1. Introduction
Nano HPLC and mass spectrometry (MS) are analytical methods of choice for separation and detection of peptides generated through enzymatic digestion of proteins. Samples’ complexity depends on its origin and the enzymatic method employed for proteins’ digestion; however, the number of peptides generated largely surpasses the capacity of separation columns applied [
1,
2,
3,
4,
5,
6], which needs to be optimized.
Separation of peptides for proteomics analysis is performed using nano HPLC columns with inner diameters (ID) of 50 µm to 100 µm. These small ID will contribute to increased sensitivity of the separation and detection method, which is electrospray ionization—mass spectrometry (ESI-MS) but will also limit the amount of sample in terms of volume that can be injected [
7] on a nano-separation column. The use of trap columns is a method, which can provide help. By applying trap columns, injections of volumes larger than 5 µL are possible without risking the damage of the separation column or peak broadening due to the enforced peak displacement on the column.
The effect of temperature on separation performance of reversed phase columns for peptides has been published in several publications [
8,
9,
10,
11,
12,
13,
14,
15,
16]. There are several studies addressing the use of elevated column temperature but only a limited number of experiments have investigated the effect of low temperature on peptide trapping. The most remarkable study for using cooling and heating phases of the entire separation column or only a column segment was performed by Eghbali
et al. [
8]. The authors described the use of selective cooling of the predefined section of the separation column, which enabled peak focusing at the end of the column. Authors correctly noted that peaks are broadening during the trapping procedure and that the lower temperature of the column enables zone focusing. Very detailed information is provided on how temperature changes affect the trapping efficiency and retention time behavior of both proteins and peptides on the separation column. It was effectively shown that peptides and proteins can be trapped on the stationary phase even under gradient conditions, and being eluted with significantly better peak shape in comparison to heated column only. However, the method described is significantly different that the approach described in this manuscript. Here, columns, the trapping column and the separation column are operated at elevated temperature but the loading mobile phase is cooled and the cooling effect applies only to the trapping column and does not directly influence the separation performance. Interested readers are encouraged to read this paper and compare the effects described with those described in the current manuscript.
Modern nano-chromatographic systems for separations of peptides are currently operated using UHPLC conditions, which implies the use of particles with small diameters packed in long separation columns. Although columns packed with sub-3 µm and sub-2 µm particles are getting more ubiquitous, the use of separation columns packed with 3 µm-particles (conventional) are more broadly spread. The use of columns packed with sub-2 µm particles induces a high backpressure, and separation at elevated column temperature is often employed [
