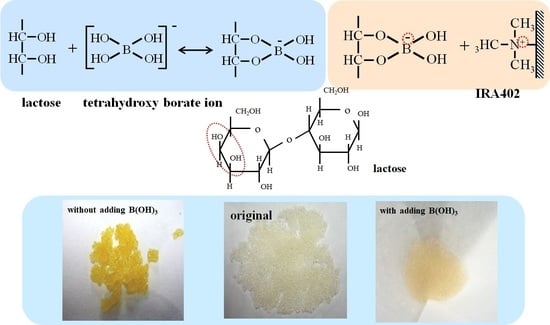
Adsorption of Lactose Using Anion Exchange Resin by Adding Boric Acid from Milk Whey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Whey Solution
2.3. Preparation of Anion Exchange Resin
2.4. Procedure of Adsorption Experiment in the Model System
2.5. Procedure of Adsorption Experiments Using Whey Solution
2.6. Procedure of Desorption Experiments
2.7. Determination Method of Lactose, Boric Acid, Phosphate, and Proteins
3. Results and Discussion
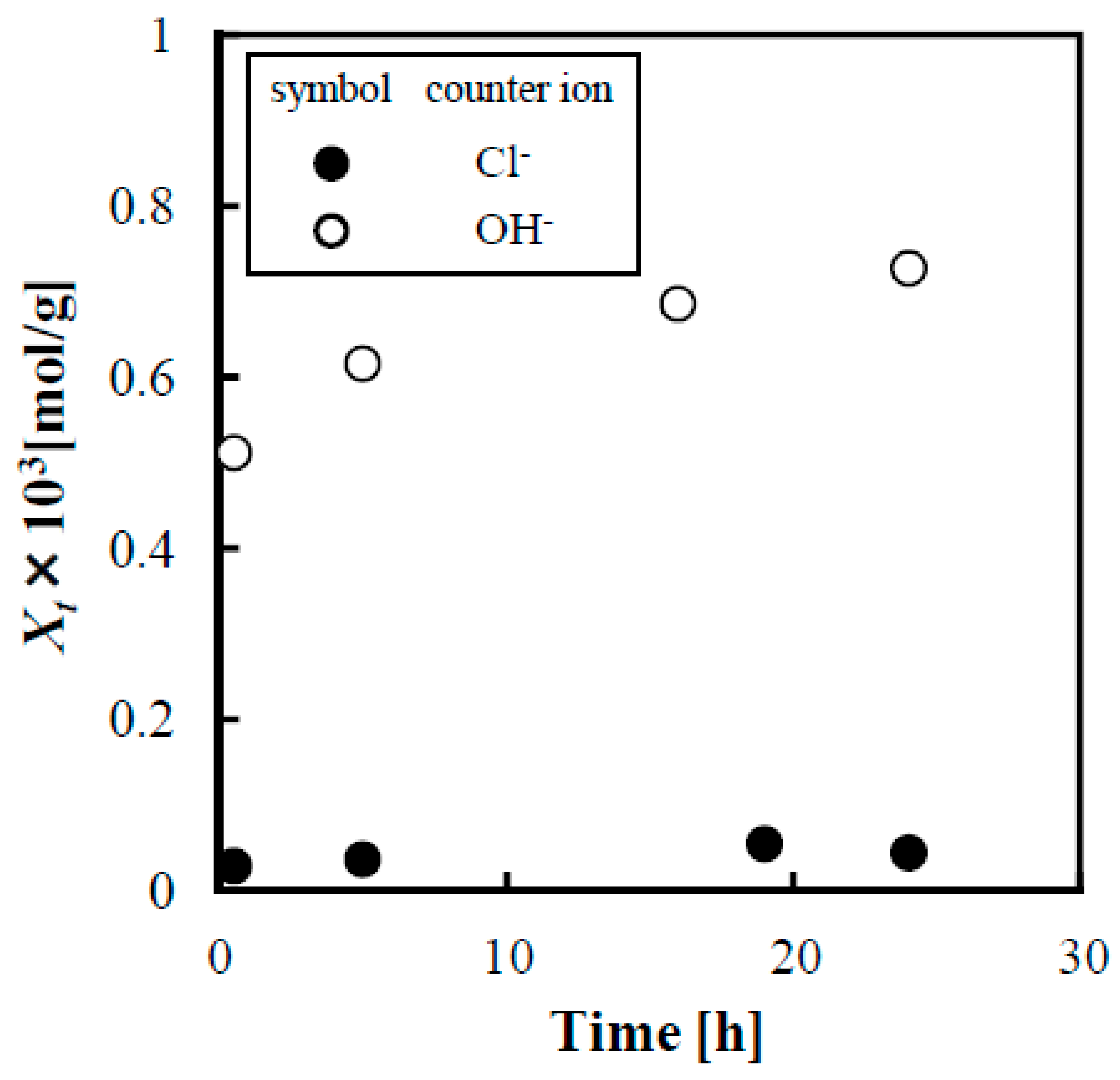
3.1. Effect of Adding Boric Acid on Adsorption of Lactose onto Anion Exchange Resin
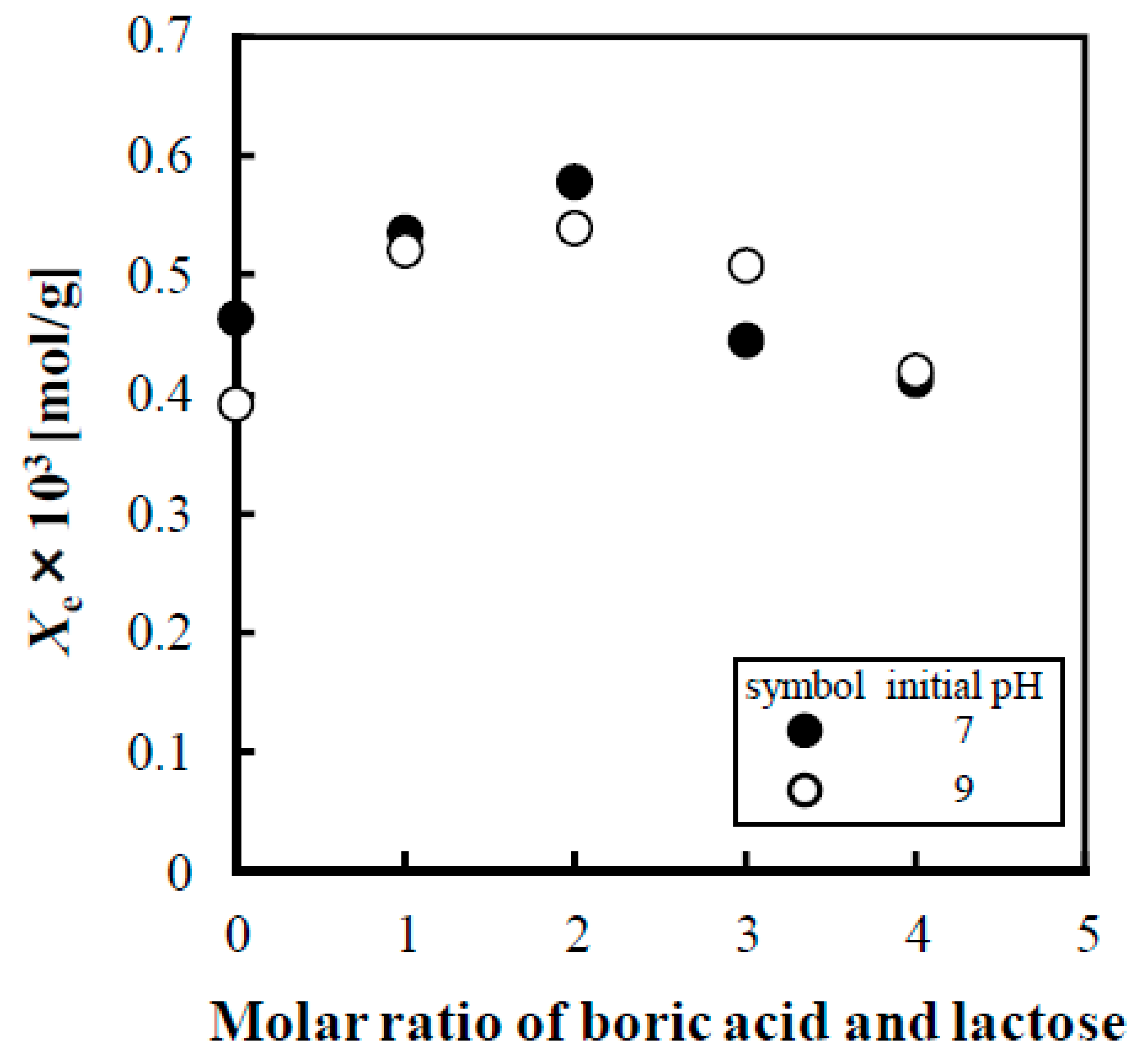
3.2. Influence of the Molar Ratio of Boric Acid and Lactose and the Initial and Equilibrium pH Values on the Amount of Lactose Adsorbed
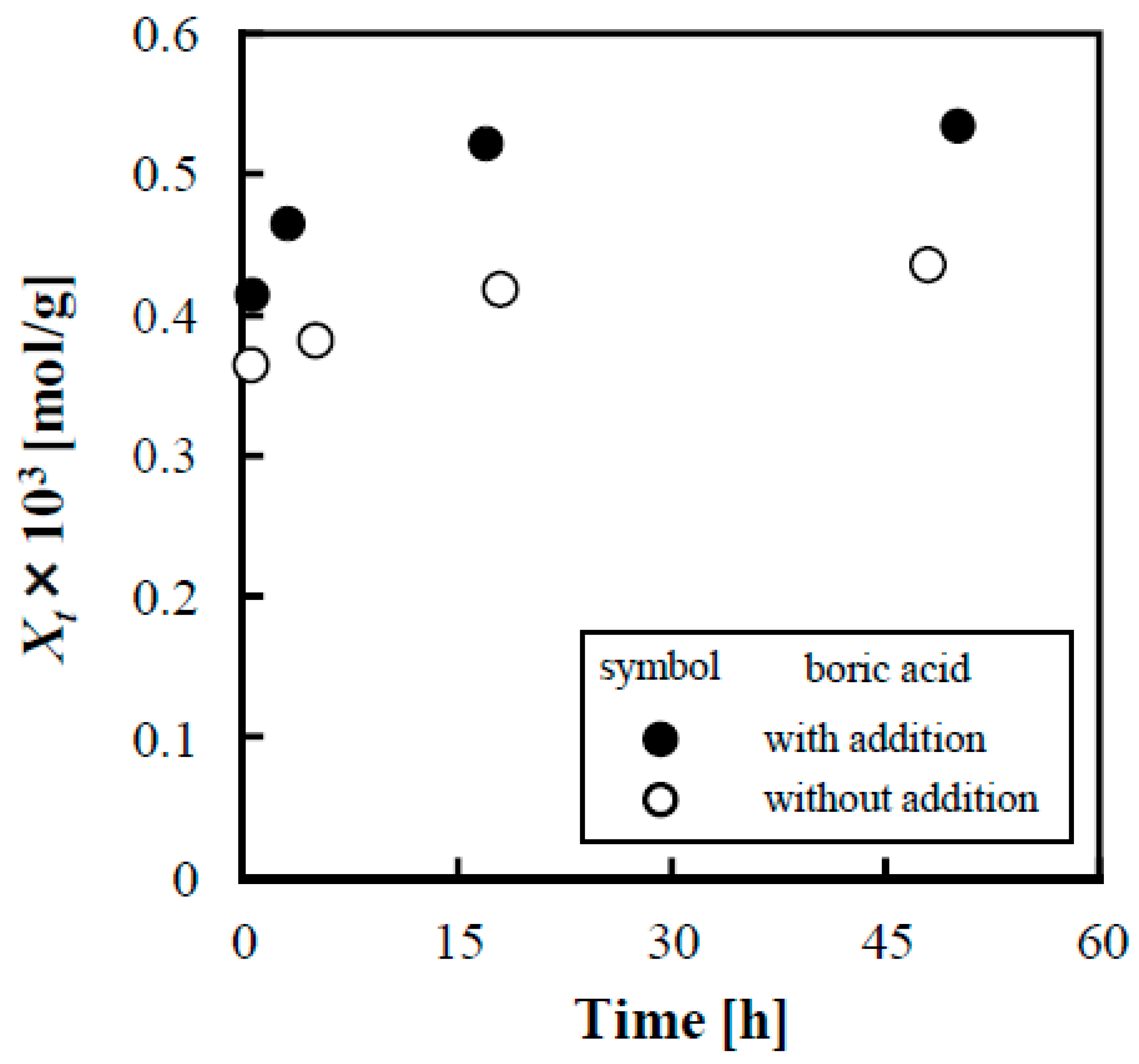
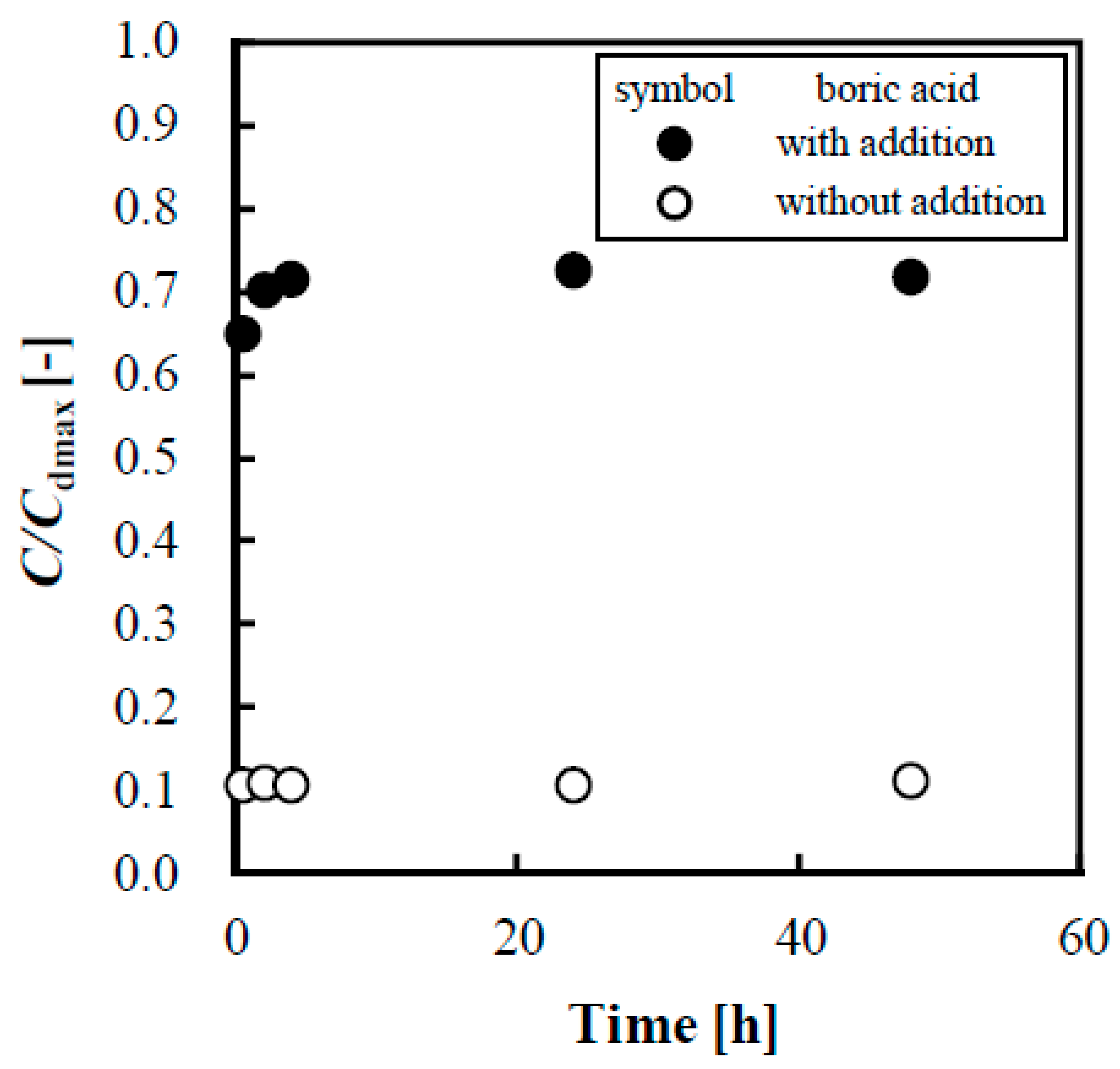
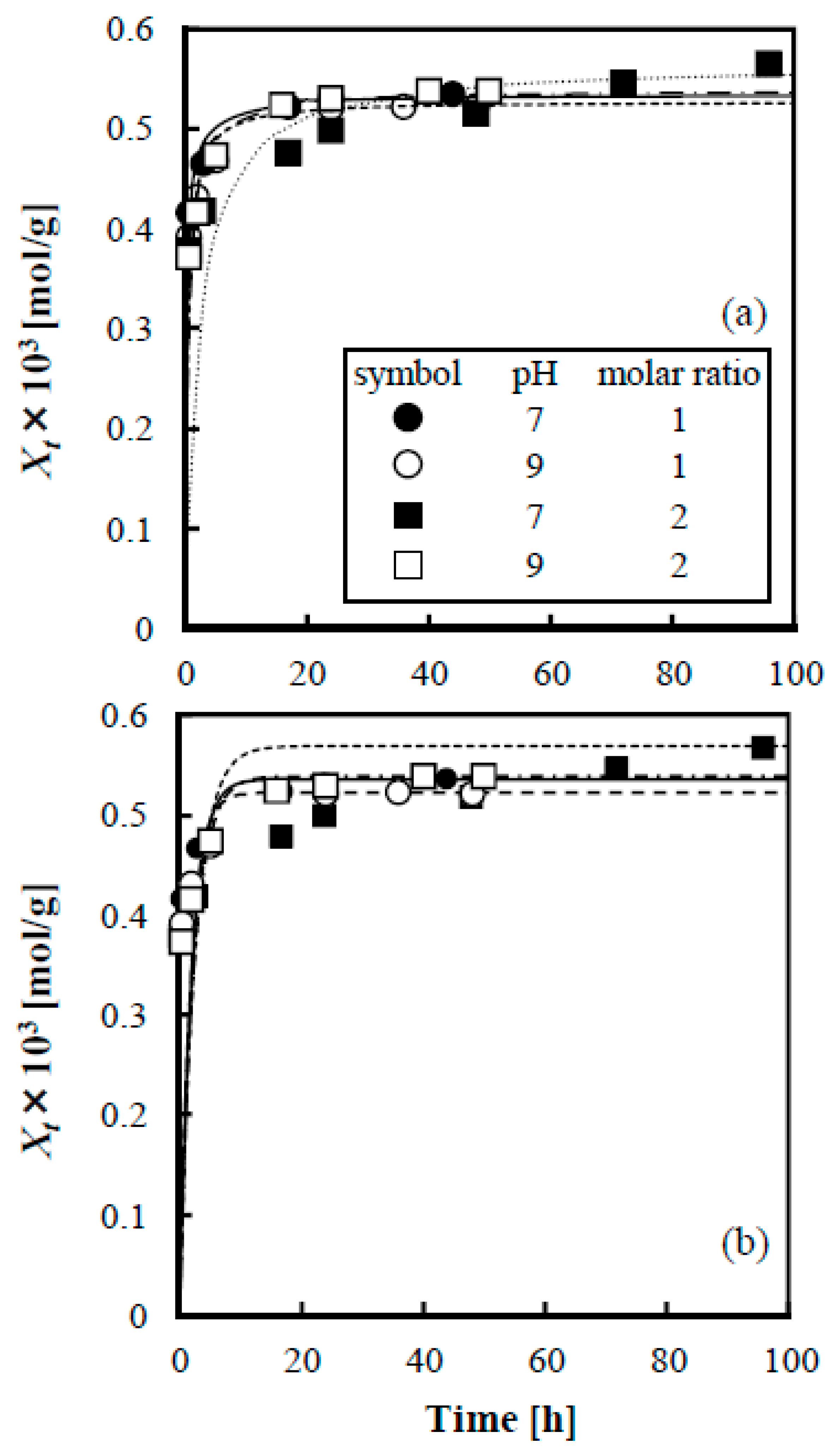
3.3. Evaluation of Adsorption Kinetics of Lactose on Anion Exchange Resin when Adding Boric Acid
3.4. Evaluation of Adsorption Equilibrium of Lactose on Anion Exchange Resin when Adding Boric Acid
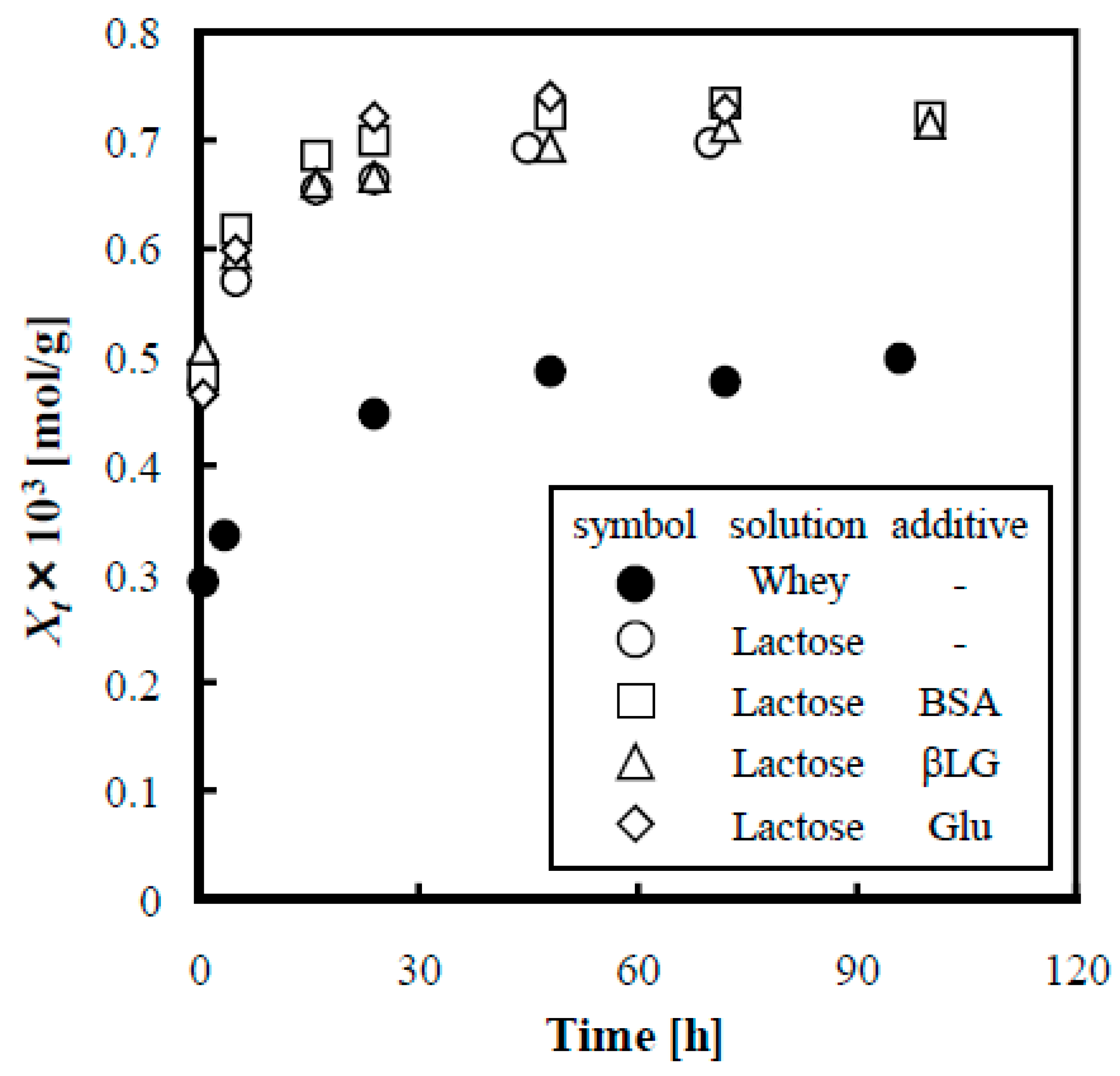
3.5. Influence of Coexisting Substances in Whey Solution on Adsorption of Lactose on Anion Exchange Resin when Adding Boric Acid
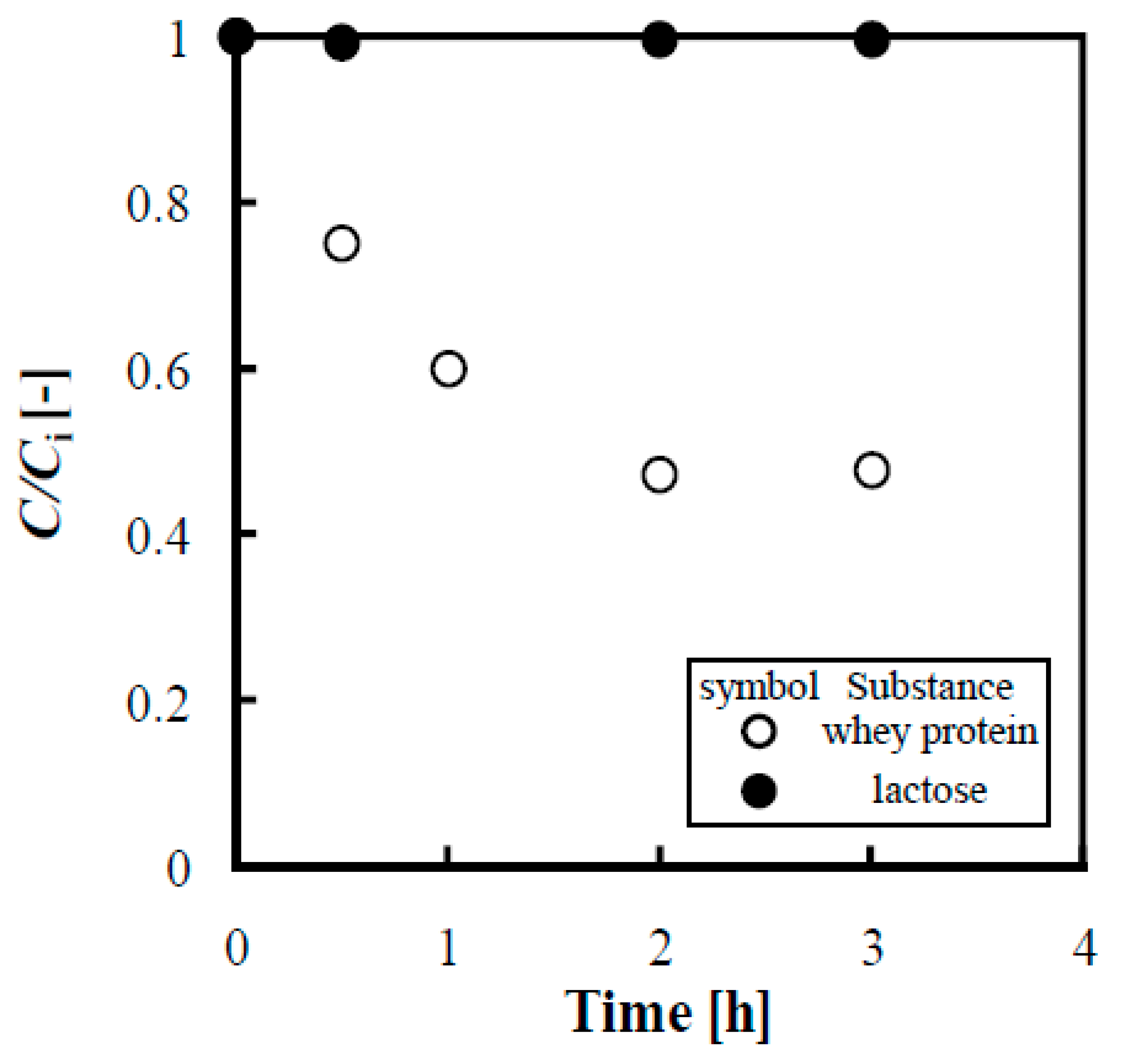
3.5.1. Influence of Phosphate Ion on Adsorption of Lactose
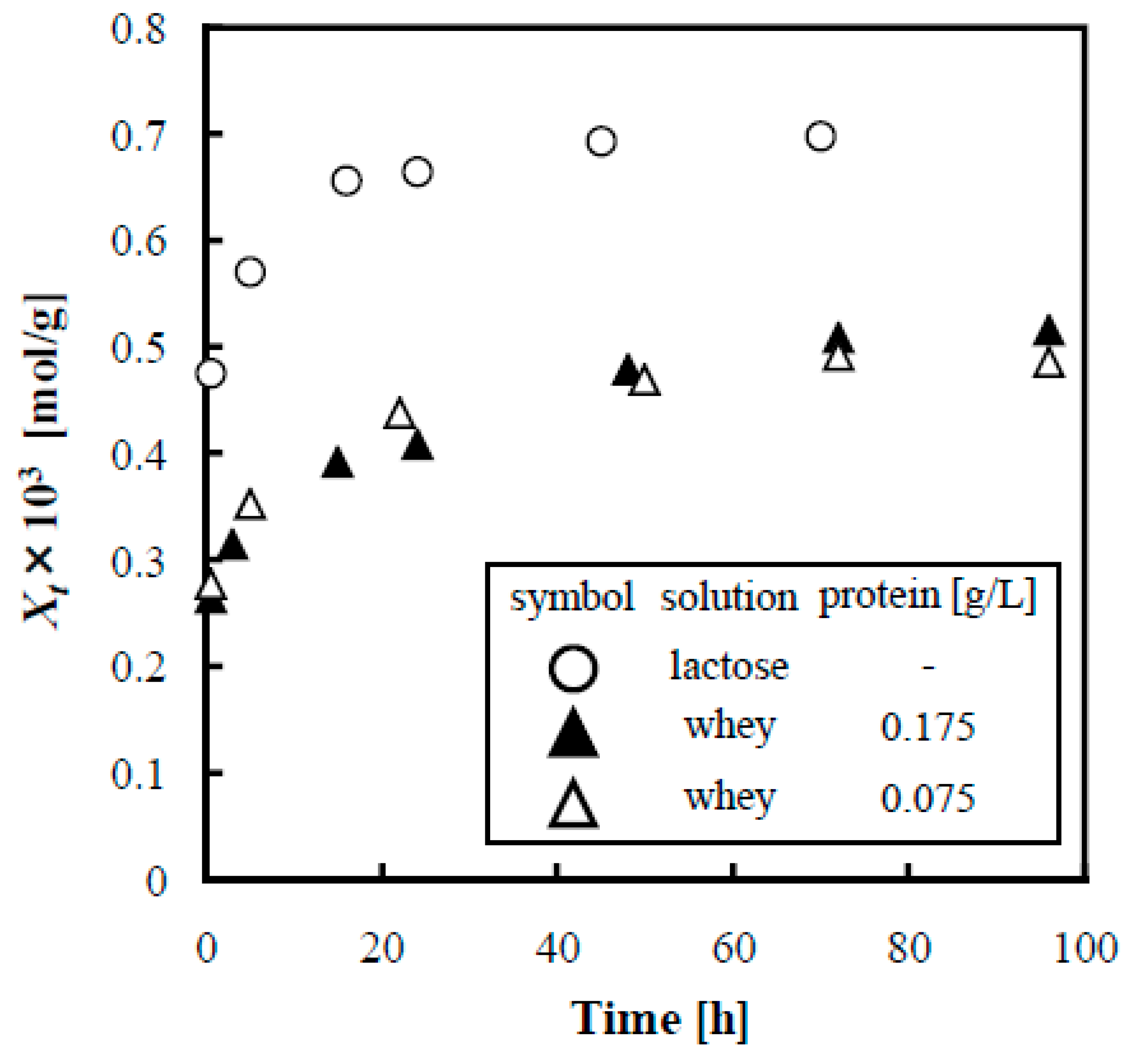
3.5.2. Influence of Whey Proteins on Adsorption of Lactose
3.5.3. Influence of Amino Acid and Kinds of Protein on Adsorption of Lactose
3.6. Limitation of the Present Adsorption Process
4. Conclusions
- By exchanging the counter ion of the anion exchange resin (IRA402) from chloride ion to hydroxide ion, lactose could be adsorbed onto IRA402, and the amount of lactose adsorbed was 14.2 times larger.
- The addition of boric acid enabled the desorption of lactose from anion exchange resins, and resulted in 72% desorption of lactose from IRA402, and the desorption rate (%) increased 6.7-fold.
