Removal of Azo Dyes from Aqueous Effluent Using Bio-Based Activated Carbons: Toxicity Aspects and Environmental Impact
Abstract
:1. Introduction
2. Classification of Dyes
2.1. Structure and Properties of Azo Dyes
2.2. Toxicity Aspects of Azo Dyes
2.3. The Environmental Impact of Azo Dye
2.4. The Health Impact of Azo Dyes
3. Treatments Methods for Dye-Contaminated Effluents
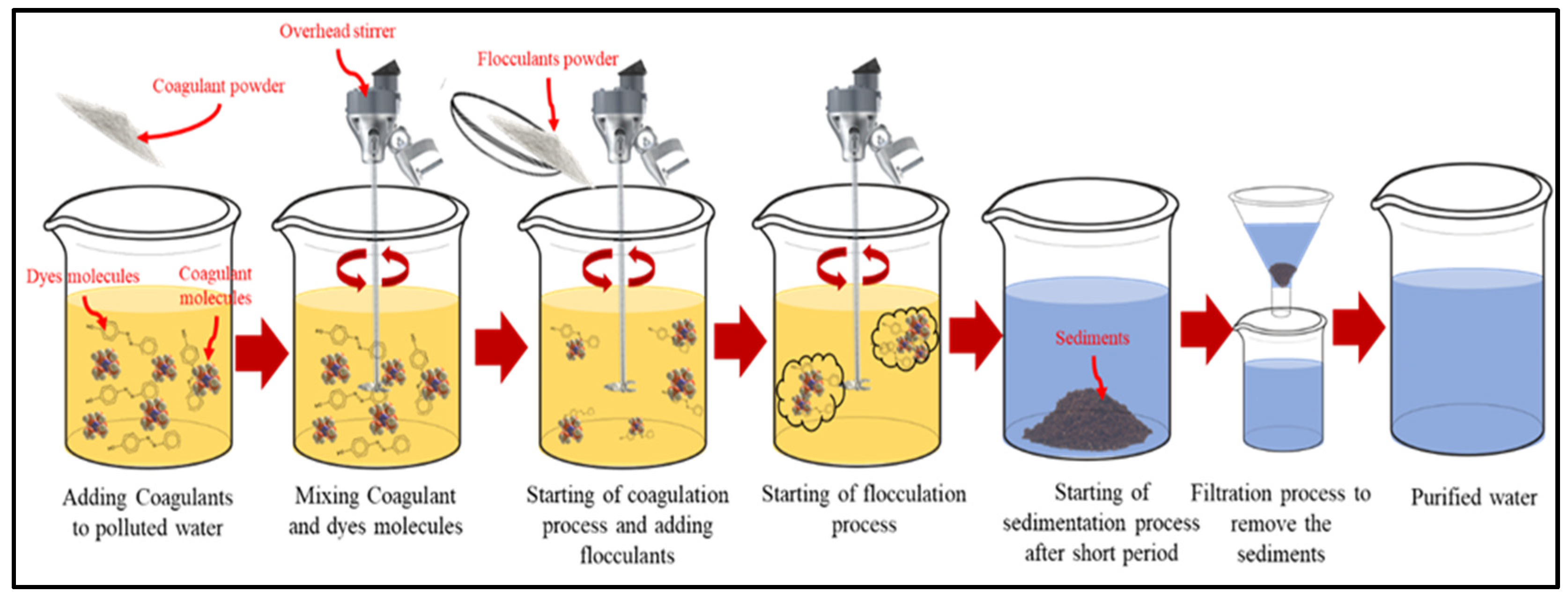
3.1. Coagulation and Flocculation
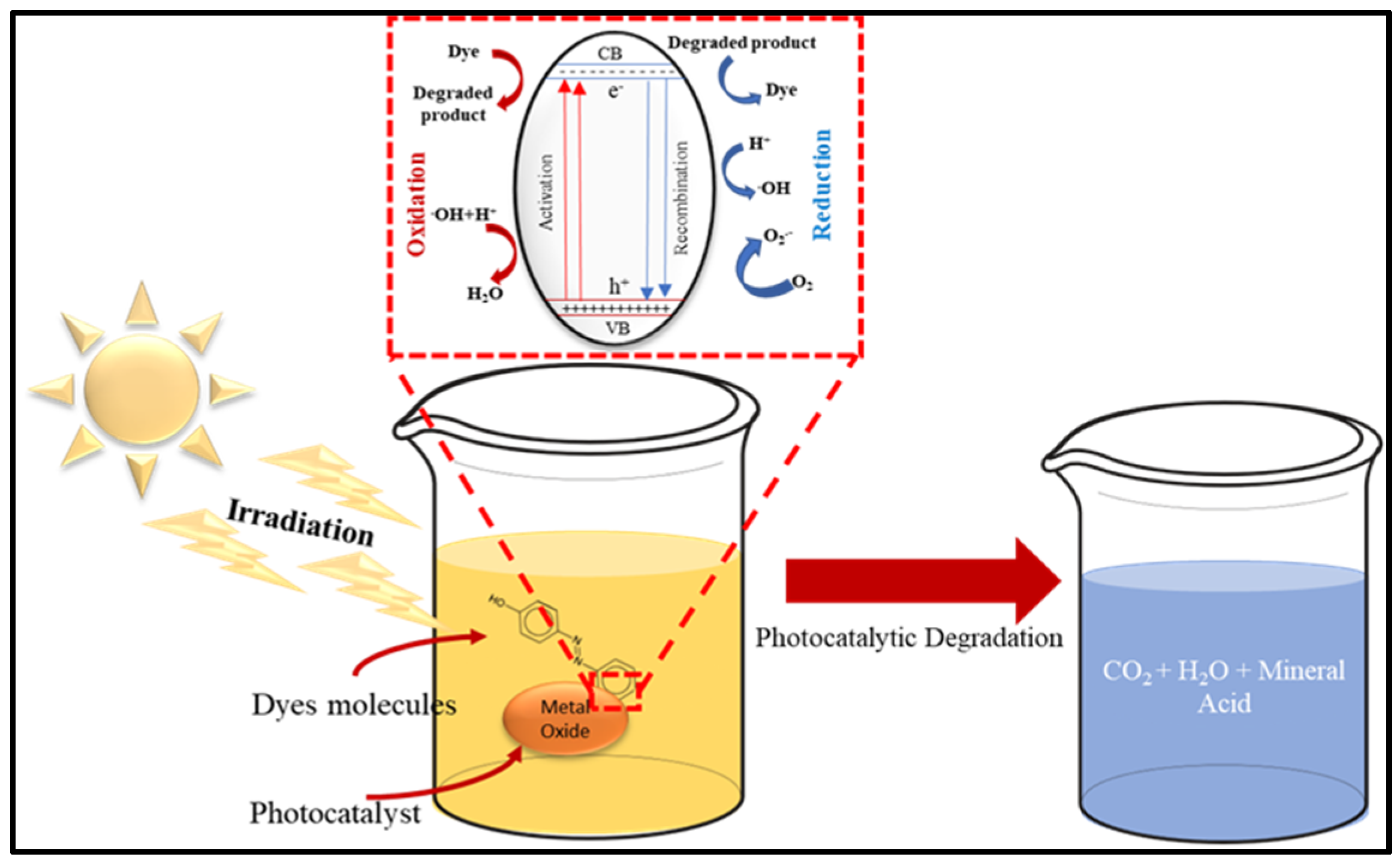
3.2. Photocatalytic Degradation
3.3. Ion Exchange
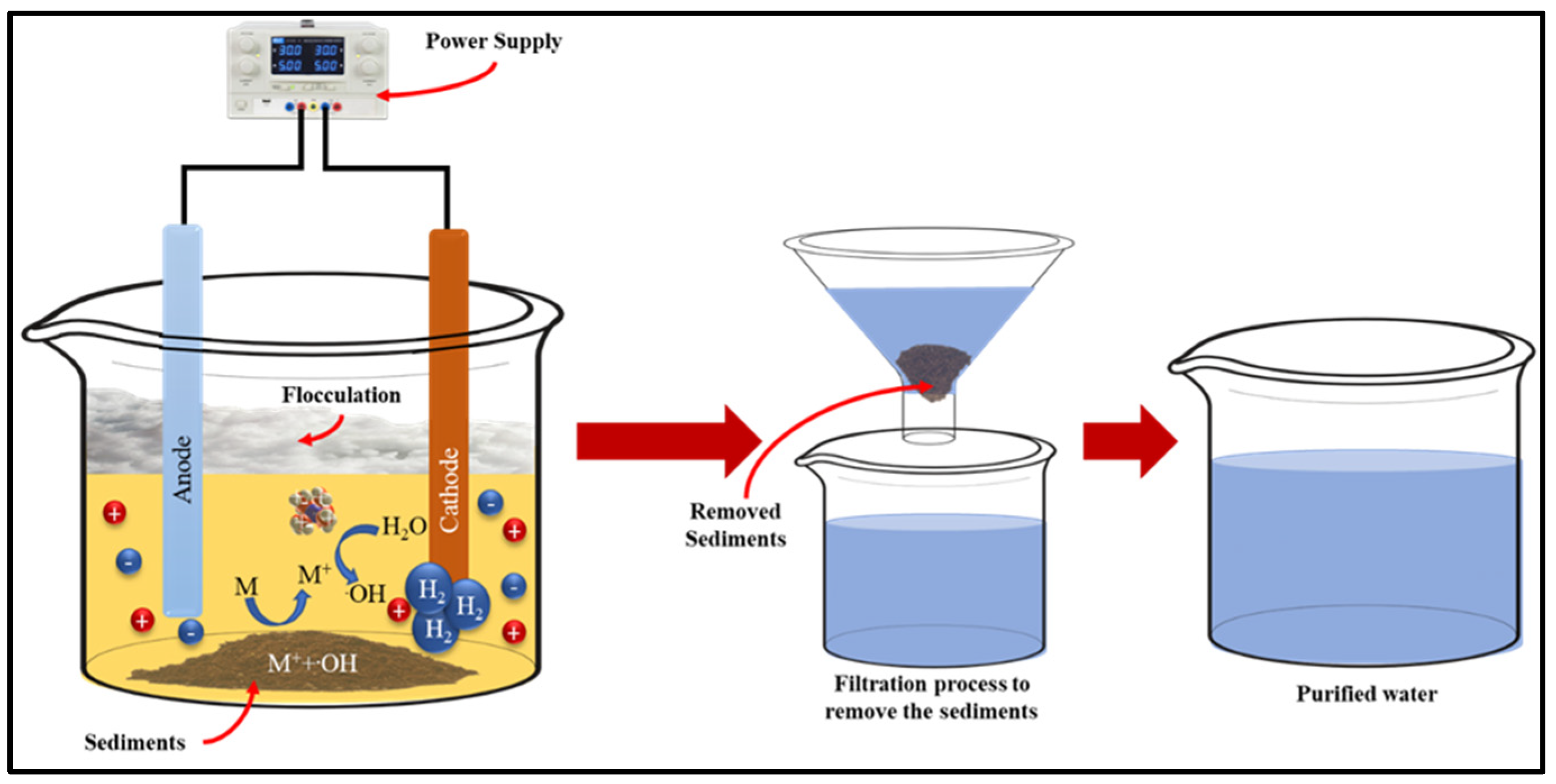
3.4. Electrochemical Technique
3.5. Membrane Filtration
3.6. Electrodialysis Process
3.7. Biodegradation Techniques
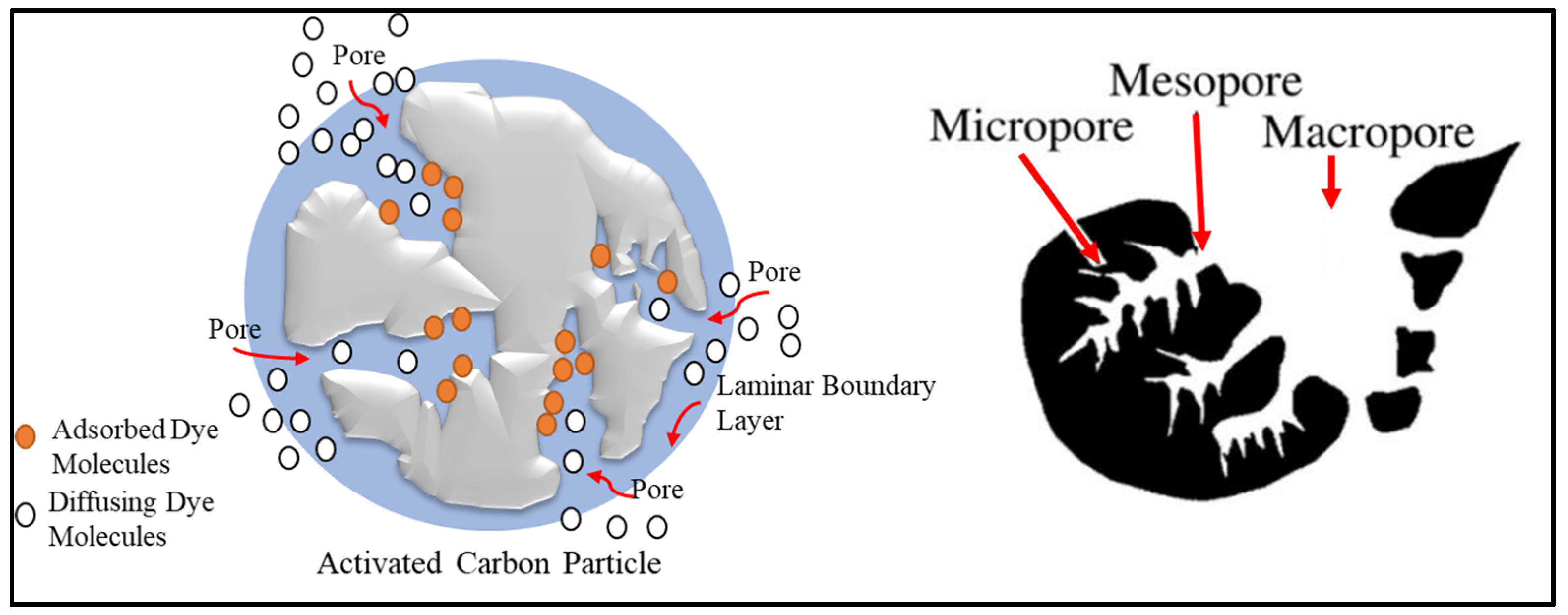
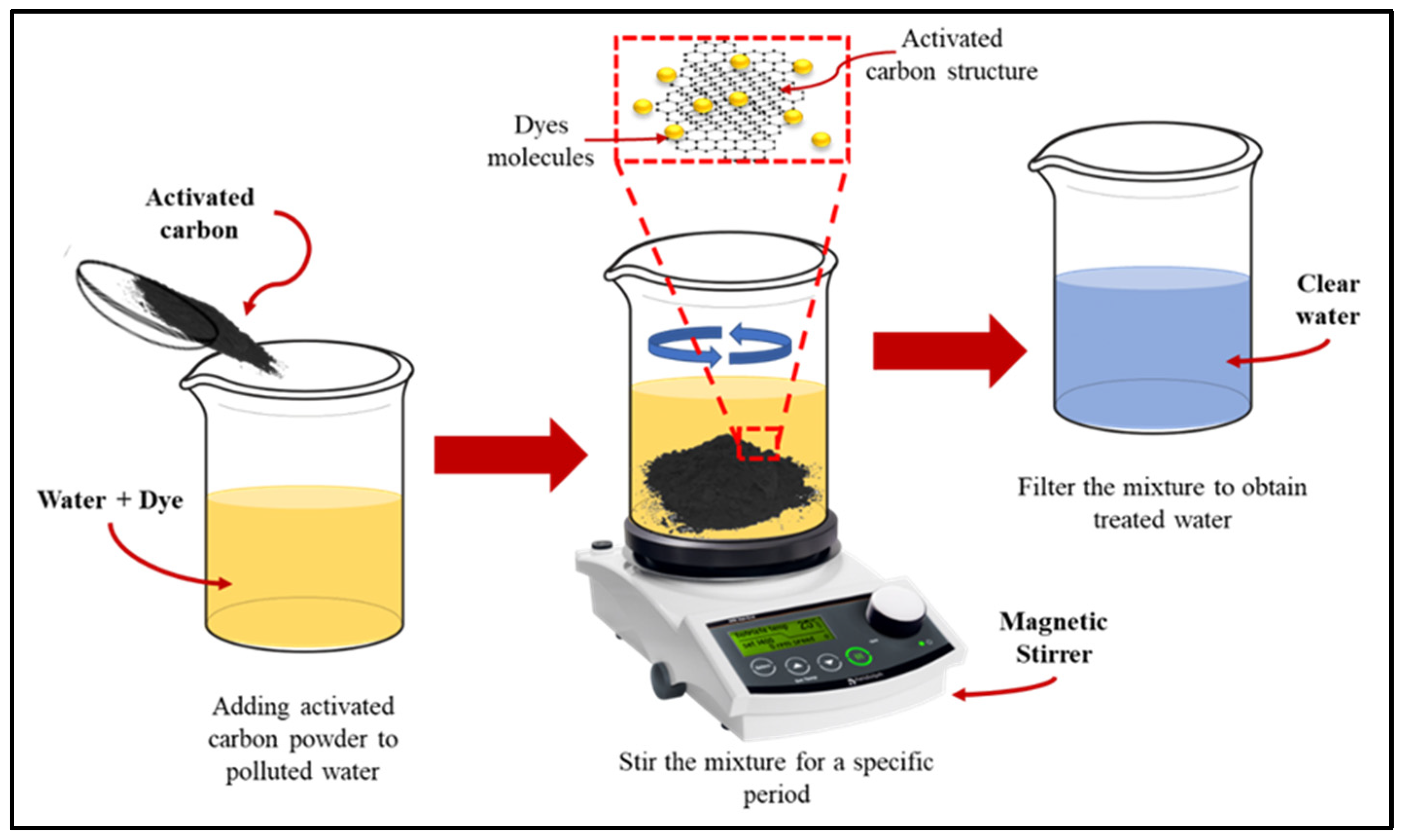
3.8. Adsorption
3.9. Application of Activated Carbon for Dye Removal from Wastewater
3.9.1. Renewable Bio-Based Precursors for Synthesis of Activated Carbon
Cellulose
Hemicellulose
Lignin
3.9.2. Synthesis Protocol of Activated Carbon
Physical Activation Process
Chemical Activation Process