17,
18,
19,
20,
21,
22,
23] in order to reduce the backpressure and improve column performance. Separation columns packed with conventional particles can also be applied for UHPLC separations, without loss of separation performance when higher flow rates and higher operational temperatures are applied.
As for the current study, a conventionally-packed separation column with 3 µm particles was applied using conventional nano-separation approach.
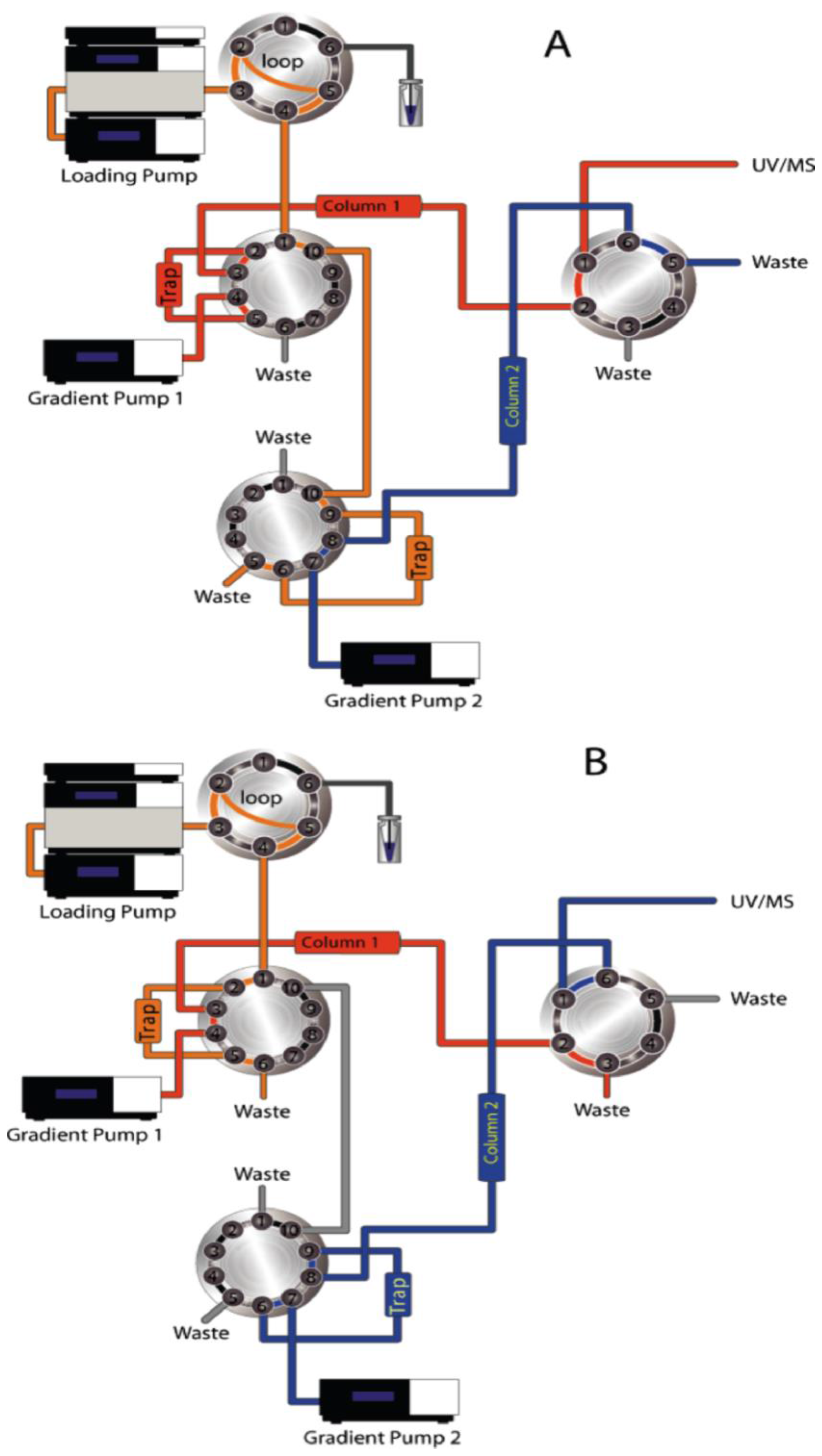
In modern chromatographic systems, trap columns are always mounted on the same switching valve as the separation column,
Figure 1. This approach is needed in order to minimize the void volume and enable fast sample transfer from the trap column to the separation column and avoid additional separation on the trap column. These settings require trap columns to be operated in the column oven along with the separation column, using the same operating temperature. In cases where separation column is operated at elevated temperature, this set-up can lead to insufficient sample trapping and subsequent sample loss. Mounting the column outside of the column oven is not a reasonable solution in this case due to long connecting tubing and subsequently increased void volume. Separate temperature control for the trap column and for the separation column would be an option for improved sample trapping at elevated temperatures. However, this solution is difficult to implement and operate; therefore, other options to maintain optimal sample loading must be considered.
The most convenient option is the use of precooled mobile phases for sample loading, which would enable the lowering of the trapping column’s temperature and enhancing the trapping efficiency. Here, we describe the use of standard loading phase in our laboratory, aqueous trifluoroacetic acid (TFA), for sample loading at different operating temperatures of the column oven. The temperature of the elution mobile phase (nano flow) was not altered, enabling it to reach the trap column and the separation column at the temperature of the column oven during the elution.
All experiments were carried out using an RSLC nano HPLC system (ThermoFisher, Germering, Germany) with the C18 trapping and separation column having an inner diameter (ID) of 300 µm and 75 µm, respectively.
Figure 1.
Plumbing scheme showing the (dual) nano HPLC separation system with (A) trap column and nano column 1 operated in eluting loading position while trap column and nano column 2 are equilibrated and prepared for the following sample injection; and (B) trap column 1 and nano column 1 are being equilibrated while the analytes are being eluted from trap column 2 and nano column 2. The trap column was operated in a back-flush mode ensuring minimal peak broadening and preventing peptide separation before the transfer on the nano separation column.
Figure 1.
