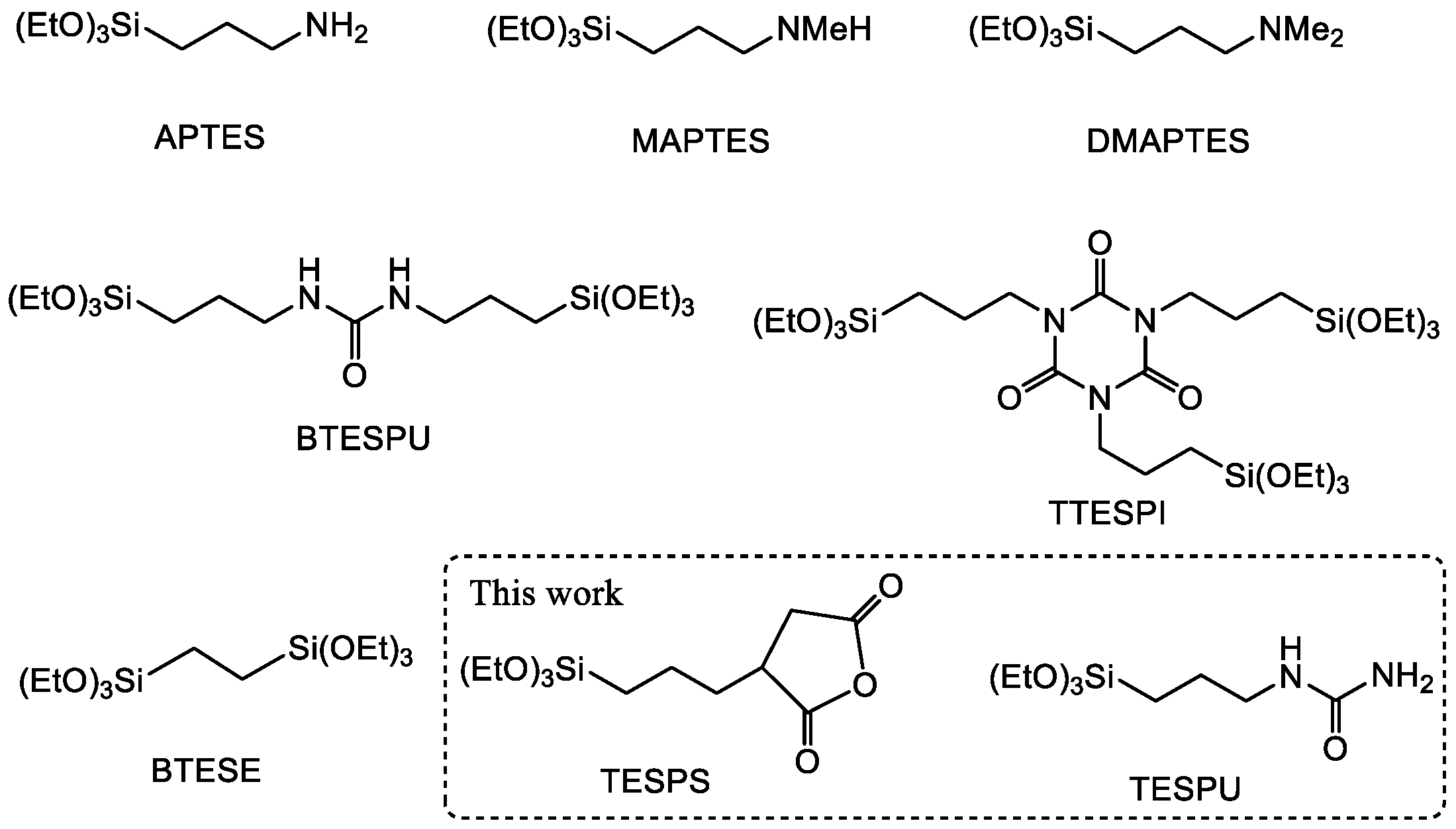
3.1. Design of CO2-Philic Groups
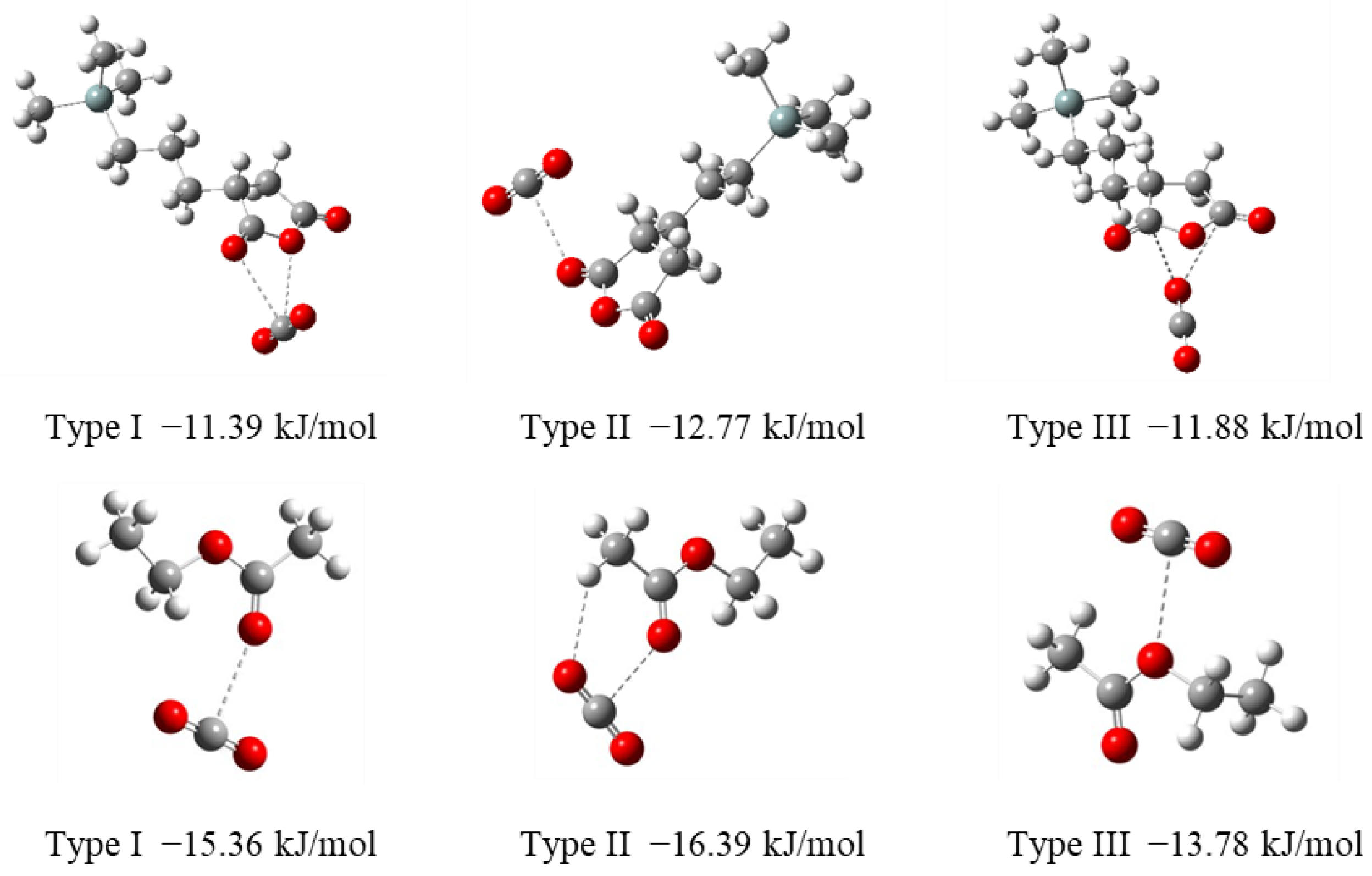
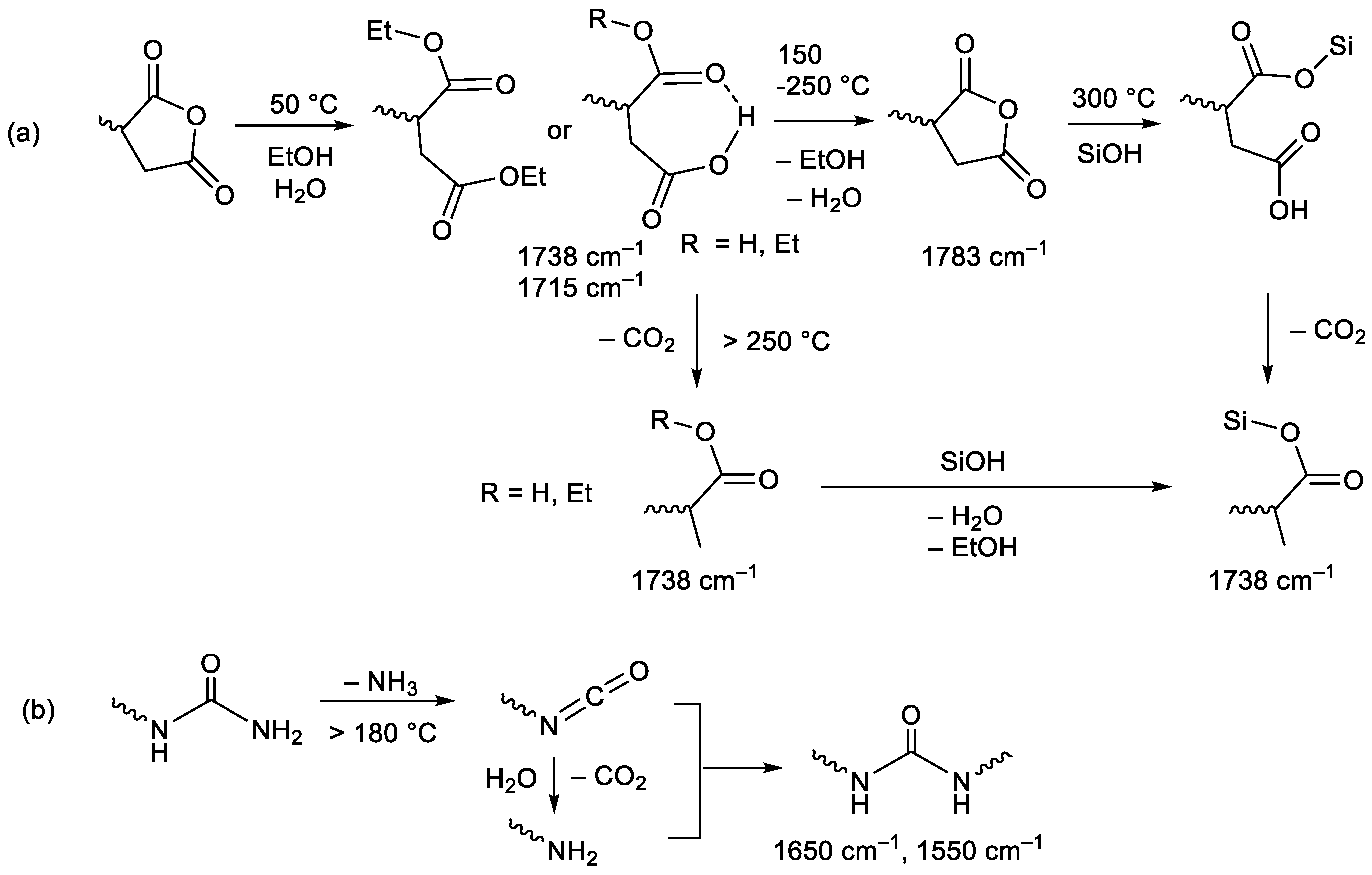
The formation of 1:1 complexes of a CO
2 molecule and trimethylsilylpropylsuccinic anhydride (i.e., model of TESPS) was investigated by DFT calculations at the B3LYP/6-31G level, revealing three thermodynamically stable complexes with different kinds of interactions (Types I–III), as shown in
Figure 1. In Type I complex, the CO
2 carbon atom is chelated by the carbonyl and ether oxygens of the succinic anhydride unit, whereas in Type II complex, there is only a single site interaction between the CO
2 carbon atom and one of the succinic anhydride carbonyl oxygens. A coordination through the chelate interaction between the CO
2 oxygen and anhydride carbonyl carbons is observed in a Type III complex. The coordination energy of CO
2 to succinic anhydride was calculated to be approximately −12 kJ/mol for each complex formation.