- The binding between tetrahydroxyboronate ion and the cis-diol of lactose is considered to be the most likely adsorption species.
- The optimal amount of boric acid and pH were 1:1 in the molar ratio of boric acid to lactose and pH 7–9.
- Langmuir-type adsorption equilibrium relationship was established.
- The recovery of the adsorption method in this study was 58–60% in the model system for lactose, and 40–42% in the actual system with whey.
- The amount of lactose adsorbed from the actual whey solution was 35% lower than in the model system. It was suggested that coexisting minerals, vitamins, and chlorine ions influenced the amount of lactose adsorbed from the whey solution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| C | = concentration of metal ion within the column | [mol/m3] |
| Cdmax | = the maximum concentration of lactose by releasing lactose | [mol/m3] |
| Ci | = initial concentration of of lactose | [mol/m3] |
| Ct | = concentration of lactose at time, t | [mol/m3] |
| k | = adsorption rate constant defined in Equation (5) | [kg/(mol min)] |
| K | = equilibrium adsorption constant defined in Equation (7) | [m3/mol] |
| ka | = adsorption rate constant defined in Equation (6) | [m3/(mol min)] |
| kd | = desorption rate constant defined in Equation (6) | [min−1] |
| m | = mass of anion exchange resin | [kg] |
| R | = removal efficiency of metal ion | [-] |
| t | = time | [min] |
| Xe | = equilibrium amount of lactose adsorbed | [mol/kg] |
| Xs | = saturated amount of lactose adsorbed | [mol/kg] |
| Xt | = amount of lactose adsorbed at time, t | [mol/kg] |
| V | = volume of liquid | [m3] |
References
- Siso, M.I.G. The biotechnological utilization of cheese whey: A review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Illanes, A. Whey upgrading by enzyme biocatalysis. Electron. J. Biotechnol. 2011, 14, 9. [Google Scholar] [CrossRef]
- Chourasia, R.; Phukon, L.C.; Abedin, M.; Padhi, S.; Singh, S.P.; Rai, A.K. Whey valorization by microbial and enzymatic bioprocesses for the production of nutraceuticals and value-added products. Bioresour. Technol. Rep. 2022, 19, 101144. [Google Scholar] [CrossRef]