Physiochemical Activation Process
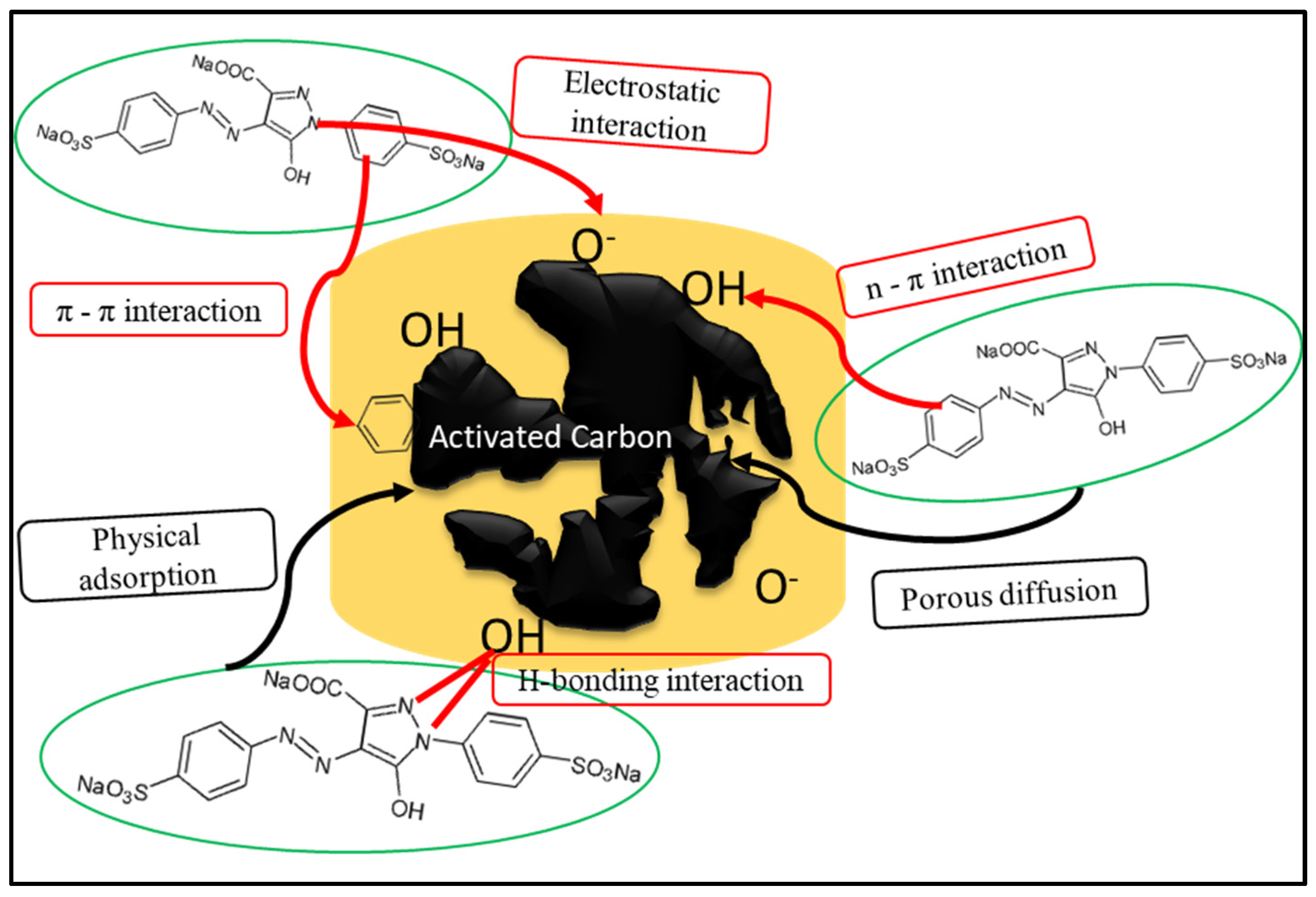
3.9.3. Activated Carbon for the Elimination of Azo Dyes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chequer, F.M.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Carvalho, J.; Zanoni, M.B.; de Oliveir, D.P. Textile Dyes: Dyeing Process and Environmental Impact. In Eco-Friendly Textile Dyeing and Finishing; InTechOpen: London, UK, 2013. [Google Scholar]
- Krishnamoorthy, R.; Choudhury, A.R.; Jose, P.A.; Suganya, K.; Senthilkumar, M.; Prabhakaran, J.; Gopal, N.O.; Choi, J.; Kim, K.; Anandham, R.; et al. Long-term exposure to azo dyes from textile wastewater causes the abundance of Saccharibacteria population. Appl. Sci. 2021, 11, 379. [Google Scholar] [CrossRef]
- Chequer, F.M.D.; Junqueira, D.; de Oliveir, D.P. Azo Dyes and Their Metabolites: Does the Discharge of the Azo Dye into Water Bodies Represent Human and Ecological Risks? In Advances in Treating Textile Effluent; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Uddin, F.; Umer, K.; Anjum, S.T. Textile solid waste in product development studies. Chem. Rep. 2022, 3, 203–209. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, D.; Alam Khan, T. Recent Advances in Synthetic Dyes. In Innovative and Emerging Technologies for Textile Dyeing and Finishing; Wiley: New York, NY, USA, 2021; pp. 91–111. [Google Scholar] [CrossRef]
- Saini, R.D. Textile Organic Dyes: Polluting Effects and Elimination Methods from Textile Waste Water. 2017. Available online: http://www.ripublication.com (accessed on 27 January 2023).
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’Rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Brice, D.N.C.; Manga, N.H.; Arnold, B.S.; Daouda, K.; Victoire, A.A.; Giresse, N.N.A.; Nangah, C.R.; Nsami, N.J. Adsorption of Tartrazine onto Activated Carbon Based Cola Nuts Shells: Equilibrium, Kinetics, and Thermodynamics Studies. Open J. Inorg. Chem. 2021, 11, 1–19. [Google Scholar] [CrossRef]
- Bensedira, A.; Haddaoui, N.; Doufnoune, R.; Meziane, O.; Labidi, N.S. Study of methylene blue dye elimination from water using polyaniline (PANI) and PANI/SiO2 composite. Polym. Polym. Compos. 2022, 30, 09673911221141747. [Google Scholar] [CrossRef]
- Van Hung, N.; Nguyet, B.T.M.; Nghi, N.H.; Thanh, N.M.; Quyen, N.D.V.; Nguyen, V.T.; Nhiem, D.N.; Khieu, D.Q. Highly effective adsorption of organic dyes from aqueous solutions on longan seed-derived activated carbon. Environ. Eng. Res. 2022, 28, 220116. [Google Scholar] [CrossRef]
- Chung, K.-T. Accepted Manuscript Accepted Manuscript Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar]
- Ernawati, L.; Reza, M.; Synthia, A.C.; Kartikasari, D.A.; Maharsih, I.K.; Halim, A. Role of Chemical Activating Agent on the Characteristics of Activated Carbon Derived from Fruit-Peel Waste for Aqueous Dye Removal. Key Eng. Mater. 2022, 937, 165–180. [Google Scholar] [CrossRef]
- Benkhaya, S.; El Harfi, S.; El Harfi, A. Classifications, Properties and Applications of Textile Dyes: A Review Textile Dayes View Project Treatment of Residual Waters View Project Said Benkhaya Université Ibn Tofail. 2017. Available online: https://www.researchgate.net/publication/323960391 (accessed on 27 January 2023).