The coordination energies of CO
2 to ethyl acetate, a model of the ester unit that would be formed by the thermal degradation of succinic anhydride in PSQ (vide infra), were also calculated and were found to have larger negative value than those of the succinic anhydride model (
Figure 1). In Type I–III complexes, a CO
2 molecule interacts with the carbonyl or ether oxygen of ethyl acetate at the central carbon atom. The formation of the Type II complex involves the interaction between the alkyl C–H unit and the CO
2 oxygen atom, as reported for the formation of a methyl acetate–CO
2 complex [
20]. On the other hand, the coordination energy of CO
2 to
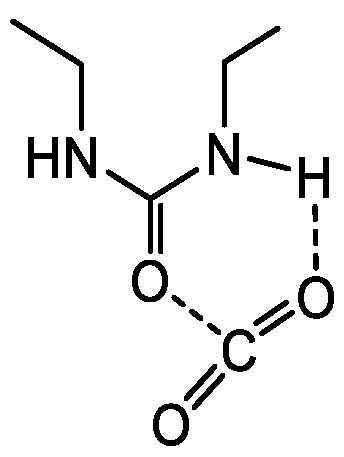
N,
N’-diethylurea (i.e., model of TESPU), which involves a chelate-type interaction as presented in
Chart 2, was previously computed to be approximately −28 kJ/mol [
15].
3.2. Membrane Preparation
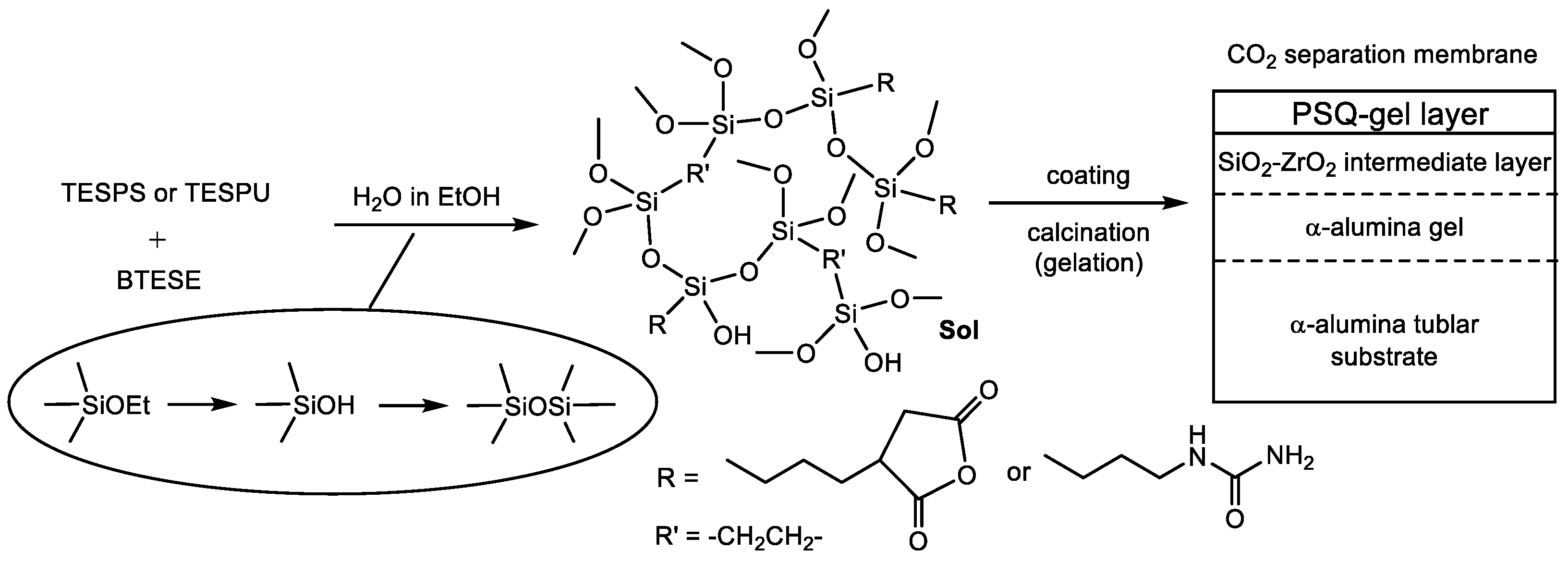
The sols prepared by the hydrolysis/condensation of the precursors in ethanol were coated on the intermediate layer of the support membrane and calcined in nitrogen to produce the PSQ gel layers, as shown in
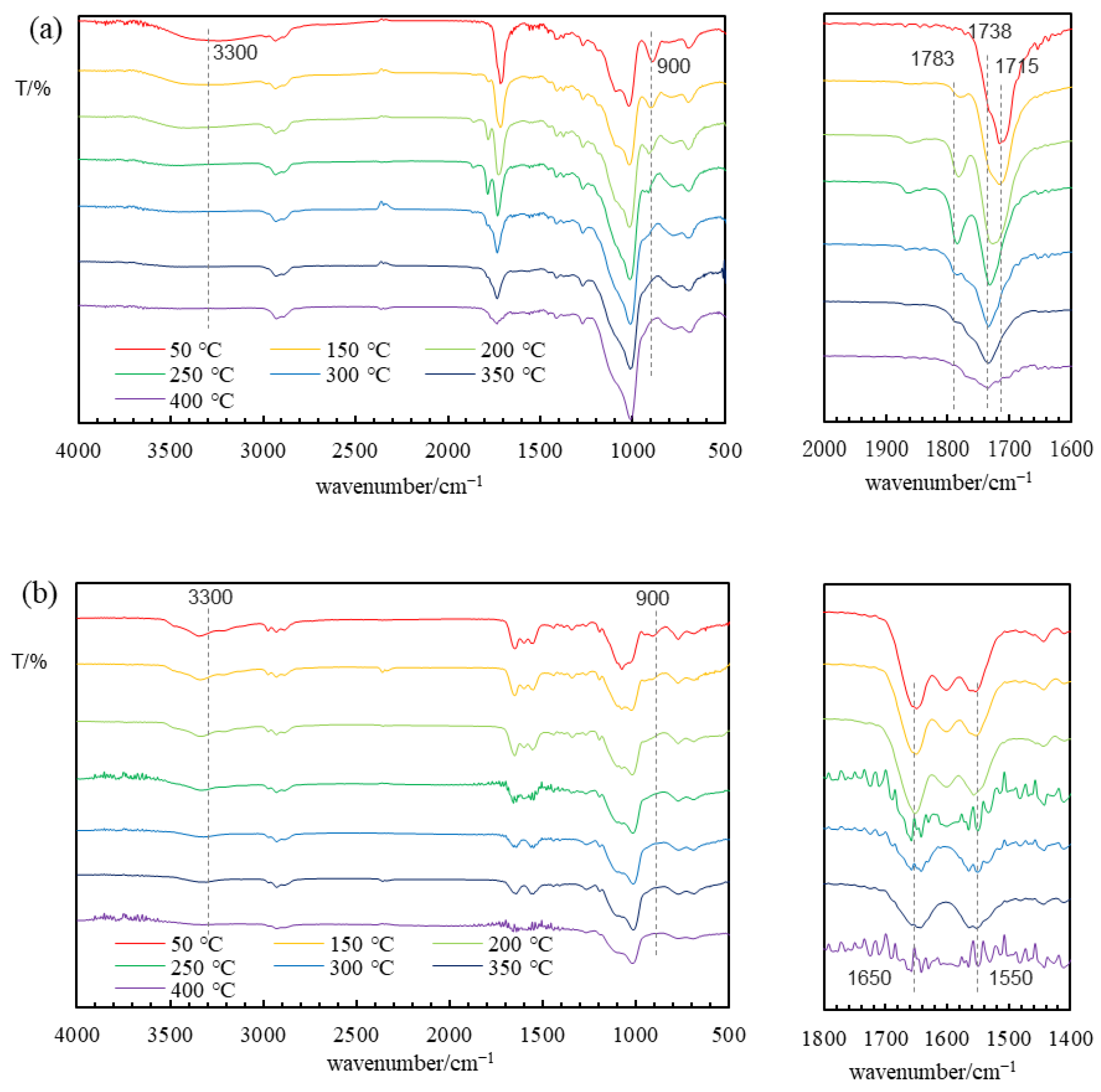
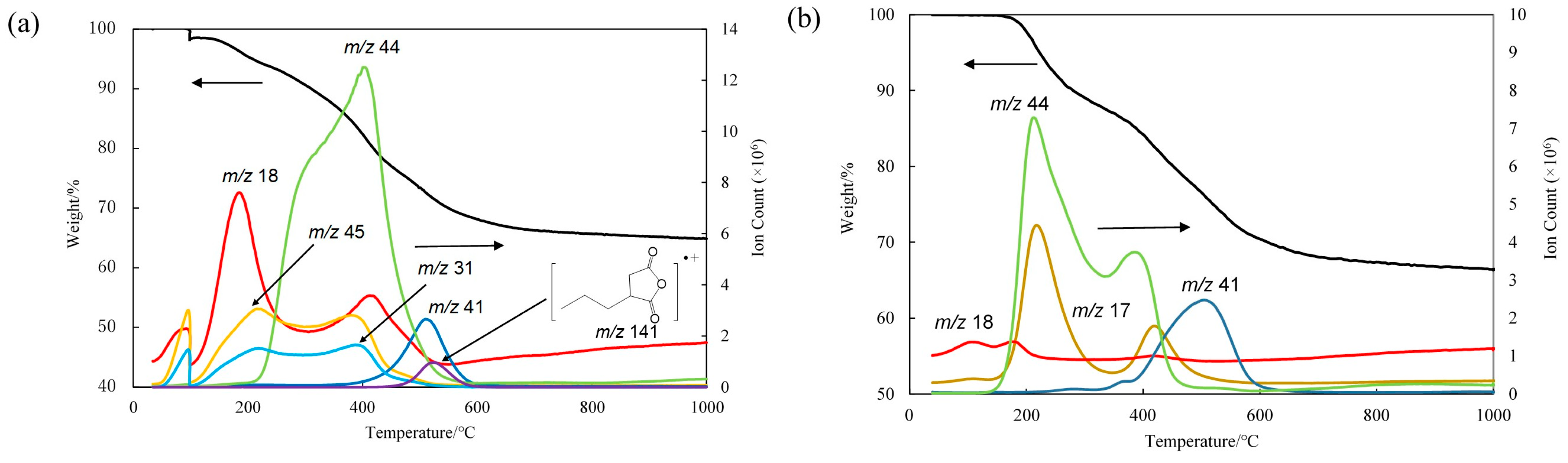
Figure 2. The calcination temperature was determined on the basis of the IR spectra measured after heating at each temperature for 10 min and the results of TGA, as shown in
Figure 3 and
Figure 4, respectively. The TGA of the gels prepared from TESPS-BTESE and TESPU-BTESE revealed no significant weight loss up to 200 °C, with a gradual decrease thereafter up to 700 °C.
