Optimised Extraction of Trypsin Inhibitors from Defatted Gac (Momordica cochinchinensis Spreng) Seeds for Production of a Trypsin Inhibitor-Enriched Freeze Dried Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents and Chemicals
2.1.2. Gac Seeds
2.1.3. Preparation of Defatted Gac Seed Kernel Powder
2.2. Methods
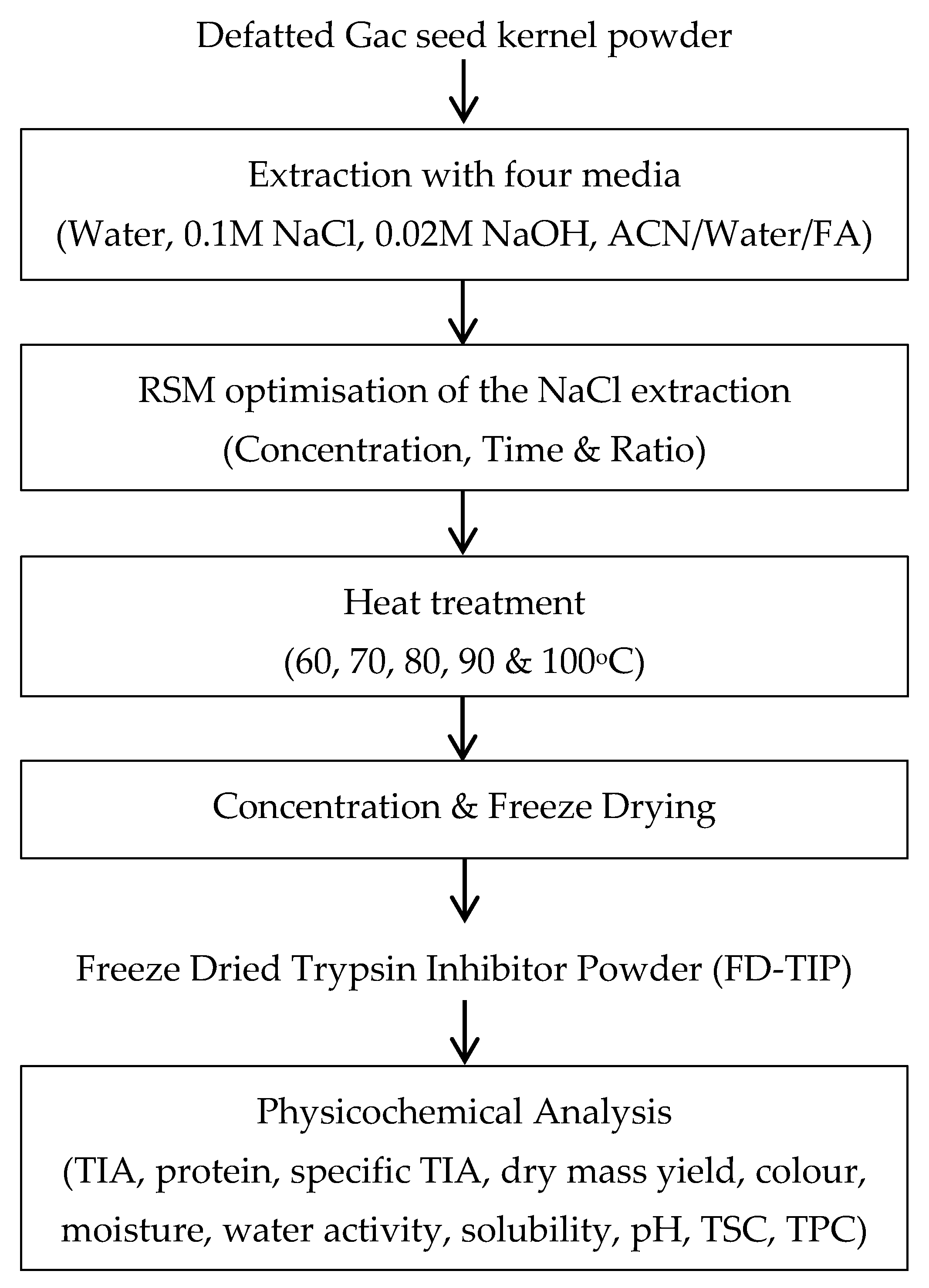
2.2.1. Experiment Design
2.2.2. Extraction with Four Aqueous Media
2.2.3. Optimisation of the Extraction Conditions Using RSM
2.2.4. Heat Treatment of the Optimal TI Extract
2.2.5. Concentrating and Freeze Drying the Extract Prepared with the Optimal Parameters
2.2.6. Determination of Trypsin-Inhibiting Activity (TIA)
- , subscripts as per Table 2.