- Walstra, P.; Jenness, R. Dairy Chemistry and Physics; John Wiley & Sons: NewYork, NY, USA, 1984. [Google Scholar]
- Sánchez-Moya, T.; Hidalgo, A.M.; Ros-Berruezo, G.; López-Nicolás, R. Screening ultrafiltration membranes to separate lactose and protein from sheep whey: Application of simplified model. J. Food Sci. Technol. 2020, 57, 3193–3200. [Google Scholar] [CrossRef]
- Qi, T.; Yang, D.; Chen, X.; Qiu, M.; Fan, Y. Rapid removal of lactose for low-lactose milk by ceramic membranes. Sep. Purif. Technol. 2022, 289, 120601. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Díaz-Guridi, P.; Otero, J.A.; Ibáñez, R. Modeling of lactic acid rejection from lactose in acidified cheese whey by nanofiltration. J. Dairy Sci. 2023, 106, 4533–4544. [Google Scholar] [CrossRef]
- Khani, M.H.; Khamseh, A.G. Statistical analysis, equilibrium and dynamic study on the biosorption of strontium ions on Chlorella vulgaris. J. Radioanal. Nucl. Chem. 2023, 332, 3325–3334. [Google Scholar] [CrossRef]
- Khamseh, A.A.G.; Ghorbanian, S.A.; Amini, Y.; Shadman, M.M. Investigation of kinetic, isotherm and adsorption efficacy of thorium by orange peel immobilized on calcium alginate. Sci. Rep. 2023, 13, 8393. [Google Scholar] [CrossRef]
- Khamseh, A.G.; Ghorbanian, S.A. Experimental and modeling investigation of thorium biosorption by orange peel in a continuous fixed-bed column. J. Radioanal. Nucl. Chem. 2018, 317, 871–879. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Abdel-Salam, A.H.; Khalil, T.E. The impact of an Amberlite XAD-16-based chelating resin for the removal of aqueous Cd(II) and Pb(II)ions. Microchem. J. 2021, 165, 106097. [Google Scholar] [CrossRef]
- Shaaban, A.; Fadel, D.; Mahmoud, A.; Elkomy, M.; Elbahy, S. Synthesis of a new chelating resin bearing amidoxime group for adsorption of Cu(II), Ni(II) and Pb(II) by batch and fixed-bed column methods. J. Environ. Chem. Eng. 2014, 2, 632–641. [Google Scholar] [CrossRef]