For the TESPS-BTESE gel, the IR spectrum obtained after heating at 50 °C showed a carbonyl signal at 1715 cm
−1 with a shoulder at 1738 cm
−1, as shown in
Figure 3a. These signals, which were too low in energies to be assigned as succinic anhydride signals, are attributable to the ester or carboxylic acid unit with and without hydrogen bonding, respectively. The hydrogen bonding was probably formed intramolecularly and intermolecular hydrogen bonding with water, ethanol, and silanol units would be also involved when calcined at this temperature. On the basis of the IR spectrum, it is likely that the succinic anhydride unit underwent reactions with ethanol or water under hydrolysis/condensation conditions to provide, unexpectedly, a ring-opened ester or a carboxylic acid unit. It is likely that the silanol (Si–OH) unit, which was formed by the hydrolysis of the ethoxy–Si bond, acted as a weak acid catalyst for the hydrolysis of the succinic anhydride unit. Indeed, the
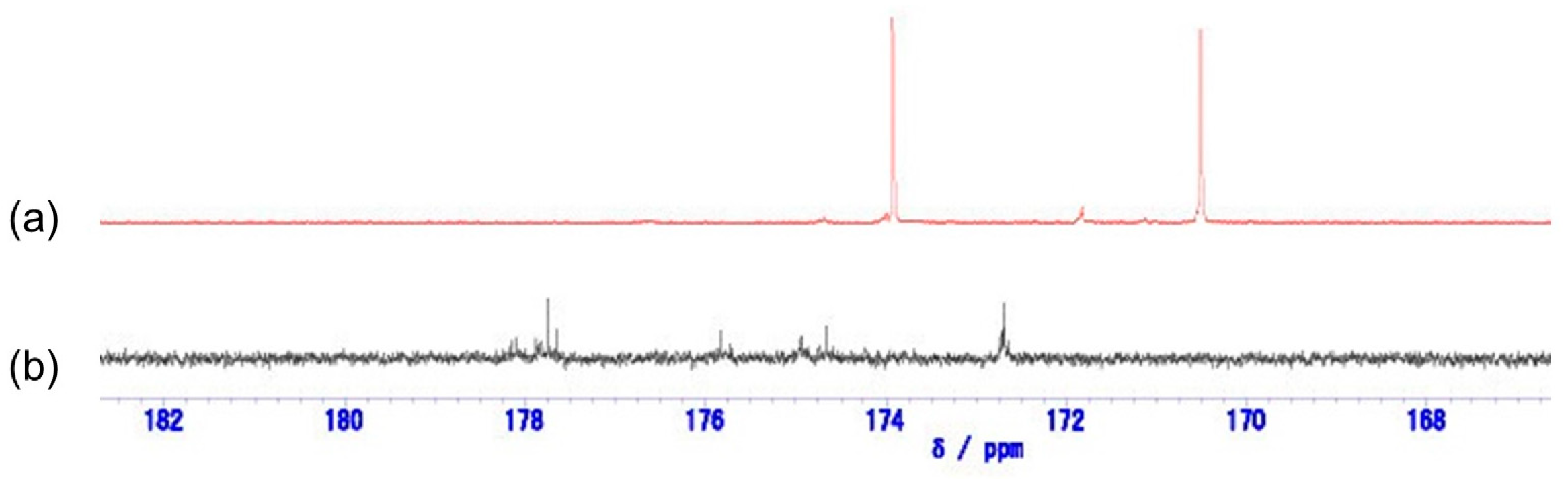
13C NMR spectrum of the mixture of TESPS-BTESE revealed multiple carbonyl carbon signals at 173–178 ppm after treatment with water in ethanol, as shown in
Figure 5. These signals were low-field sifted from those of TESPS in accordance with the fact that succinic acid and diethyl succinate show the signals at 179.9 ppm and 172.4 ppm, respectively, lower than that of succinic anhydride (170.7 ppm). From 150 °C to 250 °C, the 1715 cm
−1 peak gradually decreased in intensity and a new peak at 1783 cm
−1, likely due to the succinic anhydride unit, appeared. However, the succinic anhydride peak that appeared at 1783 cm
−1 was weakened at 300 °C and disappeared at 350 °C. During this process, the signal at 1738 cm
−1, which was observed as a shoulder from 50 °C to 200 °C and attributable to the ester or carboxylic acid unit without hydrogen bonding, appeared as a sharp peak at 250 °C, but became broad at 350 °C and weakened at 400 °C. A broad peak centered at 3300 cm
−1, which corresponds to the O–H stretching of water, carboxylic acid, and/or silanol, almost disappeared at 250 °C. At the same time, the Si–OH vibration band around 900 cm
−1 decreased in intensity as the heating temperature increased, suggesting the formation of siloxane linkages by the condensation of the silanol units. However, the Si–OH band was observed even at 400 °C, although the intensity was low.
To obtain more information about this process, we carried out a TG-MS analysis of the gels, as shown in
Figure 4a. The TG-MS profiles of the TESPS-BTESE gel revealed the formation of water (
m/
z 18), ethanol (
m/
z 45 and 31), and CO
2 (
m/
z 44) at elevated temperatures (
Figure 4a). On the basis of these spectral observations, we speculated that the ring-opened products underwent ring closure at temperatures higher than 150 °C (
Figure 6b). Thermal decarbonylation also occurred at temperatures higher than 250 °C, giving ethyl ester and/or carboxylic acid units, which might react with silanol units to produce silyl ester units. The ethyl ester units seemed to be thermally stable even at a high temperature. In fact, related poly(ethyl acrylate) is known to be stable up to 300 °C, and only an approximately 9% weight loss was observed by TGA in nitrogen [
21]. The ethyl ester units might remain at high temperatures unless they reacted with silanol units. The decomposition of the acid anhydride units observed at 300 °C was likely due to the reaction with the silanol units, followed by spontaneous decarbonylation, yielding silyl ester units, since it did not appear that large amounts of other nucleophiles such as water and ethanol remained in the gel at high temperatures. The TG-MS profiles also revealed a small peak at
m/
z 141, which corresponds to a fragment containing a succinic anhydride unit, at 300 °C or higher temperatures, indicating that the succinic anhydride units were not all degraded but remained to some extent. An unidentified peak of
m/
z 41 was observed at temperatures higher than 390 °C.
