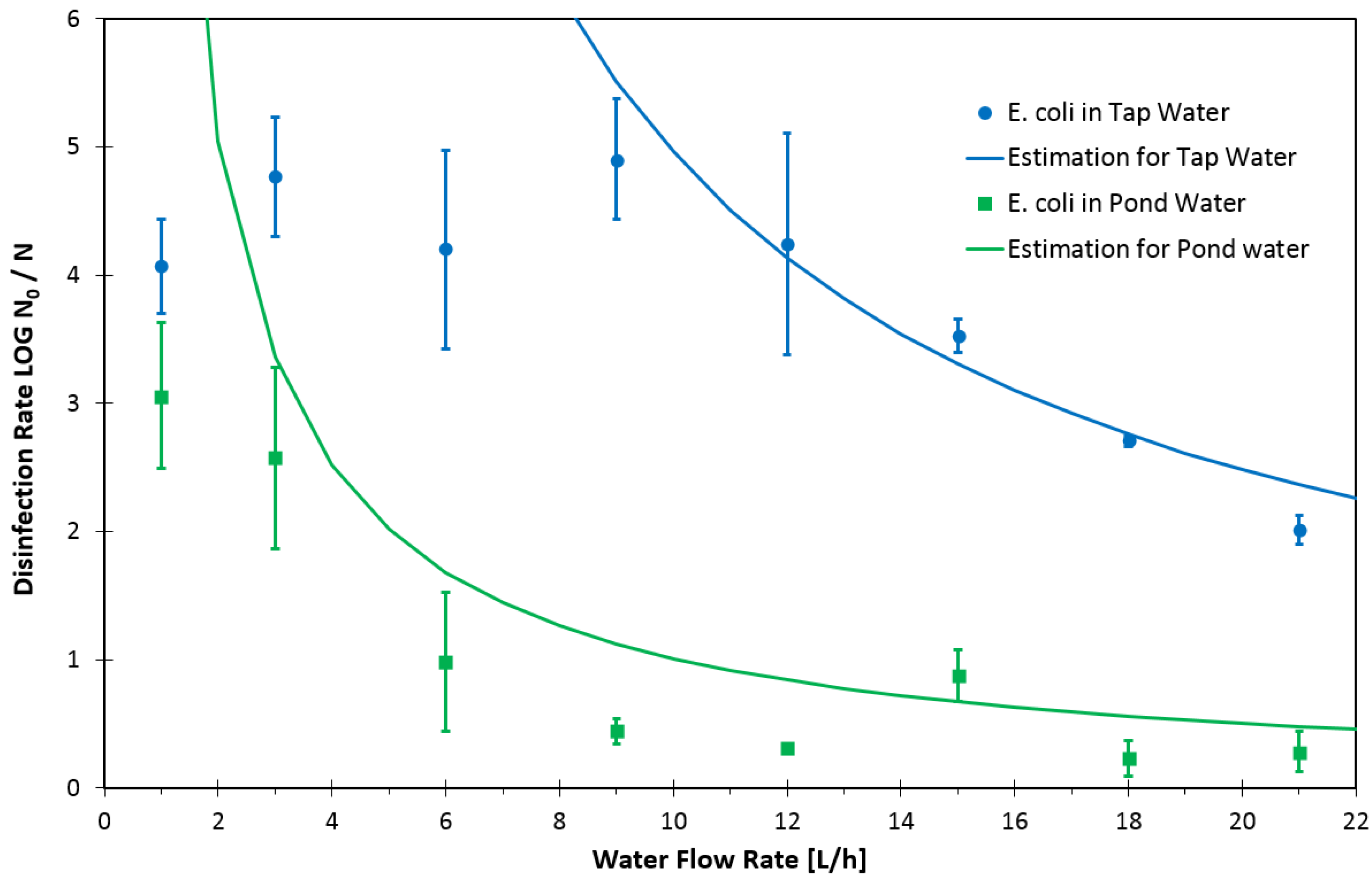
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lazarova, V.; Savoye, P.; Janex, M.L.; Blatchley, E.R.; Pommepuy, M. Advanced wastewater disinfection technologies: State of the art and perspectives. Water Sci. Technol. 1999, 4–5, 203–213. [Google Scholar] [CrossRef]
- Borella, P.; Montagna, M.T.; Romano-Spica, V.; Stampi, S.; Stancanelli, G.; Triassi, M.; Neglia, R.; Marchesi, I.; Fantuzzi, G.; Tatò, D.; et al. Legionella infection risk from domestic hot water. Emerg. Infect. Dis. 2004, 10, 457–464. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Legionnaires’ Disease in Europe 2013; ECDC: Stockholm, Sweden, 2015. [Google Scholar] [CrossRef]
- Szymańska, J.; Sitkowska, J.; Dutkiewicz, J. Microbial contamination of dental unit waterlines. Ann. Agric. Environ. Med. 2008, 15, 173–179. [Google Scholar] [PubMed]
- Cervia, J.S.; Ortolano, G.A.; Canonica, F.P. Hospital tap water—A reservoir of risk for health care-associated infection. Infect. Dis. Clin. Prac. 2008, 16, 349–353. [Google Scholar] [CrossRef]
- Decker, B.K.; Palmore, T.N. The role of water in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.; Yu, V.L.; Stout, J.E. Waterborne nosocomial infections. Curr. Infect. Dis. Rep. 2000, 2, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Bain, R.; Cronk, R.; Hossain, R.; Bonjour, S.; Onda, K.; Wright, J.; Yang, H.; Slaymaker, T.; Hunter, P.; Prüss-Ustün, A.; et al. Global assessment of exposure to faecal contamination through drinking water based on a systematic review. Trop. Med. Int. Health 2014, 19, 917–927. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF. Joint Water Supply and Sanitation Monitoring Programme: Progress on Drinking Water and Sanitation; WHO/UNICEF: Geneva, Switzerland; New York, NY, USA, 2014. [Google Scholar]
- WHO/UNICEF. Joint Water Supply and Sanitation Monitoring Programme: Progress on Drinking Water and Sanitation; WHO/UNICEF: Geneva, Switzerland; New York, NY, USA, 2012. [Google Scholar]
- Acra, A.; Karahagopian, Y.; Raffoul, Z.; Dajani, R. Disinfection of oral rehydration solutions by sunlight. Lancet 1980, 2, 1257–1258. [Google Scholar] [CrossRef]
- Mäusezahl, D.; Christen, A.; Pacheco, G.D.; Tellez, F.A.; Iriarte, M.; Zapata, M.E.; Cevallos, M.; Hattendorf, J.; Cattaneo, M.D.; Arnold, B.; et al. Solar drinking water disinfection (SODIS) to reduce childhood diarrhoea in rural Bolivia: A cluster-randomized, controlled trial. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Henri, V.; Helbronner, A.; Von Recklinghausen, M. Stérilization de grandes quantités d’Eau par les rayons Ultraviolets. Compt. Rend. Acad. Sci. 1910, 150, 932–934. [Google Scholar]
- Hayward, K. UV LEDs light the way for disruptive technologies. Water 2013, 21, 41–42. [Google Scholar]
- Gross, A.; Stangl, F.; Hoenes, K.; Sift, M.; Hessling, M. Improved drinking water disinfection with UVC-LEDs for Escherichia coli and Bacillus subtilis utilizing quartz tubes as light guide. Water 2015, 7, 4605–4621. [Google Scholar] [CrossRef]