- Gossuin, Y.; Hantson, A.-L.; Vuong, Q.L. Low resolution benchtop nuclear magnetic resonance for the follow-up of the removal of Cu2+ and Cr3+ from water by amberlite IR120 ion exchange resin. J. Water Process Eng. 2020, 33, 101024. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Bian, Y.; Bian, Z.; Zhang, J.; Ding, A.; Liu, S.; Zheng, L.; Wang, H. Adsorption of cadmium ions from aqueous solutions by activated carbon with oxygen-containing functional groups. Chin. J. Chem. Eng. 2015, 23, 1705–1711. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.; Magro, J.D. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Kasai, M.; Kobayashi, Y.; Togo, M.; Nakahira, A. Synthesis of zeolite-surface-modified perlite and their heavy metal adsorption capability. Mater. Today Proc. 2019, 16, 232–238. [Google Scholar] [CrossRef]
- Tanan, W.; Panpinit, S.; Saengsuwan, S. Comparison of microwave-assisted and thermal-heated synthesis of P(HEMA-co-AM)/PVA interpenetrating polymer network (IPN) hydrogels for Pb(II) removal from aqueous solution: Characterization, adsorption and kinetic study. Eur. Polym. J. 2021, 143, 110193. [Google Scholar] [CrossRef]
- Gokmen, F.; Yaman, E.; Temel, S. Eco-friendly polyacrylic acid based porous hydrogel for heavy metal ions adsorption: Characterization, adsorption behavior, thermodynamic and reusability studies. Microchem. J. 2021, 168, 106357. [Google Scholar] [CrossRef]
- Zumreoglu-Karan, B.; Köse, D.A. Boric acid: A simple molecule of physiologic, therapeutic and prebiotic significance. Pure Appl. Chem. 2015, 87, 155–162. [Google Scholar] [CrossRef]
- Kunin, R.; Preuss, A.F. Characterization of a Boron-Specific Ion Exchange Resin. I&EC Prod. Res. Dev. 1964, 3, 304–306. [Google Scholar] [CrossRef]
- Bai, S.; Li, J.; Ding, W.; Chen, S.; Ya, R. Removal of boron by a modified resin in fixed bed column: Breakthrough curve analysis using dynamic adsorption models and artificial neural network model. Chemosphere 2022, 296, 134021. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Seki, H. Recovery of milk whey proteins by foam separation. Process Saf. Environ. Prot. 2022, 159, 566–574. [Google Scholar] [CrossRef]