Plumbing scheme showing the (dual) nano HPLC separation system with (A) trap column and nano column 1 operated in eluting loading position while trap column and nano column 2 are equilibrated and prepared for the following sample injection; and (B) trap column 1 and nano column 1 are being equilibrated while the analytes are being eluted from trap column 2 and nano column 2. The trap column was operated in a back-flush mode ensuring minimal peak broadening and preventing peptide separation before the transfer on the nano separation column.
2. Experimental Section
2.1. Chemicals
HPLC Grade water was obtained from an in-house Millipore water purification system (Millipore, Wien, Austria), acetonitrile (AcN), methanol (MeOH), both as Chromasolv, dithiothreitol, iodoacetamide, trifluoroacetic acid, formic acid, and bovine serum albumin (BSA) was purchased from Sigma-Aldrich (Sigma-Aldrich, Wien, Austria). Trypsin (sequencing grade) was purchased from Promega GmbH (Wien, Austria). Trifluoroethanol (TFE) was purchased from Merck-Millipore (Wien, Austria).
2.2. LC/MS Instrumentation
All experiments were performed using an Ultimate 3000 RSLC nano HPLC system, PepMap C18 trapping column (TC, 5 µm particle size, 300 Å pore size, 300 µm ID × 5 mm length), and the PepMap C18 nano separation column (3 µm particle size, 100 Å pore size, 75 µm ID × 250 mm length), all purchased from ThermoFisher Scientific, Vienna, Austria. Both columns were operated in a column oven at:
Loading mobile phase was always aqueous 0.1% TFA, which was pumped through the trap column at 30 µL/min using:
Mixing following mobile phases composed reversed phase separation gradient:
The separation gradient for BSA peptides was generated as follows: 3% B for 10 min rising to 35% B at 40 min, 35% B at 70 min, kept at 90% B from 51 min to 55 min. Finally, the separation column was equilibrated at 3% B from 56 min to 60 min. The trap column was switched online with the separation column at 8 min and switched back to the load position at 50 min.
The separation gradient for peptides from the biological sample was generated as follows: 3% B for 15 min rising to 30% B at 70 min, rising to 60% B at 90 min, keeping at 90% B from 91 min to 100 min. Finally, the separation column was equilibrated using 3% B from 101 min to 125 min. The trap column was switched online with the separation column at 10 min and switched back to load position at 115 min.
The trap column was operated in a back-flush mode, meaning that the sample was eluted using the nano-flow, pumped in opposite direction of the loading flow. This elution method ensures low void volume, prevents peak broadening, and prevents unwanted separation on the trap column.
All peptides were detected using UV detection at 214 nm and electrospray ionization with a maXis Impact q-ToF mass spectrometer (Bruker, Bremen, Germany) equipped with a captive spray source. Ionization was performed at 1600 V, MS/MS using top ten of all ions was performed for all species with two or more positive charges while single charged ions were excluded.
2.3. Samples
Tryptically-digested BSA was used as a standard sample to investigate the influence of the trap column temperature on separation efficiency. For the complex sample, proteins obtained from human urine were tryptically digested upon protein precipitation using slightly modified Wessel-Fluegge method [
24,
25].
2.4. Data Analysis
All HPLC–UV data was analyzed using Chromeleon 6.8 (ThermoFisher, Germering, Germany), Raw MS data was transformed into Mascot
mgf files using Data Analysis 4.2 (Bruker, Bremen, Germany). Database search of MS/MS data was performed using ProteinScape 3.1 (Bruker, Bremen, Germany) and Mascot 2.5.1. (Matrix Science, London, UK), SwissProt protein databases (
www.uniprot.org) at the most current version was used for MS/MS data search. Trypsin was selected as digesting enzyme with two miscleavages allowed, carbamidomethylation on C and oxidation on M were selected as fixed and variable modifications, respectively.
3. Results and Discussion
The relation between the retention factor
k and the absolute temperature in a chromatographic system is described by van’t Hoff equation [
26]:
Here, ΔH0 is the enthalpy of the retention and ΔS0 is the entropy of the retention at the given temperature T, R is the universal gas constant, and ϕ the phase ration. Obviously, the ln(k) is inversely proportional to the operating temperature of the separation column and retention of analytes is influenced by operating temperature of the column and the mobile phase. According to the Equation (1), retention time will be higher for separations performed at lower temperatures, which is also true for the trapping efficiency of a column. Thus, an analyte will be trapped more efficiently on a trap column when loaded at lower temperature. Since the trapping column was operated in a column oven, it was not possible to provide efficient solution for separate cooling and heating of the trapping column within the existing column oven. Therefore, the decision was made to use the precooled loading mobile phase in order to enhance the trapping efficiency of tryptic peptides.