The IR spectrum of the TESPU-BTESE-based gel film prepared on a KBr plate showed three peaks at 1500–1700 cm
−1, which are characteristic of the monosubstituted urea units. These peaks were broadened after heating at 250 °C, and two peaks at 1650 cm
−1 and 1550 cm
−1 due to C=O stretching and N–H bending vibrations, respectively, were observed in the spectrum after heating at 300 °C (
Figure 3b). The signals at 1650 cm
−1 and 1550 cm
−1 closely resembled those of the IR spectrum of the BTESPU gel [
15] and were assignable to
N,
N′-disubstituted urea units. The signals of the
N,
N′-disubstituted urea units remained unchanged after heating at 350 °C. The silanol signal at 900 cm
−1 decreased in intensity by heating at 150 °C and 200 °C, with no further decrease at higher temperatures. The vibration peak of O–H/N–H bonds around 3300 cm
−1 also decreased, but did not disappear after heating at 350 °C. The TG-MS analysis of the TESPU-BTESE gel revealed that NH
3 and CO
2 are formed at temperatures higher than 180 °C (
Figure 4b). A possible mechanistic interpretation of this phenomenon is presented in
Figure 6b. It is known that monosubstituted urea undergo thermal degradation to form isocyanate with the liberation of NH
3 [
22,
23]. The hydrolysis of the isocyanate unit with water results in the formation of amine and CO
2. Thus-formed amine reacts with the isocyanate unit to form the
N,
N′-disubstituted urea unit. An unidentified peak of
m/
z 41 was observed in the TG-MS of TESPU-BTESE-based gels at temperatures higher than 390 °C, similarly to that of TESPS-BTESE.
On the basis of these results, we examined the CO
2 separation performance of the membranes calcined at different temperatures to observe the effects of the thermal degradation of the CO
2-philic units. The TESPS-BTESE films were calcined at 250 °C and 300 °C, at which the succinic anhydride unit reformation and the ester unit formation would complete, respectively. Calcination at 350 °C was also examined for TESPS-BTESE. For TESPU-BTESE, the membranes prepared by calcination at 200 °C and 300 °C were investigated, because the monoalkylurea units seemed to be converted to dialkylurea units between 200 °C and 300 °C. The membrane calcined at 300 °C showed a largely decreased CO
2/N
2 permselectivity from that calcined at 200 °C (vide infra), and calcination at higher temperature was not examined for TESPU-BTESE. The gels obtained from TESPS-BTESE and TESPU-BTESE were not porous according to the nitrogen adsorption analysis, regardless of the calcination temperature, as shown in
Figure S2. However, void spaces would be formed in the PSQ layers by the thermal degradation of the organic units, affecting the CO
2 separation properties of the membranes.
3.3. Gas Permeation
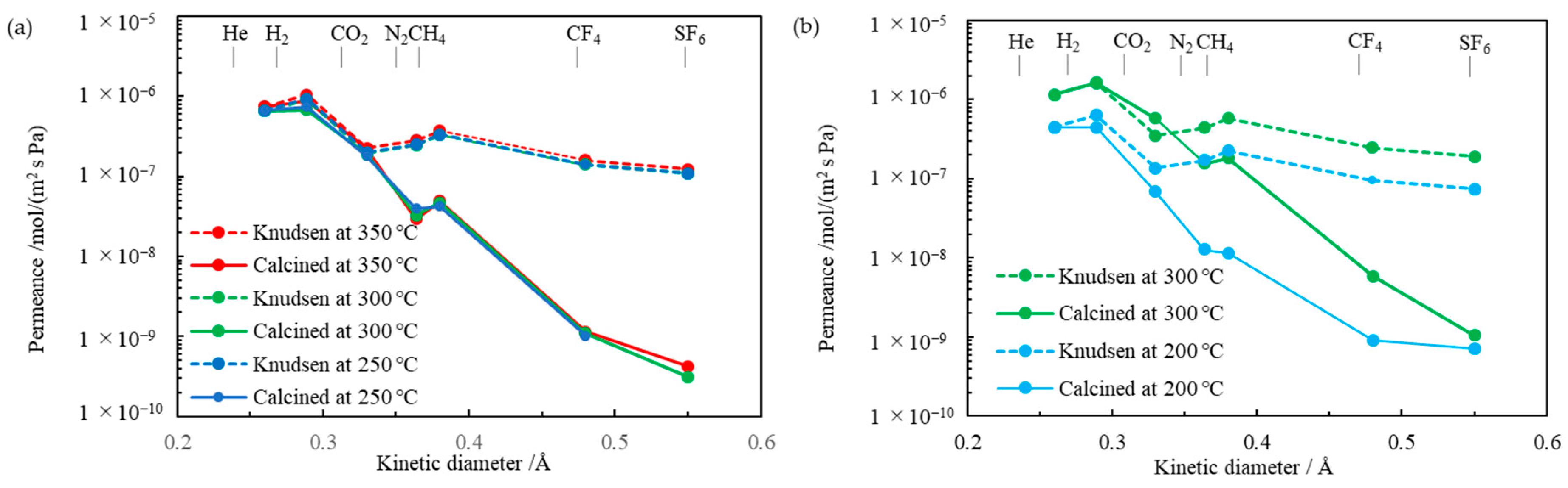
The pure gas permeance data of the TESPS-BTESE- and TESPU-BTESE-based PSQ membranes calcined at different temperatures are shown in
Figure 7. The SF
6 permeation of the TESPS-BTESE-based membrane calcined at 250 °C (TESPS-BTESE(250)) was too slow to determine the permeance. The plots of the gas permeance of the membranes vs. the kinetic diameter of gas molecules from N
2 to SF
6 had larger negative slopes than the Knudsen plots, as shown in
Figure 7, suggesting the molecular sieving effects of the membranes for gas separation in this region.