- Maruyama, H.; Seki, H.; Suzuki, A.; Inoue, N. Batch foam separation of a soluble protein. Water Res. 2007, 41, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Seki, H. Enhancement of separation rate and recovery efficiency of milk whey proteins by addition of calcium and magnesium ions in batch foam separation. Process Saf. Environ. Prot. 2022, 162, 1102–1106. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Smith, W.C.; Goudie, A.J.; Sivertson, J.N. Colorimetric Determination of Trace Quantities of Boric Acid in Biological Materials. Anal. Chem. 1955, 27, 295–297. [Google Scholar] [CrossRef]
- Crouch, S.R.; Malmstadt, H.V. Mechanistic investigation of molybdenum blue method for determination of phosphate. Anal. Chem. 1967, 39, 1084–1089. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Maillard, L.C. Action of amino acids on sugars. Formation of melanoidins in a methodical way. C. R. 1912, 154, 66–68. (In French) [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Smith, J.T.; Nashabeh, W.; El Rassi, Z. Micellar electrokinetic capillary chromatography with in situ charged micelles. Evaluation of N-D-Gluco-N-methylalkanamide surfactants as anionic borate complexes. Anal. Chem. 1994, 66, 1119–1133. [Google Scholar] [CrossRef]
- Van Duin, M.; Peters, J.; Kieboom, A.; Van Bekkum, H. Studies on borate esters II: Structure and stability of borate esters of polyhydroxycarboxylates and related polyols in aqueous alkaline media as studied by 11B NMR. Tetrahedron 1985, 41, 3411–3421. [Google Scholar] [CrossRef]
- Makkee, M.; Kieboom, A.P.G.; van Bekkum, H. Studies on borate esters III. Borate esters of D-mannitol, D-glucitol, D-fructose and D-glucose in water. Recl. Trav. Chim. Pays-Bas 1985, 104, 230–235. [Google Scholar] [CrossRef]