Trapping column was constantly flushed with the loading mobile phase, but its temperature was not assessed separately. It can also be assumed that a certain increase of the temperature of the precooled loading mobile phase occurs during the loading process within the heated column oven. The temperature difference of the loading mobile phase before and after the trapping column was, however, not measured.
Separation column’s operational temperature has significant impact on the thermodynamic process of peptide binding and elution influencing retention time and peak shape. In case of nano-separations, as used for proteomics analysis, it can be assumed that the temperature of the column oven is actually the operational temperature of both the trap and separation column due to their small dimensions and thin outer walls, which enables fast heating and fast thermal equilibration.
Tryptically-digested BSA is routinely used in our laboratory to test the performance of the separation and detection system.
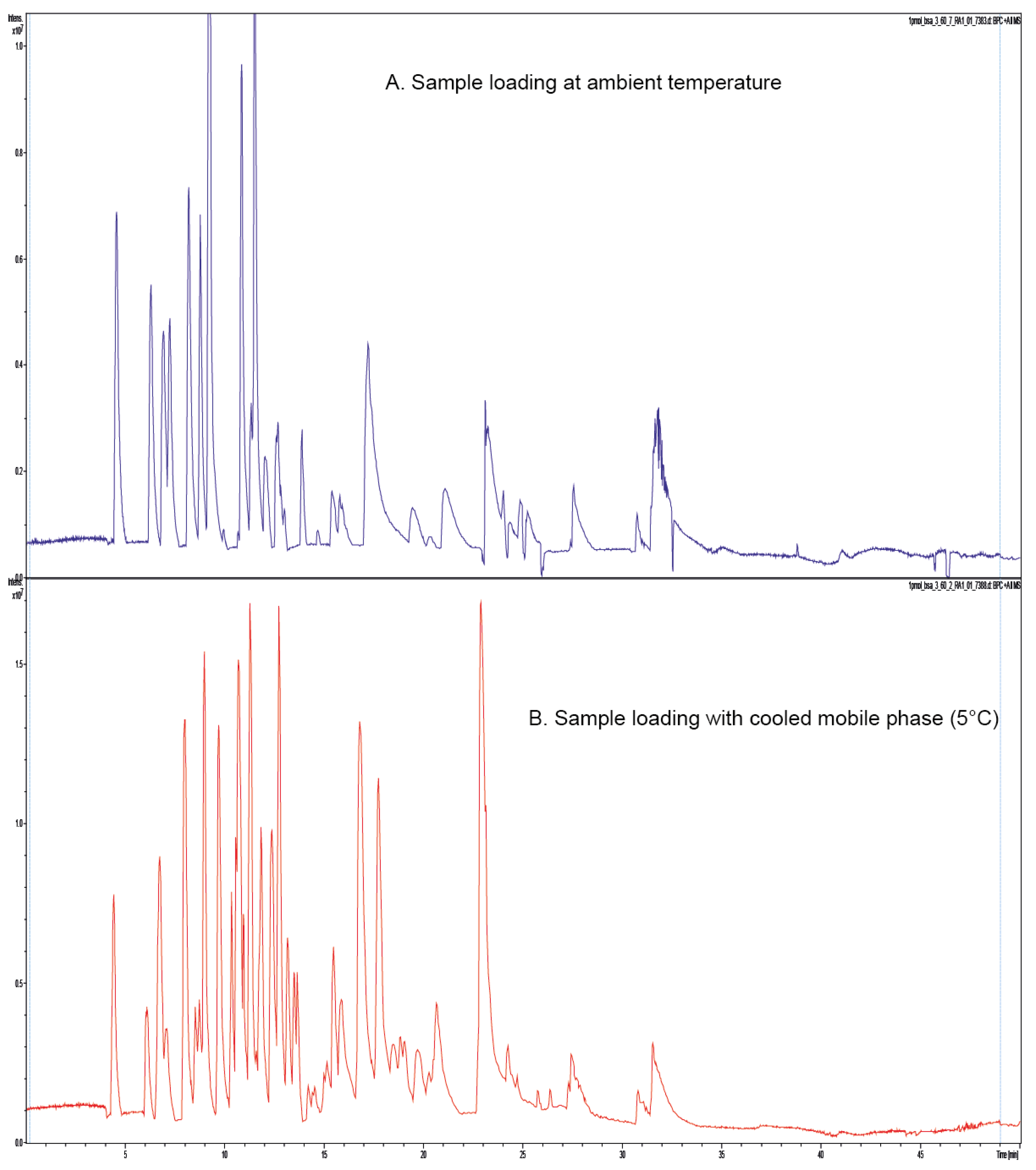
Figure 2A shows the separation of the BSA tryptic digest performed with both the trap column and separation column operated at 60 °C without a precooled loading mobile phase. In
Figure 2B, the same separation was performed using a precooled loading mobile phase (5 °C) and otherwise identical conditions.