- V: Volume of undiluted extract (mL)
- D: Dilution factor of the extract
- S: Weight of defatted Gac seed kernel powder or FD-TIP in V mL (g)
- 19: Constant figure based on the absorbance given by 1 mg of pure trypsin
- m%: moisture content of defatted Gac seed kernel powder or freeze-dried extract powder
2.2.7. Determination of Total Protein Yield (TPY)
2.2.8. Physicochemial Analyses on the FD-TIP
2.2.9. Statistical Analyses
3. Results and Discussion
3.1. Extraction with four Aqueous Media
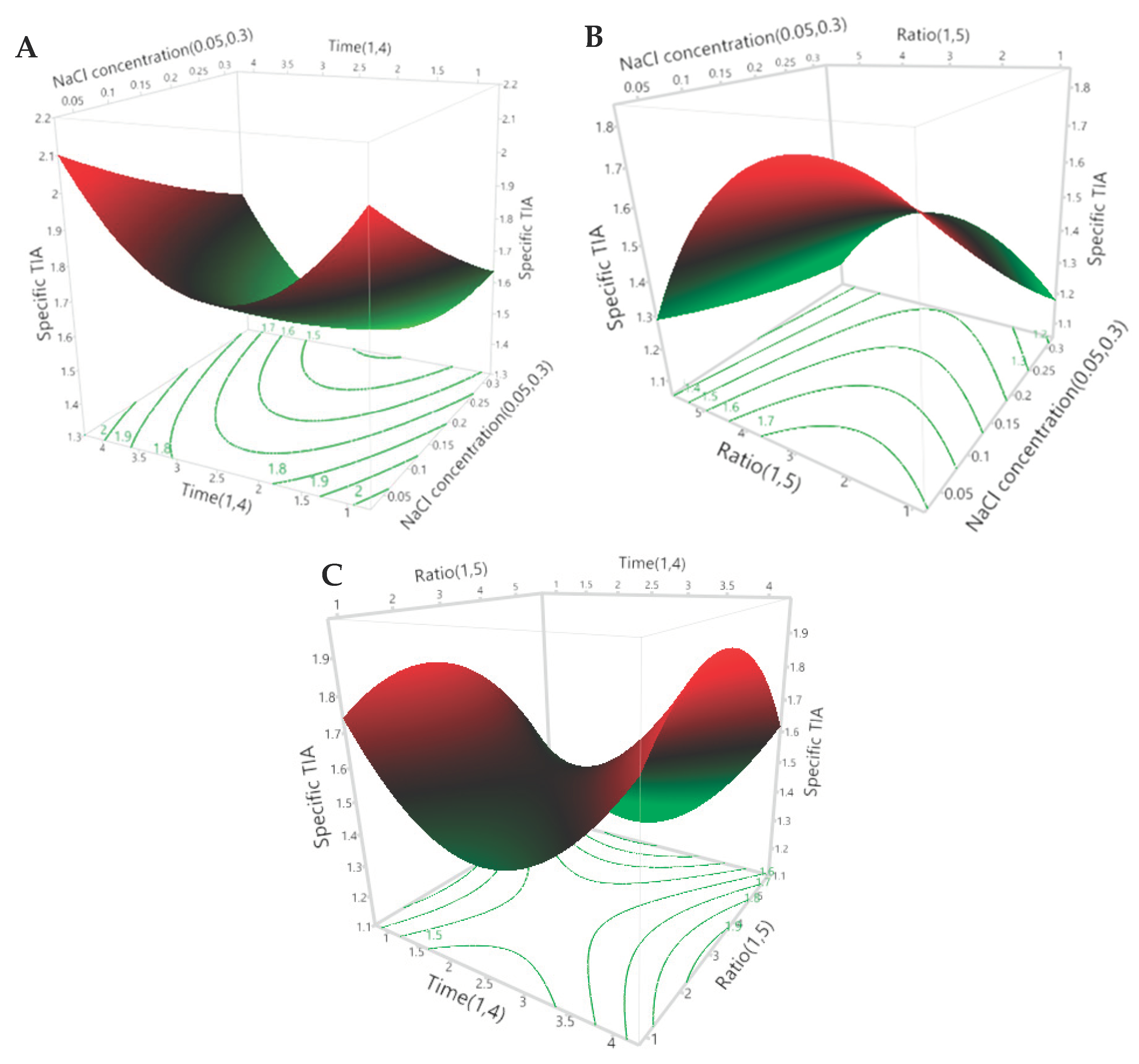
3.2. Optimisation of the Extraction Conditions Using RSM
3.2.1. Fitting the Response Surface Model
3.2.2. Effects of the Extraction Parameters on the Specific TIA
3.2.3. Optimal Extraction Parameters and Validation of the Model
3.3. Heat Treatment of the Optimal TI Extract
3.4. The Physicochemical Properties of the Freeze Dried Trypsin-Inhibitor Powder (FD-TIP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| aw | water activity |
| BAPNA | Benzyl-DL-arginine-para-nitroanilide |
| DI | Deionised |
| DMSO | Dimethyl sulfoxide |
| DW | Dry weight |
| FA | Formic acid |
| FD-TIP | Freeze dried trypsin-inhibitor powder |
| MCoTI | Momordica cochinchinensis trypsin inhibitor |
| RSM | Response surface methodology |
| TI | Trypsin inhibitor |
| TIA | Trypsin-inhibitor activity |
| TPC | Total phenolic content |
| TPY | Total protein yield |
| TSC | Total saponin content |
References
- Wong, R.C.; Fong, W.; Ng, T. Multiple trypsin inhibitors from Momordica cochinchinensis seeds, the Chinese drug mubiezhi. Peptides 2004, 25, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ng, T.; Fong, W.; Wan, C.; Yeung, H. Isolation of a trypsin inhibitor with deletion of N-terminal pentapeptide from the seeds of Momordica cochinchinensis, the Chinese drug mubiezhi. Int. J. Biochem. Cell Biol. 1999, 31, 707–715. [Google Scholar] [CrossRef]
- Chan, L.Y.; Wang, C.K.L.; Major, J.M.; Greenwood, K.P.; Lewis, R.J.; Craik, D.J.; Daly, N.L. Isolation and characterization of peptides from Momordica cochinchinensis seeds. J. Nat. Prod. 2009, 72, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.F.; Gagnon, J.; Chiche, L.; Nguyen, T.M.; Andrieu, J.P.; Heitz, A.; Hong, T.T.; Pham, T.T.C.; Nguyen, D.L. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry 2000, 39, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Felizmenio-Quimio, M.E.; Daly, N.L.; Craik, D.J. Circular Proteins in Plants: Solution structure of a novel macrocyclic trypsin inhibitor from Momordica Cochinchinensis. J. Biol. Chem. 2001, 276, 22875–22882. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y. Protein proteinase inhibitors in legume seeds-overview. Arch. Latinoam. Nutr. 1996, 44, 26S–30S. [Google Scholar] [PubMed]