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Anliker, R. Ecotoxicology of Dyestuffs-A Joint Effort by Industry. Ecotoxicol. Environ. Saf. 1979, 3, 59–74. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. 2019. Available online: https://www.intechopen.com (accessed on 27 January 2023).
- Carmen, Z.; Daniela, S. Textile Organic Dyes-Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. 2011. Available online: https://www.intechopen.com (accessed on 27 January 2023).
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef]
- Pelley, J.W. Protein Synthesis and Degradation. In Elsevier’s Integrated Review Biochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 149–160. [Google Scholar] [CrossRef]
- Singh, N.; De, A. Lignocellulosics as adsorbents for removal of dyes from water. In Natural Polymers-Based Green Adsorbents for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 195–222. [Google Scholar] [CrossRef]
- Namgung, S.; Park, H.A.; Kim, J.; Lee, P.-G.; Kim, B.-G.; Yang, Y.-H.; Choi, K.-Y. Ecofriendly one-pot biosynthesis of indigo derivative dyes using CYP102G4 and PrnA halogenase. Dye. Pigment. 2019, 162, 80–88. [Google Scholar] [CrossRef]
- Sabnis, W.R. PHTHALEIN DYES. 2010. Available online: https://www.researchgate.net/publication/278307582_Phthalein_Dyes (accessed on 27 January 2023).
- Salveson, P.J.; Haerianardakani, S.; Thuy-Boun, A.; Yoo, S.; Kreutzer, A.G.; Demeler, B.; Nowick, J.S. Repurposing Triphenylmethane Dyes to Bind to Trimers Derived from Aβ. J. Am. Chem. Soc. 2018, 140, 11745–11754. [Google Scholar] [CrossRef]
- Cheriaa, J.; Khaireddine, M.; Rouabhia, M.; Bakhrouf, A. Removal of Triphenylmethane Dyes by Bacterial Consortium. Sci. World J. 2012, 2012, 512454. [Google Scholar] [CrossRef]
- Mizutani, T. Toxicity of Xanthene Food Dyes by Inhibition of Human Drug-Metabolizing Enzymes in a Noncompetitive Manner. J. Environ. Public Health 2009, 2009, 953952. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Dome, R.N.; Hazra, S.; Ghosh, D.; Ghosh, S. Beneficial effects of ethanolic leaf extract of coriandrum sativum on metanil yellow induced alteration inactivity of catalase and level of lipid peroxidation in hercine cardiac tissue in vitro. Int. J. Pharm. Pharm. Sci. 2017, 9, 203–209. [Google Scholar] [CrossRef]
- Ghosh, D.; Singha, P.S.; Firdaus, S.B.; Ghosh, S. Metanil yellow: The toxic food colorant. Asian Pac. J. Health Sci. 2017, 4, 65–66. [Google Scholar] [CrossRef]
- Bor, B.; Bedree, J.K.; Shi, W.; McLean, J.S.; He, X. Saccharibacteria (TM7) in the Human Oral Microbiome. J. Dent. Res. 2019, 98, 500–509. [Google Scholar] [CrossRef]
- Leulescu, M.; Rotaru, A.; Pălărie, I.; Moanţă, A.; Cioateră, N.; Popescu, M.; Morîntale, E.; Bubulică, M.V.; Florian, G.; Hărăbor, A.; et al. Tartrazine: Physical, thermal and biophysical properties of the most widely employed synthetic yellow food-colouring azo dye. J. Therm. Anal. Calorim. 2018, 134, 209–231. [Google Scholar] [CrossRef]
- Rajaram Patil, P.; Ladhe, U.; Patil, P. Removals of Sudan Red g Dye from Aqueous Solution by Adsorption on To Activated Carbon Prepared from Mosambi and Cotton an Agricultural Waste. Int. J. Sci. Environ. Technol. 2014, 3, 546–555. [Google Scholar]
- Fernandes, F.H.; Bustos-Obregon, E.; Salvadori, D.M.F. Disperse Red 1 (textile dye) induces cytotoxic and genotoxic effects in mouse germ cells. Reprod. Toxicol. 2015, 53, 75–81. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Wastewater Treatment: An Overview. In Green Adsorbents for Pollutant Removal, Environmental Chemistry for a Sustainable World; Springer Nature: Cham, Switzerland, 2018; Volume 18, pp. 1–21. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Saratale, R.; Saratale, G.; Chang, J.; Govindwar, S. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Nanjan Bellie, P.; Sockan, V. Waste Water Treatment by Coagulation and Flocculation. Int. J. Eng. Sci. Innov. Technol. 2014, 3, 479–484. [Google Scholar]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Ghernaout, D. Water Treatment Coagulation: Dares and Trends. Oalib 2020, 7, e6636. [Google Scholar] [CrossRef]
- Pakharuddin, N.H.; Fazly, M.N.; Sukari, S.H.A.; Tho, K.; Zamri, W.F.H. Water treatment process using conventional and advanced methods: A comparative study of Malaysia and selected countries. IOP Conf. Ser. Earth Environ. Sci. 2021, 880, 012017. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Chiang, P.-C.; Shah, K.J. Evaluation and optimization of enhanced coagulation process: Water and energy nexus. Water-Energy Nexus 2019, 2, 25–36. [Google Scholar] [CrossRef]
- Mathuram, M.; Meera, R.; Vijayaraghavan, G. Application of Locally Sourced Plants as Natural Coagulants for Dye Removal from Wastewater: A Review. J. Mater. Environ. Sci. 2018, 2508, 2058–2070. Available online: http://www.jmaterenvironsci.com (accessed on 15 February 2023).
