Turn a Shrimp into a Firefly: Monitoring Tissue pH in Small Crustaceans Using an Injectable Hydrogel Sensor with Infrared Excitation and Visible Luminescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensor Preparation
2.2. Signal Acquisition and Sensor Calibration
2.3. Amphipod Sampling and Performed Experiments
2.4. Obtaining Hemocyte Primary Culture and Performed Tests
2.5. Phenoloxidase Assay
2.6. Histological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Calibration of the IR-Excited pH-Sensors
3.2. Testing Influence of the Sensor Components on Amphipods
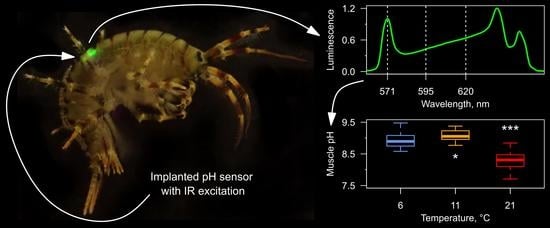
3.3. Tissue pH Monitoring under Temperature Increase
3.4. Long-Term Immune Response to the Injected Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rong, G.; Tuttle, E.E.; Neal Reilly, A.; Clark, H.A. Recent developments in nanosensors for imaging applications in biological systems. Annu. Rev. Anal. Chem. 2019, 12, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Wang, S.; Jin, X.; Bakh, N.A.; Nguyen, F.T.; Dong, J.; Silmore, K.S.; Gong, X.; Pham, C.; Jones, K.K.; et al. Implantable Nanosensors for Human Steroid Hormone Sensing In Vivo Using a Self-Templating Corona Phase Molecular Recognition. Adv. Healthc. Mater. 2020, 9, 2000429. [Google Scholar] [CrossRef] [PubMed]
- Kaefer, K.; Krüger, K.; Schlapp, F.; Uzun, H.; Celiksoy, S.; Flietel, B.; Heimann, A.; Schroeder, T.; Kempski, O.; Sonnichsen, C.; et al. Implantable sensors based on gold nanoparticles for continuous long-term concentration monitoring in the body. Nano Lett. 2021, 21, 3325–3330. [Google Scholar] [CrossRef]
- Bian, S.; Zhu, B.; Rong, G.; Sawan, M. Towards wearable and implantable continuous drug monitoring: A review. J. Pharm. Anal. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Q.; Shi, C.; Chen, M.; Ma, K.; Wan, J.; Liu, R. Dealing with the foreign-body response to implanted biomaterials: Strategies and applications of new materials. Adv. Funct. Mater. 2021, 31, 2007226. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Kanick, S.C.; Schneider, P.A.; Klitzman, B.; Wisniewski, N.A.; Rebrin, K. Continuous monitoring of interstitial tissue oxygen using subcutaneous oxygen microsensors: In vivo characterization in healthy volunteers. Microvasc. Res. 2019, 124, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Jin, X.; Muthupalani, S.; Bakh, N.A.; Gong, X.; Strano, M.S. In-Vivo fluorescent nanosensor implants based on hydrogel-encapsulation: Investigating the inflammation and the foreign-body response. J. Nanobiotechnol. 2023, 21, 133. [Google Scholar] [CrossRef]
- Balaconis, M.K.; Luo, Y.; Clark, H.A. Glucose-sensitive nanofiber scaffolds with an improved sensing design for physiological conditions. Analyst 2015, 140, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Rong, G.; Corrie, S.R.; Clark, H.A. In vivo biosensing: Progress and perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.A.; Nguyen, F.T.; Scott, K.; Chan, N.Y.; Bakh, N.A.; Jones, K.K.; Pham, C.; Garcia-Salinas, P.; Garcia-Parraga, D.; Fahlman, A.; et al. Implanted nanosensors in marine organisms for physiological biologging: Design, feasibility, and species variability. ACS Sens. 2018, 4, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rzhechitskiy, Y.; Gurkov, A.; Bolbat, N.; Shchapova, E.; Nazarova, A.; Timofeyev, M.; Borvinskaya, E. Adipose Fin as a Natural “Optical Window” for Implantation of Fluorescent Sensors into Salmonid Fish. Animals 2022, 12, 3042. [Google Scholar] [CrossRef]
- Herring, P.J.; Gaten, E.; Shelton, P.M. Are vent shrimps blinded by science? Nature 1999, 398, 116. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, T.; Yakovleva, M.; Lindström, M.; Donner, K.; Ostrovsky, M. Eye adaptation to different light environments in two populations of Mysis relicta: A comparative study of carotenoids and retinoids. J. Crust. Biol. 2010, 30, 636–642. [Google Scholar] [CrossRef] [Green Version]