- Greenwood, K.P.; Daly, N.L.; Brown, D.L.; Stow, J.L.; Craik, D.J. The cyclic cystine knot miniprotein MCoTI-II is internalized into cells by macropinocytosis. Int. J. Biochem. Cell Biol. 2007, 39, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.; Elnagar, A.Y.; Hamm-Alvarez, S.F.; Camarero, J.A. Cellular uptake of cyclotide MCoTI-I follows multiple endocytic pathways. J. Control. Release 2011, 155, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Cascales, L.; Henriques, S.T.; Kerr, M.C.; Huang, Y.-H.; Sweet, M.J.; Daly, N.L.; Craik, D.J. Identification and characterization of a new family of cell-penetrating peptides cyclic cell-penetrating peptides. J. Biol. Chem. 2011, 286, 36932–36943. [Google Scholar] [CrossRef] [PubMed]
- Mahatmanto, T.; Mylne, J.S.; Poth, A.G.; Swedberg, J.E.; Kaas, Q.; Schaefer, H.; Craik, D.J. The evolution of Momordica cyclic peptides. Mol. Biol. Evol. 2014, 32, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Swedberg, J.E.; Mylne, J.S.; Cemazar, M. Cyclotides as a basis for drug design. Exp. Opin. Drug Discov. 2012, 7, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Gruber, C.W.; Cemazar, M.; Siatskas, C.; Tagore, P.; Payne, N.; Sun, G.; Wang, S.; Bernard, C.C.; Craik, D.J. Molecular grafting onto a stable framework yields novel cyclic peptides for the treatment of multiple sclerosis. ACS Chem. Biol. 2013, 9, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mahatmanto, T.; Poth, A.G.; Mylne, J.S.; Craik, D.J. A comparative study of extraction methods reveals preferred solvents for cystine knot peptide isolation from Momordica cochinchinensis seeds. Fitoterapia 2014, 95, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Effect of solvents and extraction methods on recovery of bioactive compounds from defatted Gac (Momordica cochinchinensis Spreng.) seeds. Separations 2018, 5, 39. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Kishimura, H.; Chaijan, M. Extraction, purification and properties of trypsin inhibitor from Thai mung bean (Vigna radiata (L.) R. Wilczek). Food Chem. 2011, 129, 1348–1354. [Google Scholar] [CrossRef]
- Pesoti, A.R.; de Oliveira, B.M.; de Oliveira, A.C.; Pompeu, D.G.; Gonçalves, D.B.; Marangoni, S.; da Silva, D.A.; Granjeiro, P.A. Extraction, purification and characterization of inhibitor of trypsin from Chenopodium quinoa seeds. Food Sci. Technol. 2015, 35, 588–597. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Campbell, C.G. Effect of different solvents on protein recovery and neurotoxin and trypsin inhibitor contents of grass pea (Lathyrus sativus). J. Sci. Food Agric. 1992, 60, 245–249. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Wimalasiri, D.; Piva, T.; Urban, S.; Huynh, T. Morphological and genetic diversity of Momordica cochinchinenesis (Cucurbitaceae) in Vietnam and Thailand. Genet. Resour. Crop Evol. 2016, 63, 19–33. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Tan, S.P.; Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J. Food Sci. 2014, 79, E1372–E1381. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.; Siddhuraju, P.; Becker, K. Trypsin Inhibitor. Plant Secondary Metabolites; Humana Press: New York, NY, USA, 2007; pp. 1–6. [Google Scholar]
- Stauffer, C.E. Measuring trypsin inhibitor in soy meal: Suggested improvements in the standard method. Cereal Chem 1990, 67, 296–302. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Anderson, R. Water absorption and solubility and amylograph characteristics of roll-cooked small grain products. Cereal Chem. 1982, 59, 265–269. [Google Scholar]
- Le, A.; Huynh, T.; Parks, S.; Nguyen, M.; Roach, P. Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds. Medicines 2018, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Marfo, E.; Oke, O. Effect of sodium chloride, calcium chloride and sodium hydroxide on Denolix regia protein solubility. Food Chem. 1989, 31, 117–127. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Thummaratwasik, P. Isolation and characterization of trypsin inhibitors from some Thai legume seeds. J. Food Biochem. 2000, 24, 107–127. [Google Scholar] [CrossRef]
- Sathe, S.; Salunkhe, D. Solubilization and electrophoretic characterization of the Great Northern bean (Phaseolus vulgaris L.) proteins. J. Food Sci. 1981, 46, 82–87. [Google Scholar] [CrossRef]
- Hamato, N.; Koshiba, T.; Pham, T.-N.; Tatsumi, Y.; Nakamura, D.; Takano, R.; Hayashi, K.; Hong, Y.-M.; Hara, S. Trypsin and elastase inhibitors from bitter gourd (Momordica charantia LINN.) seeds: purification, amino acid sequences, and inhibitory activities of four new inhibitors. J. Biochem. 1995, 117, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Mikola, J.; Suolinna, E.M. Purification and properties of a trypsin inhibitor from barley. Eur. J. Biochem. 1969, 9, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Oh, H.-J.; Han, S.-H.; Lim, S.-B. Effects of hot air and freeze drying methods on physicochemical properties of citrus ‘hallabong’powders. Food Sci. Biotechnol. 2012, 21, 1633–1639. [Google Scholar] [CrossRef]
- Mai, H.C.; Truong, V.; Haut, B.; Debaste, F. Impact of limited drying on Momordica cochinchinensis Spreng. aril carotenoids content and antioxidant activity. J. Food Eng. 2013, 118, 358–364. [Google Scholar] [CrossRef]