- Badawi, A.K.; Zaher, K. Hybrid treatment system for real textile wastewater remediation based on coagulation/flocculation, adsorption and filtration processes: Performance and economic evaluation. J. Water Process Eng. 2021, 40, 101963. [Google Scholar] [CrossRef]
- Othmani, A.; Kadier, A.; Singh, R.; Igwegbe, C.A.; Bouzid, M.; Aquatar, O.; Khanday, W.A.; Bote, M.E.; Damiri, F.; Gökkuş, Ö.; et al. A comprehensive review on green perspectives of electrocoagulation integrated with advanced processes for effective pollutants removal from water environment. Environ. Res. 2022, 215, 114294. [Google Scholar] [CrossRef]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.-Á. Efficient treatment for textile wastewater through sequential electrocoagulation, electrochemical oxidation and adsorption processes: Optimization and toxicity assessment. J. Electroanal. Chem. 2020, 878, 114578. [Google Scholar] [CrossRef]
- Awad, S.; Eldemerdash, U. Novel Method for Breakthrough Removal of Azo Dye from Aqueous Environment Using Integrated Coagulation and Fenton Process. Int. J. Photochem. 2014, 2014, 934850. [Google Scholar] [CrossRef]
- Vall, M.; Strømme, M.; Cheung, O. Amine-Modified Mesoporous Magnesium Carbonate as an Effective Adsorbent for Azo Dyes. ACS Omega 2019, 4, 2973–2979. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.; Wu, K.; Liu, Z.; Yang, S.; Yang, Q.; Dzakpasu, M. Coagulation performance of cucurbit[8]uril for the removal of azo dyes: Effect of solution chemistry and coagulant dose. Water Sci. Technol. 2018, 78, 415–423. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Mabuea, B.P.; Swart, H.C.; Erasmus, E. Photocatalytic Decomposition of an Azo Dye Using Transition-Metal-Doped Tungsten and Molybdenum Carbides. ACS Omega 2022, 7, 23401–23411. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, S.; Kan, C.; Zhang, Y.; Liang, Y.; Cui, G.; Li, J.; Yang, S. Application of Ion Exchange Resin in the Advanced Treatment of Condensate Water. E3S Web Conf. 2021, 272, 01005. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S. History, Introduction, and Kinetics of Ion Exchange Materials. J. Chem. 2013, 2013, 957647. [Google Scholar] [CrossRef]
- Kansara, N.; Bhati, L.; Narang, M.; Vaishnavi, R. Critical Review Wastewater treatment by ion exchange method: A review of past and recent researches. Environ. Sci. Indian J. 2016, 12, 143–150. [Google Scholar]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Akhter, M.; Habib, G.; Qamar, S.U. Application of Electrodialysis in Waste Water Treatment and Impact of Fouling on Process Performance. J. Membr. Sci. Technol. 2018, 8, 2. [Google Scholar] [CrossRef]
- Zioui, D.; Tigrine, Z.; Aburideh, H.; Hout, S.; Abbas, M.; Merzouk, N. Membrane Technology for Water Treatment Applications. 2015. Available online: https://www.researchgate.net/publication/301647575 (accessed on 15 February 2023).
- Chowdhury, Z.Z.; Ali, A.E.; Khalid, K.; Ikram, R.; Rahman, M.; Shibly, S.M.; Sagadevan, S.; Rafique, R.F.; Barua, A. Science and Technology Roadmap for Photocatalytic Membrane Separation: A Potential Route for Environmental Remediation and Fouling Mitigation. In Green Chemistry and Sustainable Technology; Springer: Cham, Switzerland, 2022; pp. 513–550. [Google Scholar] [CrossRef]
- Lima, A.; de Oliveira, A.; Demosthenes, L.; Sousa, T.; Pereira, A.; de Carvalho, R.; Anacleto, W. Reduced Graphene Oxide Filtration Membranes for Dye Removal—Production and Characterization. Mater. Proc. 2021, 4, 29. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, R.; Gaur, G. Study of Removal Techniques for Azo Dyes by Biosorption: A Review. IOSR J. Appl. Chem. 2014, 7, 6–21. [Google Scholar] [CrossRef]
- Al Prol, A.E. Study of Environmental Concerns of Dyes and Recent Textile Effluents Treatment Technology: A Review. Asian J. Fish. Aquat. Res. 2019, 3, 1–18. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.L. Bio-removal of Azo Dyes: A Review. Int. J. Appl. Sci. Biotechnol. 2017, 5, 108–126. [Google Scholar] [CrossRef]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Avila, H.E. Adsorption Processes for Water Treatment and Purification; Springer, International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Pivehzhani, O.A.; Johan, M.R. Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F.; Toyoda, M.; Konno, H. Advanced Materials Science and Engineering of Carbon; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–434. [Google Scholar] [CrossRef]
- González-García, P.; Centeno, T.; Urones-Garrote, E.; Ávila-Brande, D.; Otero-Díaz, L. Microstructure and surface properties of lignocellulosic-based activated carbons. Appl. Surf. Sci. 2013, 265, 731–737. [Google Scholar] [CrossRef]
- Feng, P.; Li, J.; Wang, H.; Xu, Z. Biomass-Based Activated Carbon and Activators: Preparation of Activated Carbon from Corncob by Chemical Activation with Biomass Pyrolysis Liquids. ACS Omega 2020, 5, 24064–24072. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.; Chowdhury, Z.Z.; Faisal, A.N.; Das, R.; Wahab, Y.A.; Ramakrishnan, S. Thermochemical Conversion of Lignocellulosic Waste to Activated Carbon: A Potential Resource for Industrial Wastewater Treatment; Springer: Cham, Switzerland, 2022; pp. 153–191. [Google Scholar] [CrossRef]