- Gurkov, A.; Shchapova, E.; Bedulina, D.; Baduev, B.; Borvinskaya, E.; Meglinski, I.; Timofeyev, M. Remote in vivo stress assessment of aquatic animals with microencapsulated biomarkers for environmental monitoring. Sci. Rep. 2016, 6, 36427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, J.X.; Zhou, W.C.; Zhu, X.G. Mitochondria as potential targets and initiators of the blue light hazard to the retina. Oxidative Med. Cell. Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef] [Green Version]
- Achatz, D.E.; Meier, R.J.; Fischer, L.H.; Wolfbeis, O.S. Luminescent sensing of oxygen using a quenchable probe and upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 260–263. [Google Scholar] [CrossRef]
- Arppe, R.; Näreoja, T.; Nylund, S.; Mattsson, L.; Koho, S.; Rosenholm, J.M.; Soukka, T.; Schäferling, M. Photon upconversion sensitized nanoprobes for sensing and imaging of pH. Nanoscale 2014, 6, 6837–6843. [Google Scholar] [CrossRef]
- Tajon, C.A.; Yang, H.; Tian, B.; Tian, Y.; Ercius, P.; Schuck, P.J.; Chan, E.M.; Cohen, B.E. Photostable and efficient upconverting nanocrystal-based chemical sensors. Opt. Mater. 2018, 84, 345–353. [Google Scholar] [CrossRef]
- Mahata, M.K.; De, R.; Lee, K.T. Near-infrared-triggered upconverting nanoparticles for biomedicine applications. Biomedicines 2021, 9, 756. [Google Scholar] [CrossRef]
- Rabet, N. Crustaceans. In Handbook of Marine Model Organisms in Experimental Biology, 1st ed.; Agnes, B., Bernd, S., Eds.; CRC Press: Boca Raton, MA, USA, 2021; pp. 271–287. [Google Scholar]
- Odeyemi, O.A.; Dabade, D.S.; Amin, M.; Dewi, F.; Waiho, K.; Kasan, N.A. Microbial diversity and ecology of crustaceans: Influencing factors and future perspectives. Curr. Opin. Food Sci. 2021, 39, 140–143. [Google Scholar] [CrossRef]
- Väinölä, R.; Witt, J.D.S.; Grabowski, M.; Bradbury, J.H.; Jazdzewski, K.; Sket, B. Global diversity of amphipods (Amphipoda; Crustacea) in freshwater. Hydrobiologia 2008, 595, 241–255. [Google Scholar] [CrossRef]
- Brown, K.P.; Gerber, A.; Bedulina, D.; Timofeyev, M.A. Human impact and ecosystemic health at Lake Baikal. Wiley Interdiscip. Rev. Water. 2021, 8, e1528. [Google Scholar] [CrossRef]
- Takhteev, V.V.; Berezina, N.A.; Sidorov, D.A. Checklist of the amphipoda (Crustacea) from continental waters of Russia, with data on alien species. Arthropoda Sel. 2015, 24, 335–370. [Google Scholar] [CrossRef] [Green Version]
- Karnaukhov, D.Y.; Dolinskaya, E.M.; Biritskaya, S.A.; Teplykh, M.A.; Ermolaeva, Y.K.; Pushnica, V.A.; Kuznetsova, I.V.; Okholina, A.I.; Bukhaeva, L.B.; Rusanovskaya, O.O.; et al. New data regarding ecology of freshwater pelagicamphipod Macrohectopus branickii (Dybowsky, 1874) (Amphipoda: Macrohectopodidae) and other crustaceans of plankton from the southern part of Lake Baikal (Russia, Southern Siberia). Acta Biol. Sib. 2021, 7, 39–48. [Google Scholar]
- Drozdova, P.; Saranchina, A.; Madyarova, E.; Gurkov, A.; Timofeyev, M. Experimental Crossing Confirms Reproductive Isolation between Cryptic Species within Eulimnogammarus verrucosus (Crustacea: Amphipoda) from Lake Baikal. Int. J. Mol. Sci. 2022, 23, 10858. [Google Scholar] [CrossRef] [PubMed]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Karnaukhov, D.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Simple and effective administration and visualization of microparticles in the circulatory system of small fishes using kidney injection. J. Vis. Exp. 2018, 136, e57491. [Google Scholar]
- Gurkov, A.; Borvinskaya, E.; Shchapova, E.; Timofeyev, M. Restraining small decapods and amphipods for in vivo laboratory studies. Crustacean 2018, 91, 517–525. [Google Scholar] [CrossRef]
- Shchapova, E.; Nazarova, A.; Gurkov, A.; Borvinskaya, E.; Rzhechitskiy, Y.; Dmitriev, I.; Meglinski, I.; Timofeyev, M. Application of PEG-covered non-biodegradable polyelectrolyte microcapsules in the crustacean circulatory system on the example of the amphipod Eulimnogammarus verrucosus. Polymers 2019, 11, 1246. [Google Scholar] [CrossRef] [Green Version]
- The R Project for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 15 May 2023).