Reproducible separation of complex biological samples is of great importance and it implies reproducible trapping of peptides generated upon enzymatic digestion.
Figure 3 shows the reproducibility of the BSA separation using cooled loading mobile phase at 5 °C. Reproducibility of the retention times is shown in
Figure 4, ranging from 0.05% for the first eluting peptide and 0.48% for the last eluting peptide for sample loading with the precooled mobile phase. The retention time stability and reproducibility is, therefore comparable with the separation performed without cooling of the mobile, which ranges from 0.2% for the first eluted peptide at 23.1 min and 0.46% for the latest eluting peptide at 55.5 min. Temperature stability of all system’s components is very important for reproducible trapping and separation, as shown in
Figure 3 for first two injections. Although the trap column was flushed with the precooled loading mobile phase, certain time was needed for a complete equilibration of the separation system.
Obviously, some peptides were lost during the loading process with the non-cooled loading mobile phase as compared to analysis with the precooled solvent.
Table 1 shows summarized results for peptide separations of the standard sample and of the biological sample. For BSA samples, the highest number of identified peptides, which implies the highest sequence coverage, was achieved when a precooled mobile phase at 5 °C was used for sample loading and the separation was performed at 60 °C. The use of the precooled loading solvent resulted with improved trapping of peptides on the trap column and higher separation temperature provided improved peptide separation on the nano column, which, again, results with better ionization and higher detection and identification rates.
Figure 2.
(A) MS-Base Peak Chromatogram showing the separation of BSA tryptic peptides on a nano separation column and trap column in operated in a column oven at 60 °C. Loading mobile phase was not precooled and it was loaded at ambient temperature; and (B) MS-Base Peak Chromatogram showing the separation of BSA tryptic peptides on a nano separation column and trap column operated in a column oven at 60 °C. Loading mobile phase was precooled for sample loading.
Figure 2.
(A) MS-Base Peak Chromatogram showing the separation of BSA tryptic peptides on a nano separation column and trap column in operated in a column oven at 60 °C. Loading mobile phase was not precooled and it was loaded at ambient temperature; and (B) MS-Base Peak Chromatogram showing the separation of BSA tryptic peptides on a nano separation column and trap column operated in a column oven at 60 °C. Loading mobile phase was precooled for sample loading.
Table 1.
Separation of BSA tryptic digest using different loading conditions resulted with identification of higher number of peptides when precooled loading mobile phase was used. The use of higher separation temperature (60 °C vs. 45 °C) also contributed to higher sequence coverage. For the biological sample, more proteins and peptides were identified when sample loading was performed using a precooled loading mobile phase.
Table 1.
Separation of BSA tryptic digest using different loading conditions resulted with identification of higher number of peptides when precooled loading mobile phase was used. The use of higher separation temperature (60 °C vs. 45 °C) also contributed to higher sequence coverage. For the biological sample, more proteins and peptides were identified when sample loading was performed using a precooled loading mobile phase.
| T (C; Loading MP/Column Oven) | Sequence Coverage (%) | Proteins # | Peptides # |
|---|
| BSA (5 °C/45 °C) | 56.3 | 1 | 49 |
| BSA (5 °C/60 °C) | 66.9 | 1 | 60 |
| BSA (RT/60 °C) | 48.1 | 1 | 39 |
| Urine (5 °C/60 °C) | n.a. | 344 | 1415 |
| Urine (RT/60 °C) | n.a. | 182 | 442 |
Figure 3.
Reproducibility of the separation of BSA tryptic peptides with both separation column and trap column in a column oven at 60 °C and the precooled loading mobile phase to 5 °C. Seven consecutive injections of 1 pMol digested BSA are shown. Two runs (from the bottom) show that the separation system needed a certain time to equilibrate.
Figure 3.
