Surface Acidification of BiOI/TiO2 Composite Enhanced Efficient Photocatalytic Degradation of Benzene
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization
2.3. Tests of Photocatalytic Activity
3. Results and Discussion
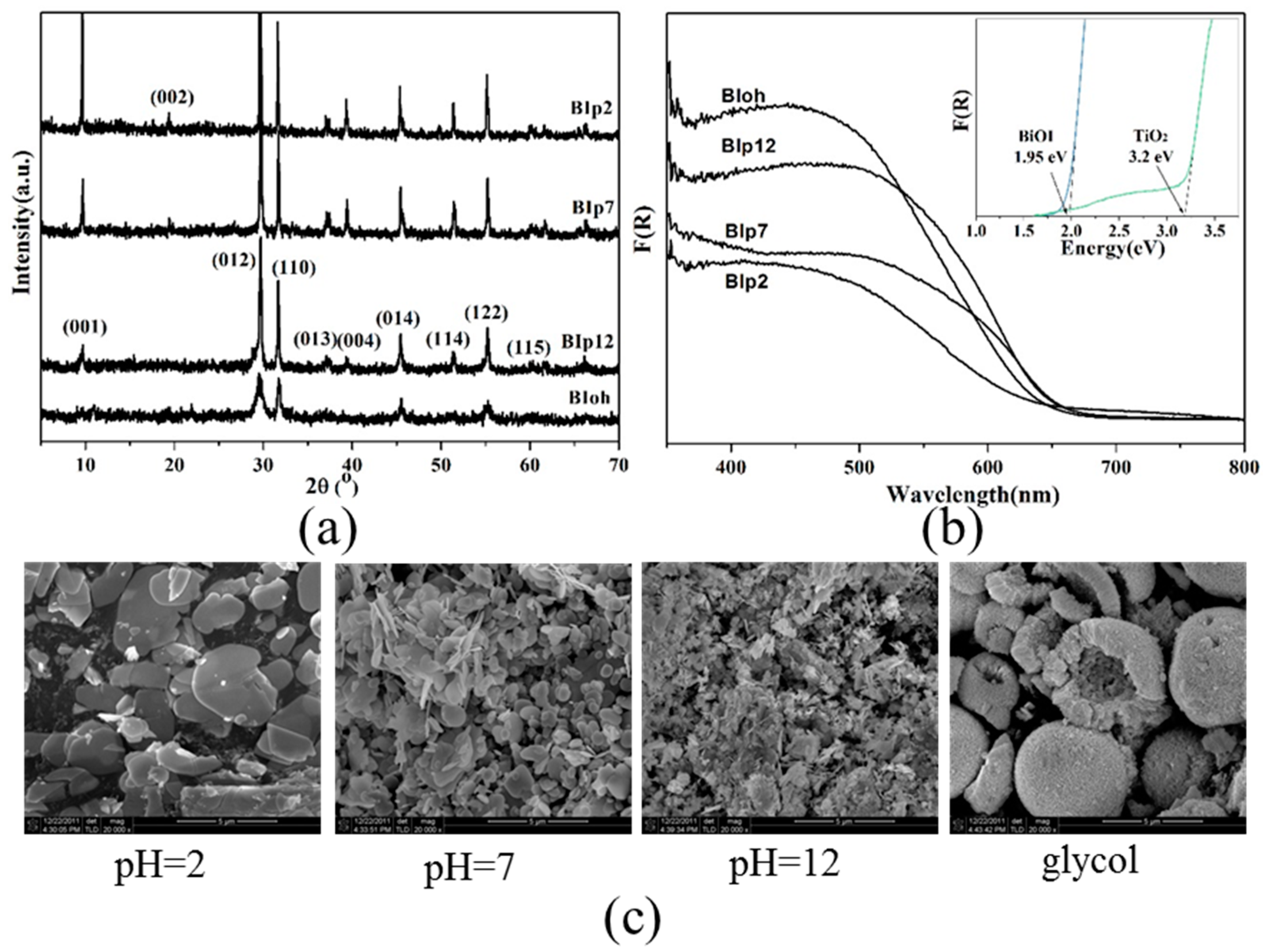
3.1. pH and Solvent Influence Photocatalytic Activity of BiOI
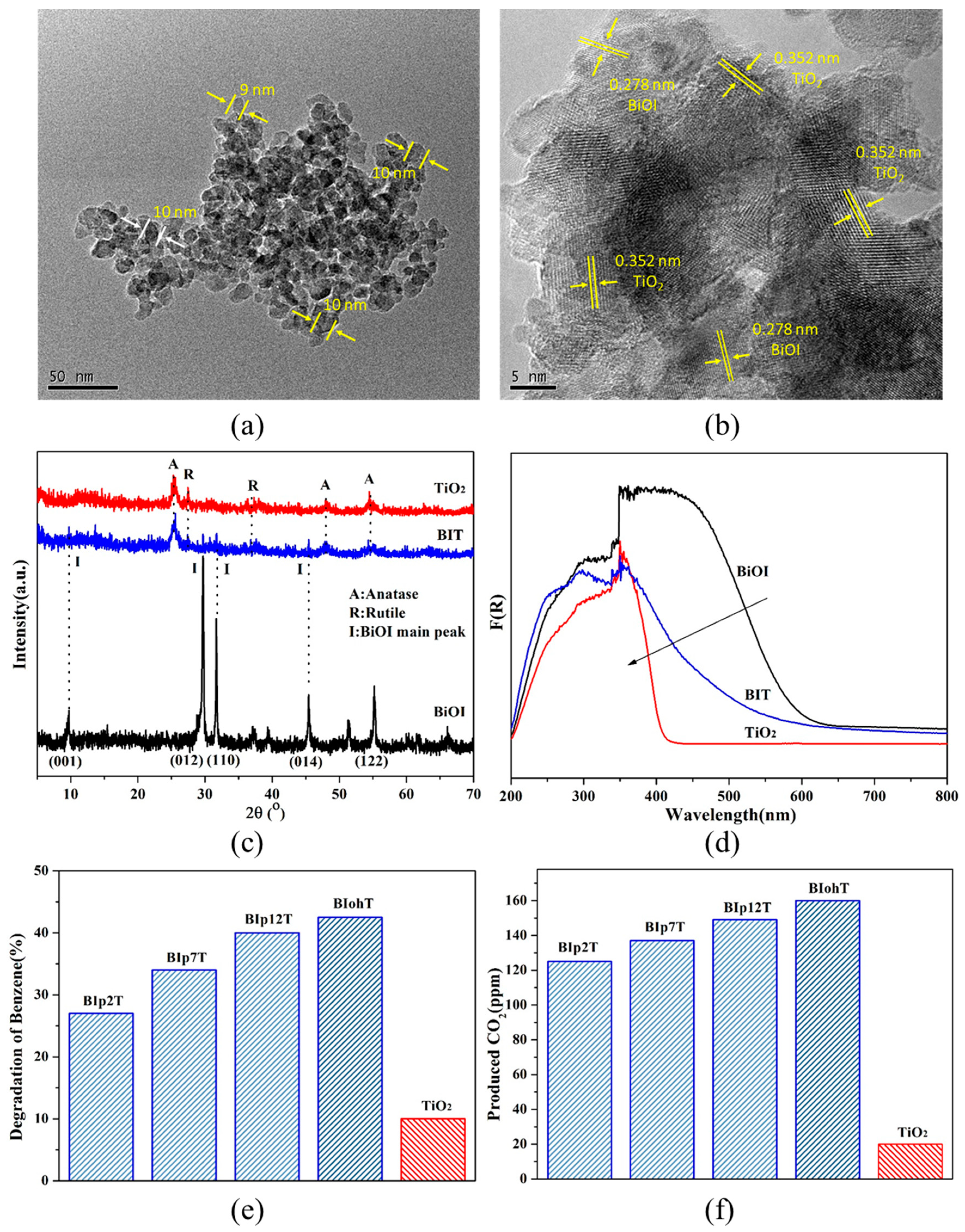
3.2. Characterization Analysis of BIT Prepared in Different Solvents
3.3. Heat Treatment Impact on BIT and BiOI
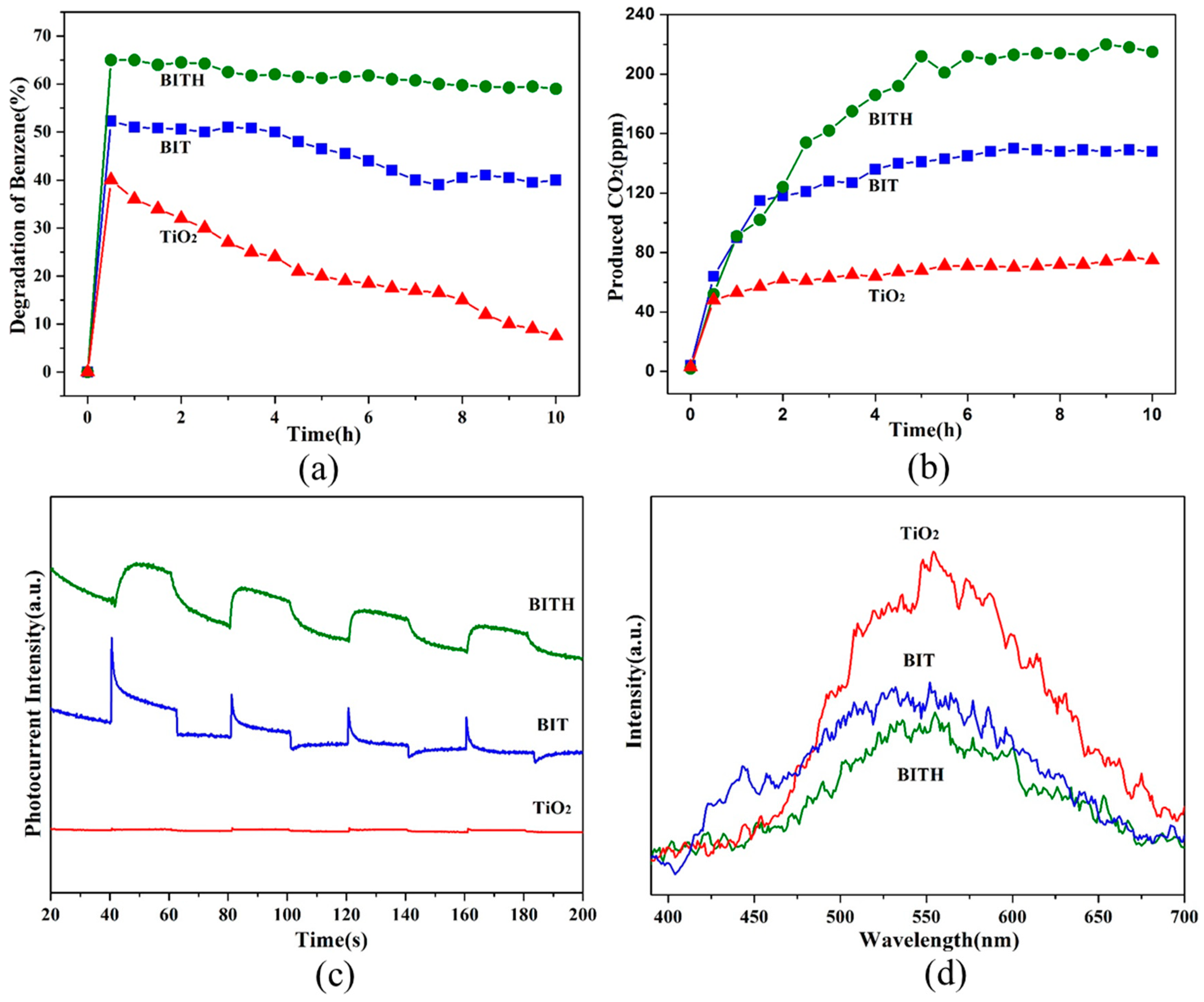
3.4. Surface Acidification Impact on the Photocatalytic Activity of BIT
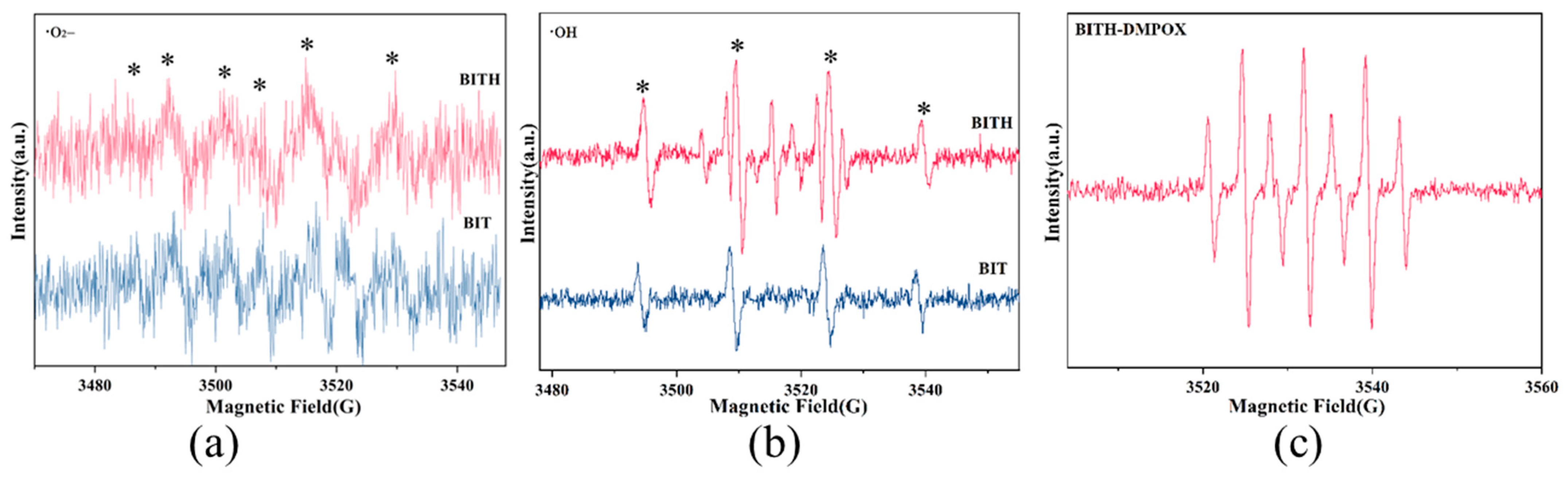
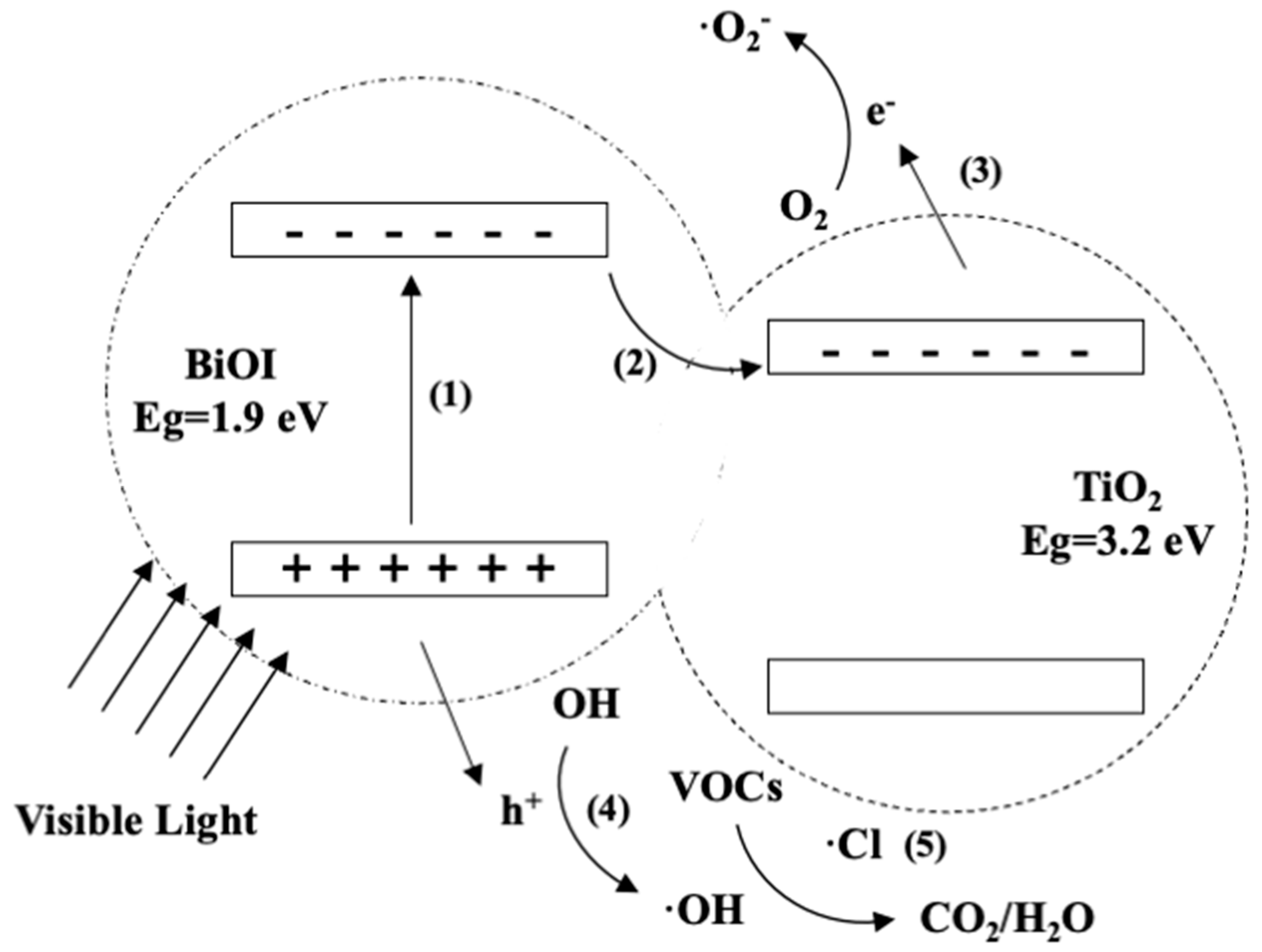
3.5. Photocatalytic Mechanism of BITH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, L.; Tang, J. Strategies and challenges on selectivity of photocatalytic oxidation of organic substances. Adv. Energy Mater. 2021, 11, 2003216. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, J.; Zhou, M.; Liang, S.; Xie, Q.; Li, S.; Liu, B.; Deng, J.; Cao, J.; Liu, S.; et al. Selective photocatalytic oxidation of gaseous ammonia at ppb level over Pt and F modified TiO2. Appl. Catal. B Environ. 2022, 300, 120688. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Chen, X.; Wang, F.; Qin, X.; Zhang, C.; He, H. High-performance of Cu-TiO2 for photocatalytic oxidation of formaldehyde under visible light and the mechanism study. Chem. Eng. J. 2020, 390, 124481. [Google Scholar] [CrossRef]