- Blessington, T.; Theofel, C.G.; Harris, L.J. A dry-inoculation method for nut kernels. Food Microbiol. 2013, 33, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, C.K.; Nguyen, M.H.; Roach, P.D. Effects of pre-treatments and air drying temperatures on colour and antioxidant properties of Gac fruit powder. Int. J. Food Eng. 2011, 7. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice, 4th ed.; Woodhead Publishing: Cambridge, UK, 2009; ISBN 9780081019078. [Google Scholar]
- Vojdani, F. Solubility. In Methods of Testing Protein Functionality; Hall, G.M., Ed.; Blackie Academic & Professional: London, UK, 1996; pp. 11–60. [Google Scholar]
- Tan, S.P.; Tuyen, K.C.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]

| Levels | Independent Factors | ||
|---|---|---|---|
| X1 NaCl Concentration (mol/L) | X2 Extraction Time (h) | X3 Ratio (g/30 mL) | |
| −1 | 0.050 | 1.0 | 1.0 |
| 0 | 0.175 | 2.5 | 3.0 |
| +1 | 0.300 | 4.0 | 5.0 |
| Component (mL) | Reagent Blank (a) | Standard (b) | Sample Blank (c) | Sample (d) |
|---|---|---|---|---|
| DI water | 1 | 1 | 0.5 | 0.5 |
| Trypsin solution | - | 1 | - | 1 |
| Trypsin-inhibitor sample | - | - | 0.5 | 0.5 |
| Trypsin solution after reaction inactivation | 1 | - | 1 | - |
| Extraction Media | Mean ± SD | ||
|---|---|---|---|
| TIA (mg trypsin/g Defatted Powder) | TPY (mg BSA/g Defatted Powder) | Specific TIA (mg trypsin/mg Protein) | |
| DI Water | 85.22 ± 0.47 c | 42.55 ± 3.15 d | 2.01 ± 0.14 a |
| 0.1 M NaCl | 140.39 ± 8.33 b | 80.58 ± 0.65 c | 1.74 ± 0.11 a |
| ACN/Water/FA | 168.11 ± 0.50 a | 152.44 ± 2.08 b | 1.10 ± 0.01 b |
| 0.02 M NaOH | 81.43 ± 2.02 c | 245.19 ± 1.06 a | 0.33 ± 0.01 c |
| Run | Pattern | X1 NaCl (mol/L) | X2 Time (h) | X3 Ratio (g/30 mL) | TIA | TPY | Specific TIA | |
|---|---|---|---|---|---|---|---|---|
| Exp. | Exp. | Exp. | Pred. | |||||
| 1 | − − 0 | 0.05 | 1 | 3 | 155.04 | 79.85 | 1.94 | 1.94 |
| 2 | − + 0 | 0.05 | 4 | 3 | 156.19 | 79.18 | 1.97 | 1.98 |
| 3 | + − 0 | 0.3 | 1 | 3 | 164.01 | 102.43 | 1.60 | 1.59 |
| 4 | + + 0 | 0.3 | 4 | 3 | 189.26 | 110.69 | 1.71 | 1.71 |
| 5 | 0 − − | 0.175 | 1 | 1 | 152.39 | 93.85 | 1.62 | 1.68 |
| 6 | 0 − + | 0.175 | 1 | 5 | 143.55 | 91.79 | 1.56 | 1.52 |
| 7 | 0 + − | 0.175 | 4 | 1 | 178.60 | 108.97 | 1.64 | 1.68 |
| 8 | 0 + + | 0.175 | 4 | 5 | 177.72 | 102.65 | 1.73 | 1.67 |
| 9 | − 0 − | 0.05 | 2.5 | 1 | 142.63 | 82.31 | 1.73 | 1.68 |
| 10 | + 0 − | 0.3 | 2.5 | 1 | 168.29 | 130.12 | 1.29 | 1.24 |
| 11 | − 0 + | 0.05 | 2.5 | 5 | 115.65 | 81.72 | 1.42 | 1.47 |
| 12 | + 0 + | 0.3 | 2.5 | 5 | 145.57 | 118.48 | 1.23 | 1.28 |
| 13 | 000 | 0.175 | 2.5 | 3 | 167.90 | 105.47 | 1.59 | 1.56 |
| 14 | 000 | 0.175 | 2.5 | 3 | 147.02 | 99.21 | 1.48 | 1.56 |
| 15 | 000 | 0.175 | 2.5 | 3 | 163.60 | 102.10 | 1.60 | 1.56 |
| Regression Coefficient | Specific Trypsin-Inhibitor Activity (mg trypsin/mg protein) | ||
|---|---|---|---|
| Regression Coefficient Values | t Ratio | p Value | |
| β0 | 1.56 | 35.23 | <0.0001 |
| Linear | |||