Reproducibility of the separation of BSA tryptic peptides with both separation column and trap column in a column oven at 60 °C and the precooled loading mobile phase to 5 °C. Seven consecutive injections of 1 pMol digested BSA are shown. Two runs (from the bottom) show that the separation system needed a certain time to equilibrate.
Enzymatic digestion of biological samples generates a large amount of peptides with a broad array of different physicochemical properties. The injected sample volume and loading time depend on the sample’s matrix, applied flow rates, and the trap column’s dimension. In our laboratory, an injection and separation protocol was established for sample loading and subsequent wash of the autosampler and transfer lines, which was also used for current measurements of peptides from urinary peptides [
25]. The amount of urinary proteins differs strongly from person to person and published results report numbers between a few hundred and several thousands of identified proteins [
27,
28,
29,
30,
31,
32,
33,
34].
Upon digestion of urinary proteins, 20 µL of digested sample was diluted with 30 µL of 0.1% aqueous TFA and 40 µL were injected on the trap column using two loading conditions, based on results obtained from tests with BSA:
- (A)
Heated trap column and separation column operated at 60 °C, using loading mobile phase at ambient temperature.
- (B)
Heated trap column and separation column operated at 60 °C, using precooled loading mobile phase at 5 °C.
Figure 5 shows the MS-BPC for separations of tryptic urinary peptides upon sample loading at different temperature of the loading solvent. Using loading solvent at ambient temperature resulted with significant sample loss (
Figure 5A) in comparison to the use of the precooled loading phase (
Figure 5B). This result is confirmed by the number of identified peptides and proteins for both experiments as shown in
Table 1. Obviously, there is a significant loss of information, which is higher when compared to analysis of BSA only. As opposed to the digest of a single protein, enzymatic digest of a complex sample results with significantly higher number of peptides, competing for a binding site on the stationary phase. Higher operating temperature weakens the bonds between the analyte (peptides) and promotes elution rather than retention. Therefore, peptides with lower binding affinity face competition from other peptides with higher binding affinity and are additionally hindered in biding to the stationary phase by high temperature. According to Equation (1), these peptides will be eluted significantly earlier from the column as compared to loading and binding at lower temperature. For urinary peptides analyzed in this experiment, it was possible to detect almost twice as many proteins using the precooled loading mobile phase as compared to the non-cooled phase.
Figure 4.
Zoom in of seven consecutive, overlapped, chromatograms showing separation of BSA tryptic peptides. The retention time’s reproducibility ranged from 0.05% for the first peak eluted at 21.2 min and 0.48% for the peak eluted at 55.3 min.
Figure 4.
Zoom in of seven consecutive, overlapped, chromatograms showing separation of BSA tryptic peptides. The retention time’s reproducibility ranged from 0.05% for the first peak eluted at 21.2 min and 0.48% for the peak eluted at 55.3 min.
Figure 5.
MS-Base Peak Chromatogram showing separation of tryptic peptides obtained from urinary proteins. (A) Trap column and the nano separation column were operated at 60 °C loading mobile phase was not precooled; and (B) tryptic peptides from urinary proteins were separated using identical gradient as in (A) but with precooled loading mobile phase.
Figure 5.
MS-Base Peak Chromatogram showing separation of tryptic peptides obtained from urinary proteins. (A) Trap column and the nano separation column were operated at 60 °C loading mobile phase was not precooled; and (B) tryptic peptides from urinary proteins were separated using identical gradient as in (A) but with precooled loading mobile phase.
In terms of reproducibility of protein identifications, two sets of samples were injected in triplicate using precooled mobile phase and loading mobile phase at ambient temperature.
Figure 6A shows the number of overlapping identifications for the precooled mobile phase and
Figure 6B for the loading mobile phase at ambient temperature. As it can be seen, the amount of proteins identified with precooled mobile phase is significantly higher and the number of overlapping identifications is higher as compared to identifications when loading solvent was used at ambient temperature. Some proteins have been identified only in one of the injections, this effect has been regularly observed for proteomics analysis and it is due to instability of electrospray ionization of peptides in complex samples.
Figure 6.
Reproducibility of protein identifications based on triplicate injections of tryptic urinary peptides using precooled (A) loading mobile phase and (B) mobile phase at ambient temperature.
Figure 6.
Reproducibility of protein identifications based on triplicate injections of tryptic urinary peptides using precooled (A) loading mobile phase and (B) mobile phase at ambient temperature.