- Seki, H.; Suzuki, A. Adsorption of Heavy Metal Ions to Floc-Type Biosorbents. J. Colloid Interface Sci. 2002, 249, 295–300. [Google Scholar] [CrossRef] [PubMed]
- González-García, C.; González-Martín, M.; Denoyel, R.; Gallardo-Moreno, A.; Labajos-Broncano, L.; Bruque, J. Ionic surfactant adsorption onto activated carbons. J. Colloid Interface Sci. 2004, 278, 257–264. [Google Scholar] [CrossRef]
- González-García, C.; González-Martín, M.; González, J.; Sabio, E.; Ramiro, A.; Gañán, J. Nonionic surfactants adsorption onto activated carbon. Influence of the polar chain length. Powder Technol. 2004, 148, 32–37. [Google Scholar] [CrossRef]
- González-García, C.; González-Martín, M.; Denoyel, R.; Gallardo-Moreno, A.; Labajos-Broncano, L.; Bruque, J. Adsorption enthalpies of sodium dodecyl sulphate onto carbon blacks in the low concentration range. Carbon 2005, 43, 567–572. [Google Scholar] [CrossRef]
- Escudero, R.R.; Robitzer, M.; Di Renzo, F.; Quignard, F. Alginate aerogels as adsorbents of polar molecules from liquid hydrocarbons: Hexanol as probe molecule. Carbohydr. Polym. 2008, 75, 52–57. [Google Scholar] [CrossRef]
- Maruyama, H.; Seki, H. Adsorption modeling by two sites Langmuir type isotherm for adsorption of bisphenol-A and diethyl phthalate onto activated carbon in single and binary system. Sep. Sci. Technol. 2022, 57, 1535–1542. [Google Scholar] [CrossRef]
- Hamza, M.F.; Guibal, E.; Althumayri, K.; Vincent, T.; Yin, X.; Wei, Y.; Li, W. New Process for the Sulfonation of Algal/PEI Biosorbent for Enhancing Sr(II) Removal from Aqueous Solutions—Application to Seawater. Molecules 2022, 27, 7128. [Google Scholar] [CrossRef] [PubMed]
- Salih, K.A.M.; Zhou, K.; Hamza, M.F.; Mira, H.; Wei, Y.; Ning, S.; Guibal, E.; Salem, W.M. Phosphonation of Alginate–Polyethyleneimine Beads for the Enhanced Removal of Cs(I) and Sr(II) from Aqueous Solutions. Gels 2023, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, L. From Langmuir Kinetics to First- and Second-Order Rate Equations for Adsorption. Langmuir 2008, 24, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Marczewski, A.W. Analysis of Kinetic Langmuir Model. Part I: Integrated Kinetic Langmuir Equation (IKL): A New Complete Analytical Solution of the Langmuir Rate Equation. Langmuir 2010, 26, 15229–15238. [Google Scholar] [CrossRef]