- Erhuan, Z.; Jia, L.; Muwei, J.; Wang, H.; Wan, X.; Rong, H.; Chen, W.; Liu, J.; Xu, M.; Zhang, J. Hollow anisotropic semiconductor nanoprisms with highly crystalline frameworks for high-efficiency photoelectrochemical water splitting. J. Mater. Chem. A 2019, 7, 8061–8072. [Google Scholar]
- Shafiq, I.; Hussain, M.; Shafique, S.; Rashid, R.; Akhter, P.; Ahmed, A.; Jeon, J.-K.; Park, Y.-K. Oxidative desulfurization of refinery diesel pool fractions using LaVO4 photocatalyst. J. Ind. Eng. Chem. 2021, 98, 283–288. [Google Scholar] [CrossRef]
- Shayegan, Z.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation of volatile organic compounds for indoor environment applications: Three different scaled setups. Chem. Eng. J. 2019, 357, 533–546. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Liang, T.; Sun, Q.; Wang, H. Photocatalytic degradation of Orange G using sepiolite-TiO2 nanocomposites: Optimization of physicochemical parameters and kinetics studies. Chem. Eng. Sci. 2018, 183, 231–239. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tong, S.; Li, W.; Liu, Y.; Tan, F.; Ge, M.; Xie, X.; Sun, J. Photocatalytic oxidation of SO2 by TiO2: Aerosol formation and the key role of gaseous reactive oxygen species. Environ. Sci. Technol. 