- Zhou, L.; He, B.; Huang, J.; Cheng, Z.; Xu, X.; Wei, C. Multihydroxy dendritic upconversion nanoparticles with enhanced water dispersibility and surface functionality for bioimaging. ACS Appl. Mater. Interfaces 2014, 6, 7719–7727. [Google Scholar] [CrossRef]
- Whitaker, J.E.; Haugland, R.P.; Prendergast, F.G. Spectral and photophysical studies of benzo[c]xanthene dyes: Dual emission pH sensors. Anal. Biochem. 1991, 194, 330–344. [Google Scholar] [CrossRef]
- Volkova, E.K.; Yanina, I.Y.; Popov, A.P.; Bykov, A.V.; Gurkov, A.N.; Borvinskaya, E.V.; Timofeyev, M.A.; Meglinski, I.V. Ecophotonics: Assessment of temperature gradient in aquatic organisms using up-conversion luminescent particles. Quantum Electrodyn. 2017, 47, 153. [Google Scholar] [CrossRef]
- Wang, K.; Ma, J.; He, M.; Gao, G.; Xu, H.; Sang, J.; Wang, Y.; Zhao, B.; Cui, D. Toxicity assessments of near-infrared upconversion luminescent LaF3: Yb, Er in early development of zebrafish embryos. Theranostics 2013, 3, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. [Google Scholar] [CrossRef]
- Bamberger, D.; Hobernik, D.; Konhäuser, M.; Bros, M.; Wich, P.R. Surface modification of polysaccharide-based nanoparticles with PEG and dextran and the effects on immune cell binding and stimulatory characteristics. Mol. Pharm. 2017, 14, 4403–4416. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, structures, and properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Baduev, B.; Shatilina, Z.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Parallel in vivo monitoring of pH in gill capillaries and uscles of fishes using microencapsulated biomarkers. Biol. Open 2017, 6, 673–677. [Google Scholar] [CrossRef] [Green Version]
- Jakob, L.; Axenov-Gribanov, D.V.; Gurkov, A.N.; Ginzburg, M.; Bedulina, D.S.; Timofeyev, M.A.; Luckenbach, T.; Lucassen, M.; Sartoris, F.J.; Pörtner, H.O. Lake Baikal amphipods under climate change: Thermal constraints and ecological consequences. Ecosphere 2016, 7, e01308. [Google Scholar] [CrossRef] [Green Version]
- Lipaeva, P.; Vereshchagina, K.; Drozdova, P.; Jakob, L.; Kondrateva, E.; Lucassen, M.; Bedulina, D.; Timofeyev, M.; Stadler, P.; Luckenbach, T. Different ways to play it cool: Transcriptomic analysis sheds light on different activity patterns of three amphipod species under long-term cold exposure. Mol. Ecol. 2021, 30, 5735–5751. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarova, A.; Gurkov, A.; Rzhechitskiy, Y.; Shchapova, E.; Mutin, A.; Saranchina, A.; Diagileva, A.; Bolbat, N.; Krivoshapkin, P.; Timofeyev, M. Turn a Shrimp into a Firefly: Monitoring Tissue pH in Small Crustaceans Using an Injectable Hydrogel Sensor with Infrared Excitation and Visible Luminescence. Photonics 2023, 10, 697. https://doi.org/10.3390/photonics10060697
Nazarova A, Gurkov A, Rzhechitskiy Y, Shchapova E, Mutin A, Saranchina A, Diagileva A, Bolbat N, Krivoshapkin P, Timofeyev M. Turn a Shrimp into a Firefly: Monitoring Tissue pH in Small Crustaceans Using an Injectable Hydrogel Sensor with Infrared Excitation and Visible Luminescence. Photonics. 2023; 10(6):697. https://doi.org/10.3390/photonics10060697
Chicago/Turabian StyleNazarova, Anna, Anton Gurkov, Yaroslav Rzhechitskiy, Ekaterina Shchapova, Andrei Mutin, Alexandra Saranchina, Anastasiia Diagileva, Nadezhda Bolbat, Pavel Krivoshapkin, and Maxim Timofeyev. 2023. "Turn a Shrimp into a Firefly: Monitoring Tissue pH in Small Crustaceans Using an Injectable Hydrogel Sensor with Infrared Excitation and Visible Luminescence" Photonics 10, no. 6: 697. https://doi.org/10.3390/photonics10060697
APA StyleNazarova, A., Gurkov, A., Rzhechitskiy, Y., Shchapova, E., Mutin, A., Saranchina, A., Diagileva, A., Bolbat, N., Krivoshapkin, P., & Timofeyev, M. (2023). Turn a Shrimp into a Firefly: Monitoring Tissue pH in Small Crustaceans Using an Injectable Hydrogel Sensor with Infrared Excitation and Visible Luminescence. Photonics, 10(6), 697. https://doi.org/10.3390/photonics10060697