- Kostag, M.; El Seoud, O.A. Sustainable biomaterials based on cellulose, chitin and chitosan composites—A review. Carbohydr. Polym. Technol. Appl. 2022, 2, 100079. [Google Scholar] [CrossRef]
- Eo, M.Y.; Fan, H.; Cho, Y.J.; Kim, S.M.; Lee, S.K. Cellulose membrane as a biomaterial: From hydrolysis to depolymerization with electron beam. Biomater. Res. 2016, 20, 16. [Google Scholar] [CrossRef]
- Hariani, P.L.; Riyanti, F.; Asmara, R.D. Extraction of Cellulose from Kepok Banana Peel (Musa parasidiaca L.) for Adsorption Procion Dye. Molekul 2016, 11, 135–142. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Xiang, Z.; Tang, N.; Jin, X.; Gao, W. Fabrications and applications of hemicellulose-based bio-adsorbents. Carbohydr. Polym. 2022, 278, 118945. [Google Scholar] [CrossRef]
- e Silva, C.F.L.; Lemões, J.S.; Romani, R.F.; de Oliveira, W.G.; Leite, G.F. Activated Carbon from Residual Lignin Used for Color Removal. Water Air Soil Pollut. 2022, 233, 177. [Google Scholar] [CrossRef]
- Yang, Z.; Gleisner, R.; Mann, D.H.; Xu, J.; Jiang, J.; Zhu, J. Lignin Based Activated Carbon Using H3PO4 Activation. Polymers 2020, 12, 2829. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Azizi, M.R.; Alavi Moghaddam, M.R.; Arami, M. Removal of a Reactive Dye using Ash of Pulp and Paper Sludge. J. Residuals Sci. Technol. 2012, 9, 159–168. [Google Scholar]
- Santos, S.C.; Boaventura, R.A. Treatment of a simulated textile wastewater in a sequencing batch reactor (SBR) with addition of a low-cost adsorbent. J. Hazard. Mater. 2015, 291, 74–82. [Google Scholar] [CrossRef]
- Ratnamala, G.M.; Shetty, K.V.; Srinikethan, G. Removal of Remazol Brilliant Blue Dye from Dye-Contaminated Water by Adsorption Using Red Mud: Equilibrium, Kinetic, and Thermodynamic Studies. Water Air Soil Pollut. 2012, 223, 6187–6199. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Jafari, S.J.; Giahi, O.; Kim, I.; Lee, S.-M.; Yang, J.-K. Removal of acid blue 113 and reactive black 5 dye from aqueous solutions by activated red mud. J. Ind. Eng. Chem. 2014, 20, 1432–1437. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass-Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Hussaro, K.; Rattanakosin. Preparation of activated carbon from palm oil shell by chemical activation with Na2CO3 and ZnCl2 as impregnated agents for H2S adsorption. Am. J. Environ. Sci. 2014, 101, 177–185. [Google Scholar] [CrossRef]
- Das, D.; Samal, D.P.; Bc, M. Preparation of Activated Carbon from Green Coconut Shell and its Characterization. J. Chem. Eng. Process Technol. 2015, 6, 1000248. [Google Scholar] [CrossRef]
- Sekirifa, M.L.; Hadj-Mahammed, M.; Pallier, S.; Baameur, L.; Richard, D.; Al-Dujaili, A.H. Preparation and characterization of an activated carbon from a date stones variety by physical activation with carbon dioxide. J. Anal. Appl. Pyrolysis 2013, 99, 155–160. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Moon, J.I.; Song, J.H.; Kim, T.N. Synthesis of activated carbon from rice husk using microwave heating induced KOH activation. Korean J. Mater. Res. 2012, 22, 321–327. [Google Scholar] [CrossRef]
- Angin, D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 2014, 115, 804–811. [Google Scholar] [CrossRef]
- Yakout, S.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2011, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Dorge, S.; Limousy, L.; Ghimbeu, C.M.; Ouederni, A. Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. Comptes Rendus Chim. 2014, 18, 63–74. [Google Scholar] [CrossRef]
- Huang, G.-G.; Liu, Y.-F.; Wu, X.-X.; Cai, J.-J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. Xinxing Tan Cailiao/New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Saad, M.J.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Misran, S.; Rahman, M.H.A.; Chin, S.X. Physical and chemical properties of the rice straw activated carbon produced from carbonization and KOH activation processes. Sains Malays. 2019, 48, 385–391. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, A.; Zhao, Y. Preparation and characterisation of activated carbon from waste tea by physical activation using steam. J. Air Waste Manag. Assoc. 2018, 68, 1269–1277. [Google Scholar] [CrossRef]
- Ahmed, M.J. Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments: Review. Process Saf. Environ. Prot. 2016, 102, 168–182. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Absi, R.S. Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Sci. Rep. 2020, 10, 15928. [Google Scholar] [CrossRef] [PubMed]
- Chukwuemeka-Okorie, H.O.; Ekuma, F.K.; Akpomie, K.G.; Nnaji, J.C.; Okereafor, A.G. Adsorption of tartrazine and sunset yellow anionic dyes onto activated carbon derived from cassava sievate biomass. Appl. Water Sci. 2021, 11, 27. [Google Scholar] [CrossRef]
- Albroomi, H.I.; Elsayed, M.A.; Baraka, A.; Abdelmaged, M.A. Batch and fixed-bed adsorption of tartrazine azo-dye onto activated carbon prepared from apricot stones. Appl. Water Sci. 2016, 7, 2063–2074. [Google Scholar] [CrossRef]
- Kumar, A.; Vyas, R.K. Adsorption of Direct Blue 5 Dye by Activated Carbon as Adsorbent-Modeling and Kinetics. Available online: https://www.ijert.org (accessed on 20 February 2023).