| β1 | −0.15 | −5.68 | 0.002 |
| β2 | 0.04 | 1.52 | 0.188 |
| β3 | −0.04 | −1.57 | 0.177 |
| Quadratic | |||
| β11 | 0.01 | 0.36 | 0.74 |
| β22 | 0.23 | 5.88 | 0.002 |
| β33 | −0.15 | −3.85 | 0.012 |
| Interaction | |||
| β12 | 0.02 | 0.52 | 0.623 |
| β13 | 0.06 | 1.63 | 0.163 |
| β23 | 0.04 | 0.98 | 0.372 |
| R2 | 0.98 | ||
| p value of lack of fit | 0.42 | ||
| Temperature (°C) | Mean ± SD | ||
|---|---|---|---|
| TIA (mg trypsin/g DW Defatted Powder) | TPY (mg BSA/g DW Defatted Powder) | Specific TIA (mg trypsin/mg Protein) | |
| Control | 169.46 ± 4.73 a | 91.17 ± 2.05 a | 1.86 ± 0.09 a |
| 60 | 155.28 ± 2.29 a | 84.96 ± 2.64 ab | 1.83 ± 0.07 a |
| 70 | 149.24 ± 2.58 b | 83.00 ± 3.09 b | 1.80 ± 0.06 a |
| 80 | 157.34 ± 6.84 ab | 84.29 ± 2.15 ab | 1.87 ± 0.13 a |
| 90 | 147.76 ± 7.98 b | 81.19 ± 3.34 b | 1.82 ± 0.05 a |
| 100 | 149.13 ± 7.31 b | 83.25 ± 2.76 b | 1.79 ± 0.08 a |
| Properties | Mean ± SD |
|---|---|
| Dry mass yield (g FD-TIP/100g DW defatted powder) | 16.3 ± 0.1 |
| Moisture content (%, w/w) | 0.43 ± 0.08 |
| Water activity | 0.18 ± 0.07 |
| Water solubility (%) | 92.4 ± 1.5 |
| pH | 5.33 ± 0.01 |
| Colour | |
| L* | 96.73 ± 0.18 |
| a* | −0.50 ± 0.03 |
| b* | 8.22 ± 0.15 |
| Trypsin-ininbitor activity (mg trypsin/g DW FD-TIP) | 695.6 ± 77.2 |
| Protein content (mg protein/g DW FD-TIP) | 444.0 ± 21.5 |
| Specific trypsin-inhibitor activity (mg trypsin/mg protein) | 1.57 ± 0.17 |
| Total saponin content (mg AE/g DW FD-TIP) | 43.6 ± 2.3 |
| Total phenolic content (mg GAE/g DW FD-TIP) | 10.5 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Optimised Extraction of Trypsin Inhibitors from Defatted Gac (Momordica cochinchinensis Spreng) Seeds for Production of a Trypsin Inhibitor-Enriched Freeze Dried Powder. Separations 2019, 6, 8. https://doi.org/10.3390/separations6010008
Le AV, Parks SE, Nguyen MH, Roach PD. Optimised Extraction of Trypsin Inhibitors from Defatted Gac (Momordica cochinchinensis Spreng) Seeds for Production of a Trypsin Inhibitor-Enriched Freeze Dried Powder. Separations. 2019; 6(1):8. https://doi.org/10.3390/separations6010008
Chicago/Turabian StyleLe, Anh V., Sophie E. Parks, Minh H. Nguyen, and Paul D. Roach. 2019. "Optimised Extraction of Trypsin Inhibitors from Defatted Gac (Momordica cochinchinensis Spreng) Seeds for Production of a Trypsin Inhibitor-Enriched Freeze Dried Powder" Separations 6, no. 1: 8. https://doi.org/10.3390/separations6010008
APA StyleLe, A. V., Parks, S. E., Nguyen, M. H., & Roach, P. D. (2019). Optimised Extraction of Trypsin Inhibitors from Defatted Gac (Momordica cochinchinensis Spreng) Seeds for Production of a Trypsin Inhibitor-Enriched Freeze Dried Powder. Separations, 6(1), 8. https://doi.org/10.3390/separations6010008