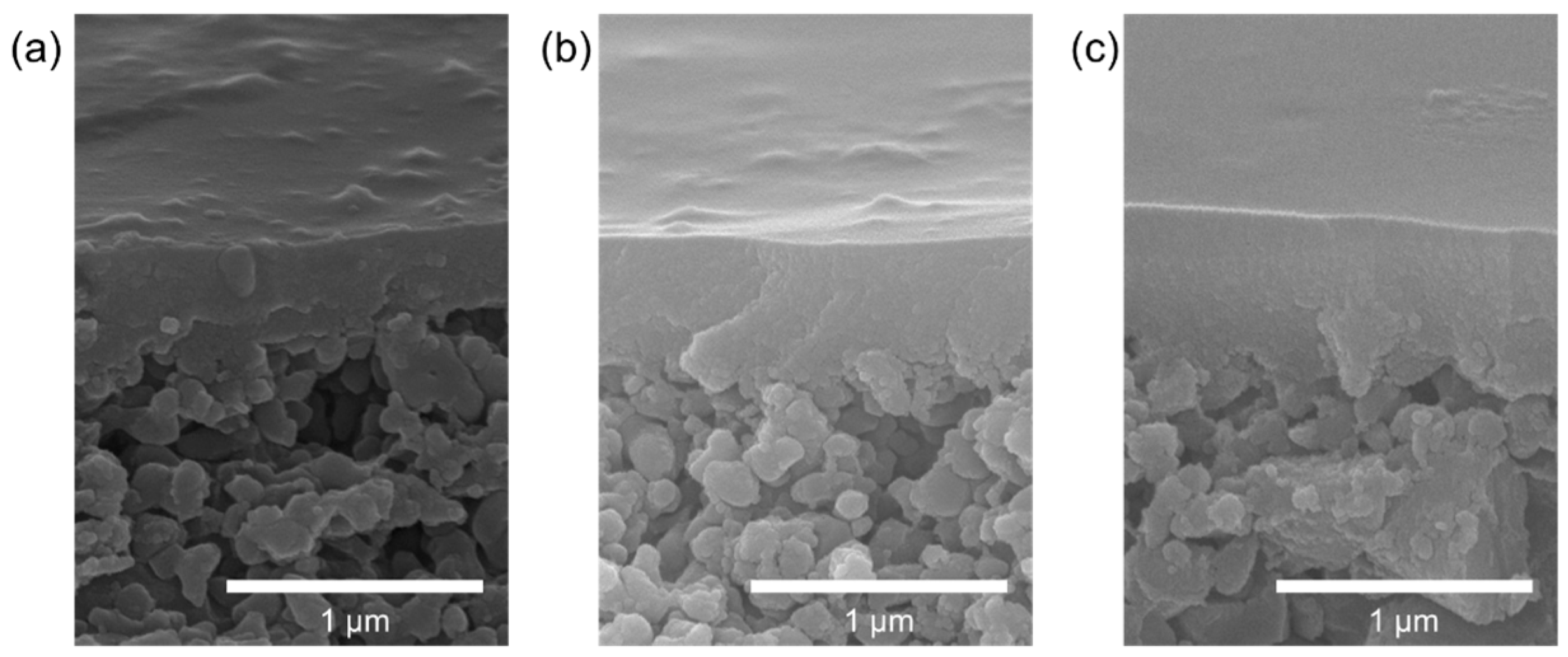
Figure 8 shows the SEM cross-sections of the TESPS-BTESE membranes calcined at different temperatures on the inorganic support. The TESPS-BTESE (250) membrane showed some gaps on the surface, which became smooth as the calcination temperature was elevated, and the TESPS-BTESE(350) membrane showed an almost flat surface. It was found that all the membranes possessed no defects such as cracks and pinholes on the surface. As the boundary of the PSQ and intermediate layers could not be clearly seen, we could not determine the thicknesses of the PSQ separation layers.
The CO
2 permeances and CO
2/N
2 permselectivities of the membranes at 50 °C are summarized in
Table 2.
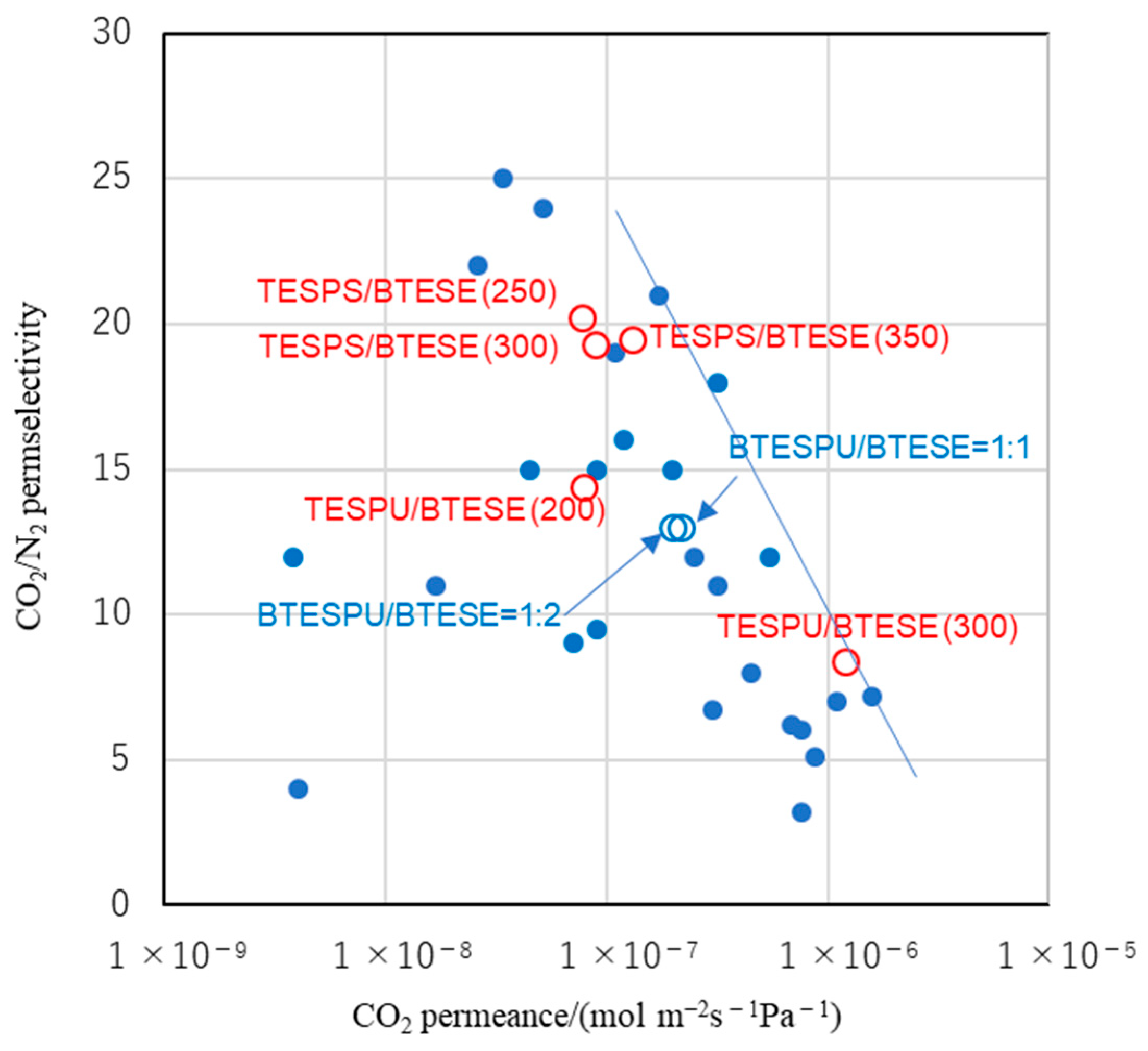
Figure 9 shows the trade-off plots of CO
2 permeance vs. CO
2/N
2 permselectivity of the membranes prepared in the present study, together with those of previously reported PSQ-based membranes. For both TESPS-BTESE- and TESPU-BTESE-based membranes, gas permeance increased with an increasing calcination temperature, possibly due to the enhanced siloxane network formation and the thermal degradation of the organic units; the former decreases the number of silanol groups that hinder the gas permeation in terms of the steric hindrance and CO
2-philicity, whereas the latter lowers the membrane density by the formation of void spaces, although the gels were found to be non-porous in the nitrogen adsorption analysis regardless of the calcination temperature as mentioned above. The trade-off relationship between CO
2 permeance and CO
2/N
2 permselectivity is generally observed for CO
2 separation membranes [
1]. However, the TESPS-BTESE-based membranes showed only slight decreases in permselectivity; increasing the calcination temperature from 250 °C to 350 °C resulted in an increase in the CO
2 permeance of the TESPS-BTESE-based membrane (TESPS-BTESE(350)) by 1.7-fold compared with that of TESPS-BTESE(250), with an almost negligible decrease in CO
2/N
2 permselectivity from 20.2 to 19.5, overcoming the general trade-off relationship between the CO
2 permeance and CO
2/N
2 permselectivity. This can be understood from the higher CO
2-philicity of the ester unit than the acid anhydride unit, as suggested by DFT calculations (vide supra). We also examined the CO
2 separation properties of PSQ membranes prepared by the homopolymerization of TESPS; however, the membranes consistently showed lower CO
2 permeances and CO
2/N
2 permselectivities than the TESPS-BTESE-based membranes (
Table 2). The membranes were found to be durable and standing the membranes at 200 °C for 5 days resulted in no obvious changes of the CO
2 permeances and CO
2/N
2 permselectivities.
The CO
2 permeance of the TESPU-BTESE-based membranes markedly increased with the increasing calcination temperature, although a decrease in CO
2/N
2 permselectivity was noted at the same time. This indicates that the TESPU-BTESE-based membranes behave as predicted by the trade-off relationship. A highly CO