- Regazzoni, A.E. Adsorption kinetics at solid/aqueous solution interfaces: On the boundaries of the pseudo-second order rate equation. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 585, 124093. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Silva, C.M. What’s wrong with Lagergreen pseudo first order model for adsorption kinetics? Chem. Eng. J. 2016, 306, 1138–1142. [Google Scholar] [CrossRef]
- Shen, L.; Liu, Y.; Paul, E. A simple geometric approach for simplification of Langmuir kinetics for adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 349, 78–82. [Google Scholar] [CrossRef]
- Tan, K.; Hameed, B. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Kinetics of Solute Adsorption at Solid/Solution Interfaces: A Theoretical Development of the Empirical Pseudo-First and Pseudo-Second Order Kinetic Rate Equations, Based on Applying the Statistical Rate Theory of Interfacial Transport. J. Phys. Chem. B 2006, 110, 16514–16525. [Google Scholar] [CrossRef]
- Zydney, A.L. Protein Separations Using Membrane Filtration: New Opportunities for Whey Fractionation. Int. Dairy J. 1998, 8, 243–250. [Google Scholar] [CrossRef]
- Vasson, M.-P.; Farges, M.-C.; Sarret, A.; Cynober, L. Free amino acid concentrations in milk: Effects of microwaveversus conventional heating. Amino Acids 1998, 15, 385–388. [Google Scholar] [CrossRef]
- Atra, R.; Vatai, G.; Bekassy-Molnar, E.; Balint, A. Investigation of ultra- and nanofiltration for utilization of whey protein and lactose. J. Food Eng. 2005, 67, 325–332. [Google Scholar] [CrossRef]
- Cuartas-Uribe, B.; Alcaina-Miranda, M.; Soriano-Costa, E.; Mendoza-Roca, J.; Iborra-Clar, M.; Lora-García, J. A study of the separation of lactose from whey ultrafiltration permeate using nanofiltration. Desalination 2009, 241, 244–255. [Google Scholar] [CrossRef]