2021, 55, 9784–9793. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Z-Scheme 2D/2D α-Fe2O3/g-C3N4 heterojunction for photocatalytic oxidation of nitric oxide. Appl. Catal. B Environ. 2021, 280, 119409. [Google Scholar] [CrossRef]
- Li, R.; Ou, X.; Zhang, L.; Qi, Z.; Wu, X.; Lu, C.; Fan, J.; Lv, K. Photocatalytic oxidation of NO on reduction type semiconductor photocatalysts: Effect of metallic Bi on CdS nanorods. Chem. Commun. 2021, 57, 10067–10070. [Google Scholar] [CrossRef] [PubMed]
- Cano-Casanova, L.; Mei, B.; Mul, G.; Lillo-Rodenas, M.A.; Roman-Martinez, M.D.C. Photocatalytic oxidation of propane using hydrothermally prepared anatase-brookite-rutile TiO2 samples. An in situ drifts study. Nanomaterials 2020, 10, 1314. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Diniz, J.; Nunes, C.D.; Monteiro, O.C. Novel approach to synthesise MoO3—TiO2 nanocomposites for the photo-assisted oxidation of benzyl alcohol to benzaldehyde. Inorg. Chem. Commun. 2020, 119, 108099. [Google Scholar] [CrossRef]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 nanocrystalline heterostructure: A wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Gopidas, K.P.; Bohorguez, M.; Kamat, P.V. Photophysical and photochemical aspects of coupled semiconductors: Charge-transfer processes in colloidal cadmium sulfide–titania and cadmium sulfide–silver (I) iodide systems. J. Phys. Chem. 1990, 94, 6435–6440. [Google Scholar] [CrossRef]