2-permeable membrane was obtained when calcined at a high temperature (TESPU-BTESE(300)), with a somewhat low CO
2/N
2 permselectivity of 8.4. Interestingly, the PSQ membrane obtained by the 1:1 copolymerization of BTESPU-BTESE having
N,
N′-disubstituted urea units [
15], similar to TESPU-BTESE(300), had a much lower CO
2 permeance than TESPU-BTESE(300). This could be due to the decreased number of CO
2-philic urea units in TESPU-BTESE(300), because one
N,
N′-disubstituted urea unit is formed from two monosubstituted urea units by thermal degradation (
Figure 6b). However, the membrane prepared by the 1:2 copolymerization of BTESPU-BTESE showed a similar performance to the membrane prepared by the 1:1 copolymerization (
Figure 9) [
15]. Thus, factors other than the chemical transformation of the CO
2-philic units—void space formation, for example—may be operative in TESPU-BTESE(300), affecting the CO
2 separation performance.
To learn more about the separation performance of the membranes, we determined their temperature-dependent gas permeances, as presented in
Figure S3. Based on these data, activation energies were calculated for each gas, as summarized in
Table S1; those of CO
2 and N
2 are also listed in
Table 2. The TESPS-BTESE-based membranes showed higher activation energies than the TESPU-BTESE-based membranes for all gases, suggesting the higher network rigidity of the TESPU-BTESE-based membranes. This may be partly due to the urea–urea interaction by hydrogen bonding, particularly for the TESPU-BTESE-based membrane with NH
2 units calcined at 200 °C (TESPU-BTESE(200)). Hydrogen bonding between the urea units in the PSQ membranes prepared from BTESPU has been reported previously [
15]. Calcination at a higher temperature (TESPU-BTESE(300)) led to even lower activation energies, likely due to an increase in structural rigidity by the enhanced network formation and the conversion of the monoalkylurea units into the dialkylurea units. Void space formation by the thermal degradation of the monoalkylurea units may also be a reason for the low activation energies. TESPS homopolymer membranes exhibited higher activation energies than the TESPS-BTESE-based membranes, suggesting the effects of the BTESE-derived units in the membranes enhancing the network rigidity.
The activation energies for the CO
2 permeation of the membranes prepared in the present study were lower than those for N
2 permeation, despite their similar molecular sizes, indicating the adsorption-controlled mechanism for CO
2 separation by these membranes, as observed for other amine, urea, and isocyanurate-containing PSQ membranes reported previously [
9,
14,
15]. The low activation energies for the CO
2 permeation of the TESPU-BTESE-based membranes compared with those of the TESPS-BTESE-based membranes are in line with the higher network rigidity of the TESPU-BTESE-based membranes. The higher CO
2-philicity of the urea unit than the ester unit may also be the reason for the lower activation energies for the CO
2 permeation of the TESPU-BTESE-based membranes (
Figure 1 and
Chart 2).