- de Souza, R.R.; Bergamasco, R.; da Costa, S.C.; Feng, X.; Faria, S.H.B.; Gimenes, M.L. Recovery and purification of lactose from whey. Chem. Eng. Process. Process Intensif. 2010, 49, 1137–1143. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, W.; Ould-Dris, A.; Jaffrin, M.Y.; Tang, B. Concentration of Milk Proteins for Producing Cheese Using a Shear-Enhanced Ultrafiltration Technique. Ind. Eng. Chem. Res. 2016, 55, 11130–11138. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, Y.; He, Y.; Pan, J.; Yang, K.; Shen, J.; Gao, C. Preparation of Low-Lactose Milk Powder by Coupling Membrane Technology. ACS Omega 2020, 5, 8543–8550. [Google Scholar] [CrossRef]



| pH | Molar Ratio | Xe × 103 | k × 10−3 | R2 |
|---|---|---|---|---|
| [mol/g] | [g/(mol h)] | |||
| 7 | 1 | 0.535 | 6.17 | 0.99 |
| 7 | 2 | 0.573 | 0.88 | 0.99 |
| 9 | 1 | 0.528 | 4.69 | 0.99 |
| 9 | 2 | 0.54 | 3.79 | 0.99 |
| pH | Molar Ratio | ka [L/(mol h)] | kd [h−1] | K [L/mol] | R2 |
|---|---|---|---|---|---|
| 7 | 1 | 104.4 | 0.169 | 617 | 0.925 |
| 7 | 2 | 97.6 | 0.103 | 1087 | 0.871 |
| 9 | 1 | 111.0 | 0.238 | 466 | 0.858 |
| 9 | 2 | 115.7 | 0.119 | 973 | 0.925 |
| pH | Molar Ratio | K [L/mol] | Xs × 103 [mol/g] | R2 |
|---|---|---|---|---|
| 7 | 1:1 | 593 | 0.791 | 0.911 |
| 7 | 1:2 | 5869 | 0.615 | 0.911 |
| 9 | 1:1 | 547 | 0.846 | 0.924 |
| 9 | 1:2 | 760 | 0.706 | 0.924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, H.; Seki, H. Adsorption of Lactose Using Anion Exchange Resin by Adding Boric Acid from Milk Whey. Separations 2023, 10, 530. https://doi.org/10.3390/separations10100530
Maruyama H, Seki H. Adsorption of Lactose Using Anion Exchange Resin by Adding Boric Acid from Milk Whey. Separations. 2023; 10(10):530. https://doi.org/10.3390/separations10100530
Chicago/Turabian StyleMaruyama, Hideo, and Hideshi Seki. 2023. "Adsorption of Lactose Using Anion Exchange Resin by Adding Boric Acid from Milk Whey" Separations 10, no. 10: 530. https://doi.org/10.3390/separations10100530