- Guanyu, C.; Kaibo, Z.; Xiaoliang, M. Preparation of ZnO/TiO2 composite nanomaterial and application in dye-sensitized solar cells. Chin. J. Vac. Sci. Technol. 2010, 30, 621–625. [Google Scholar]
- Do, Y.R.; Lee, W.; Dwight, K.; Wold, A. The effect of WO3 on the photocatalytic activity of TiO2. J. Solid State Chem. 1994, 108, 198–201. [Google Scholar] [CrossRef]
- Hu, C.C.; Nian, J.N.; Teng, H.S. Electrodeposited p-type Cu2O as photocatalyst for H2 evolution from water reduction in the presence of WO3. Sol. Energy Mater. Sol. Cells 2008, 92, 1071–1076. [Google Scholar] [CrossRef]
- Erhuan, Z.; Qianhong, Z.; Junheng, H. Visually resolving the direct Z-scheme heterojunction in CdS@ZnIn2S4 hollow cubes for photocatalytic evolution of H2 and H2O2 from pure water. Appl. Catal. B Environ. 2021, 293, 120213. [Google Scholar]
- Lei, W.; Jian, G.; Hao, H.; Yao, M.; Kang, J.; Peng, M.; Wang, D.; Xu, J.; Hao, J. Enhanced photocatalytic removal of ozone by a new chlorine-radical-mediated strategy. Appl. Catal. B Environ. 2022, 306, 121130. [Google Scholar]