- Li, Y.; Zhang, X.; Yang, R.; Li, G.; Hu, C. Removal of dyes from aqueous solutions using activated carbon prepared from rice husk residue. Water Sci. Technol. 2016, 73, 1122–1128. [Google Scholar] [CrossRef]
- Hernndez-Montoya, V.; Garca-Servin, J.; Ivn, J. Thermal Treatments and Activation Procedures Used in the Preparation of Activated Carbons. In Lignocellulosic Precursors Used in the Synthesis of Activated Carbon-Characterization Techniques and Applications in the Wastewater Treatment; InTechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated Carbon for Dyes Removal: Modeling and Understanding the Adsorption Process. J. Chem. 2020, 2020, 2096834. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, K. Removal of Acid Red 88 Using Activated Carbon Produced from Pomelo Peels by KOH Activation: Orthogonal Experiment, Isotherm, and Kinetic Studies. J. Chem. 2021, 2021, 6617934. [Google Scholar] [CrossRef]
- Sidiqua, M.A.; Priya, V. Removal of yellow dye using composite binded adsorbent developed using natural clay and activated carbon from sapindus seed. Biocatal. Agric. Biotechnol. 2021, 33, 101965. [Google Scholar] [CrossRef]










| Dye Classes | Environmental and Health Impact | Example | Ref. |
|---|---|---|---|
| Azo | -Cause bladder and liver cancer. -Reduced fertility of male and female mice. -Increase the chemical oxygen demand (COD). | -Tartrazine -Congo Red -Sudan Red -Sunset Yellow | [1] |
| Anthraquinone | -Bind to enzyme and protein fibers -Cause inhabitation of blood albumin. -Increase the chemical oxygen demand (COD). | -Alizarin Red S -Reactive Brilliant Blue R -Reactive Blue 4 | [1] |
| Acridine | -Cause damage to DNA structure. -Have mutagenic effects. -Increase the chemical oxygen demand (COD). -Inhibit the growth of some microbes. | -Basic Yellow 9 -Acridine Orange | [2,3] |
| Indigoid | -Increase the chemical oxygen demand (COD). -Inhibit the growth of some bacteria. -Extremely slow degradation in the environment | -Indigo Carmine -Ciba Blue 2B | [4] |
| Phthalein | -Increase the chemical oxygen demand (COD). | -Thymolphthalein -Phenolphthalein -Dixylenolphthalein | [5] |
| Triphenylmethane | -Increase the chemical oxygen demand (COD). | -Malachite Green -Crystal Violet -Light Green SF | [6,7] |
| Xanthene | -Increase the chemical oxygen demand (COD). -Inhibit enzymatic activities. | -Rhodamine 6G -Rhodamine 123 -Fluorescein | [8] |
| Technique | Advantages | Disadvantages |
|---|---|---|
| Coagulation and flocculation | -Inexpensive. -Simple operation procedures. | -Produces huge amount of toxic sludge. -Poor efficiency. -Requires long operation time. |
| Photocatalytic degradation | -Highly efficient. -Can be used under harsh conditions. | -Extremely expensive. -Produces highly toxic byproducts. |
| Adsorption | -High removal efficiency. -Inexpensive. -Simple operation procedures. | -Requires treatment for adsorbents. -Not efficient with all types of dye. |
| Ion exchange | -Inexpensive. -Produce small amount of byproduct. | -Not efficient with all types of dye. -Requires long time. |
| Membrane filtration | -Efficient with all types of dyes. -Requires short time. | -Expensive process. -Produces a high volume of toxic sludge. |
| Activated Carbon | Particle Size | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Physical Surface Area (m2/g) |
|---|---|---|---|---|
| PACs | 0.015–0.25 mm | 700–1600 | 0.5–1.4 | - |
| GACs | 0.6–3 mm | 700–1500 | 0.5–1.1 | ~0.001 |
| ACFs | 10–20 µm | 700–2500 | - | 0.2–2.0 |
| Activated carbon | Particle size | BET surface area (m2/g) | Pore volume (cm3/g) | Physical surface area (m2/g) |
| PACs | 0.015–0.25 mm | 700–1600 | 0.5–1.4 | - |
| GACs | 0.6–3 mm | 700–1500 | 0.5–1.1 | ~0.001 |
| ACFs | 10–20 µm | 700–2500 | - | 0.2–2.0 |
| Adsorbent | Dye | Removal Capacity | pH | Ref. |
|---|---|---|---|---|
| Activated carbon | Tartrazine | 24.57 mgg−1 | 2 | [9] |
| Pulp ash and paper sludge | Reactive Blue 19 | 95% | 12 | [82] |
| Metal hydroxide sludge | Direct Blue 85 | 98.7 mgg−1 | 10 | [83] |
| Red mud | Remazol Brilliant Blue | ~72% | 2 | [84] |
| Activated red mud | Acid blue 113 | 83.33 mgg−1 | 3 | [85] |
| Activated red mud | Reactive black 5 | 35.58 mgg−1 | 3 | [85] |
| Precursor | Activation Method | Activation Agent | Activation Temperature (°C) | Activation Time (Minutes) | SBET (m2/g) | Adsorption Capacity (ppm) | Pore Volume (cm3/g) | Reference |
|---|---|---|---|---|---|---|---|---|
| Barley straw | Physical activation | 800 | 60 | 789 | - | 0.3268 | [86] | |
| Barley straw | Physical activation | Water steam | 700 | 60 | 552 | - | 0.2304 | [86] |
| Palm oil shell | Chemical activation | 700 | 120 | 743.71 | 247.33 | 0.4210 | [87] | |
| Palm oil shell | Chemical activation | 700 | 120 | 551.05 | 241.67 | 0.3137 | [87] | |
| Green coconut shell | Chemical activation | 650 | 60 | 995.79 | - | 0.372 | [88] | |
| Date stone | Physical activation | 900 | 120 | 604 | 28,570 | 0.29 | [89] | |
| Rice husk | Chemical activation | KOH | 800 | 180 | 1505 | - | 0.42 | [90] |
| Sour cherry stones | Physio-chemical activation | with steam | 700 | 120 | 1704 | - | 0.984 | [91] |
| Olive stones | Chemical activation | 500 | 120 | 1218 | - | 0.5 | [92] | |
| Olive stones | Physical activation | Water steam | 750 | 360 | 807 | 131,000 | 0.30 | [93] |
| Garlic peel | Chemical activation | KOH | 800 | 60 | 1262 | - | 0.65 | [94] |
| Rice straw | Chemical activation | KOH | 850 | 120 | 1048.3 | - | 0.0436 | [95] |
| Tea waste | Physical activation | Water steam | 800 | 30 | 995.07 | - | 0.287 | [96] |
| Date seeds | Physio-chemical activation | HNO3/Steam | - | 180 | 950 | - | - | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.E.; Chowdhury, Z.Z.; Devnath, R.; Ahmed, M.M.; Rahman, M.M.; Khalid, K.; Wahab, Y.A.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; et al. Removal of Azo Dyes from Aqueous Effluent Using Bio-Based Activated Carbons: Toxicity Aspects and Environmental Impact. Separations 2023, 10, 506. https://doi.org/10.3390/separations10090506
Ali AE, Chowdhury ZZ, Devnath R, Ahmed MM, Rahman MM, Khalid K, Wahab YA, Badruddin IA, Kamangar S, Hussien M, et al. Removal of Azo Dyes from Aqueous Effluent Using Bio-Based Activated Carbons: Toxicity Aspects and Environmental Impact. Separations. 2023; 10(9):506. https://doi.org/10.3390/separations10090506
Chicago/Turabian StyleAli, Ahmed Elsayid, Zaira Zaman Chowdhury, Ramprosad Devnath, Md. Mostak Ahmed, Md. Mahfujur Rahman, Khalisanni Khalid, Yasmin Abdul Wahab, Irfan Anjum Badruddin, Sarfaraz Kamangar, Mohamed Hussien, and et al. 2023. "Removal of Azo Dyes from Aqueous Effluent Using Bio-Based Activated Carbons: Toxicity Aspects and Environmental Impact" Separations 10, no. 9: 506. https://doi.org/10.3390/separations10090506