- Chatterley, C.; Linden, K. Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection. J. Water Health 2010, 8, 479–486. [Google Scholar] [CrossRef]
- Harris, T.R.; Pagan, J.; Batoni, P.; Stokes, R. Optical and fluidic co-design of a UV-LED water disinfection chamber. In Proceedings of the Electrochemical Society Meeting, Seattle, WA, USA, May 2012.
- Bilenko, Y.; Shturm, I.; Bilenko, O.; Shatalov, M.; Gaska, R. New UV Technology for point-of-use water disinfection. In Proceedings of the 2010 Clean Technology Conference, Anaheim, CA, USA, 21–24 June 2010; pp. 339–342.
- Stangl, F.; Gross, A.; Hoenes, K.; Sift, M.; Schlau, D.; Hessling, M. Advanced photovoltaic water disinfection system based on efficient UVC and ultrasound generation. In Proceedings of the 4th Conference Small PV Applications, Munich, Germany, 9–10 June 2015; pp. 86–91.
- Huang, D.B.; DuPont, H.L. Enteroaggregative Escherichia coli: An emerging pathogen in children. Semin. Pediatr. Infect. Dis. 2004, 15, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Chevrefils, G.; Caron, E. UV dose required to achieve incremental log inactivation of bacteria, protozoa and viruses. IUVA News 2006, 8, 38–45. [Google Scholar]
- Gross, A.; Stangl, F.; Hoenes, K.; Hessling, M.; Brucher, R. Frequency-controlled power ultrasound unit for battery-powered UVC-LED based disinfection system. Int. J. Adv. Res. Comput. Sci. Electron. Eng. 2014, 3, 476–481. [Google Scholar]
- DIN Deutsches Institut für Normung e.V. DIN EN ISO 8199: 2007 Water Quality—General Guidance on the Enumeration of Microorganisms by Culture; Beuth: Berlin, Germany, 2008. [Google Scholar]
- Integrated DNA Technologies Inc. UV Spectrum of DNA Calculator V 1.02 (2105). Available online: http://biophysics.idtdna.com/cgi-bin/uvCalculator.cgi (accessed on 30 November 2015).
- Bowker, C.; Sain, A.; Shatalov, M.; Ducoste, J. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res. 2011, 45, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hessling, M.; Gross, A.; Hoenes, K.; Rath, M.; Stangl, F.; Tritschler, H.; Sift, M. Efficient Disinfection of Tap and Surface Water with Single High Power 285 nm LED and Square Quartz Tube. Photonics 2016, 3, 7. https://doi.org/10.3390/photonics3010007
Hessling M, Gross A, Hoenes K, Rath M, Stangl F, Tritschler H, Sift M. Efficient Disinfection of Tap and Surface Water with Single High Power 285 nm LED and Square Quartz Tube. Photonics. 2016; 3(1):7. https://doi.org/10.3390/photonics3010007
Chicago/Turabian StyleHessling, Martin, Andrej Gross, Katharina Hoenes, Monika Rath, Felix Stangl, Hanna Tritschler, and Michael Sift. 2016. "Efficient Disinfection of Tap and Surface Water with Single High Power 285 nm LED and Square Quartz Tube" Photonics 3, no. 1: 7. https://doi.org/10.3390/photonics3010007
APA StyleHessling, M., Gross, A., Hoenes, K., Rath, M., Stangl, F., Tritschler, H., & Sift, M. (2016). Efficient Disinfection of Tap and Surface Water with Single High Power 285 nm LED and Square Quartz Tube. Photonics, 3(1), 7. https://doi.org/10.3390/photonics3010007