- Zhang, L.; Sun, P.; Sun, D.; Zhou, Y.; Han, L.; Zhang, H.; Zhu, B.; Wang, B. Occupational health risk assessment of the benzene exposure industries: A comprehensive scoring method through 4 health risk assessment models. Environ. Sci. Pollut. Res. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Zhuang, Y.; Fu, X. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: A case study on degradation of benzene and methyl orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Li, T.; Li, G. Solvothermal synthesis of mesoporous TiO2: The effect of morphology, size and calcination progress on photocatalytic activity in the degradation of gaseous benzene. Chem. Eng. J. 2014, 237, 312–321. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L. Electronic and band structure tuning of ternary semiconductor photocatalysts by self doping: The case of BiOI. J. Phys. Chem. C 2010, 114, 18198–18206. [Google Scholar] [CrossRef]
- Huang, H.; Li, D.; Lin, Q.; Zhang, W.; Shao, Y.; Chen, Y.; Sun, M.; Fu, X. Efficient degradation of benzene over LaVO4/TiO2 nanocrystalline heterojunction photocatalyst under visible light irradiation. Environ. Sci. Technol. 2009, 43, 4164–4168. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhu, L.; Fu, Y.; Chu, X. Highly active Bi/BiOI composite synthesized by one-step reaction and its capacity to degrade bisphenol A under simulated solar light irradiation. Chem. Eng. J. 2013, 233, 305–314. [Google Scholar] [CrossRef]
- Ramesham, R. Determination of flatband potential for boron doped diamond electrode in 0.5 M NaCl by AC impedance spectroscopy. Thin Solid Films 1998, 322, 158–166. [Google Scholar] [CrossRef]
- Wang, W.; Huang, F.; Lin, X.; Yang, J. Visible-light-responsive photocatalysts xBiOBr-(1−x)BiOI. Cat. Commun. 2008, 9, 8–12. [Google Scholar] [CrossRef]
- Martra, G. Lewis acid and base sites at the surface of microcrystalline TiO2 anatase: Relationships between surface morphology and chemical behavior. Appl. Catal. A Gen. 2000, 200, 275–285. [Google Scholar] [CrossRef]
- Yuan, R.S.; Chen, T.; Fei, E.H.; Lin, J.; Ding, Z.; Long, J.; Zhang, Z.; Fu, X.; Liu, P.; Wu, L.; et al. Surface chlorination of TiO2-based photocatalysts: A way to remarkably improve photocatalytic activity in both UV and visible region. ACS Catal. 2011, 1, 200–206. [Google Scholar] [CrossRef]
- d’Hennezel, O.; Pichat, P.; Ollis, D.F. Benzene and toluene gas-phase photocatalytic degradation over H2O and HCl pretreated TiO2: By-products and mechanisms. J. Photochem. Photobiol. A 1998, 118, 197–204. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, J.; Hidaka, H. ESR spin-trapping detection of radical intermediates in the TiO2-assisted photo-oxidation of sul for rhodamine B under visible irradiation. J. Photochem. Photobiol. A 2000, 133, 83–88. [Google Scholar] [CrossRef]
- Xianfeng, H.; Yi, W.; Xuchun, L.; Guan, D.; Li, Y.; Zheng, X.; Zhao, M.; Shan, C.; Pan, B. Autocatalytic decomplexation of Cu(II)-EDTA and simultaneous removal of aqueous Cu(II) by UV/chlorine. Environ. Sci. Technol. 2019, 53, 2036–2044. [Google Scholar]
- Lee, S.; Dai, G.; Liang, Y.; Liu, H.; Zhang, X. Preparing BiOI/Bi2O3 photocatalyst with highly visible-light activity by in-situ dissolution-sedimentation. Chin. J. Inorg. Chem. 2011, 27, 1964–1968. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Wang, H.; Wang, C.; Sun, Y.; Han, H.; Kang, J.; Dong, Y.; Wang, L. Surface Acidification of BiOI/TiO2 Composite Enhanced Efficient Photocatalytic Degradation of Benzene. Separations 2022, 9, 315. https://doi.org/10.3390/separations9100315
Zhao Z, Wang H, Wang C, Sun Y, Han H, Kang J, Dong Y, Wang L. Surface Acidification of BiOI/TiO2 Composite Enhanced Efficient Photocatalytic Degradation of Benzene. Separations. 2022; 9(10):315. https://doi.org/10.3390/separations9100315
Chicago/Turabian StyleZhao, Ziwang, Hao Wang, Chunyu Wang, Yuan Sun, Hao Han, Jian Kang, Yanchun Dong, and Lei Wang. 2022. "Surface Acidification of BiOI/TiO2 Composite Enhanced Efficient Photocatalytic Degradation of Benzene" Separations 9, no. 10: 315. https://doi.org/10.3390/separations9100315



