CoFe2O4/MoS2@Au: Multifunction Z-Scheme Heterojunction for SERS Monitoring and Photocatalytic Degradation of Fungicides
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Instruments
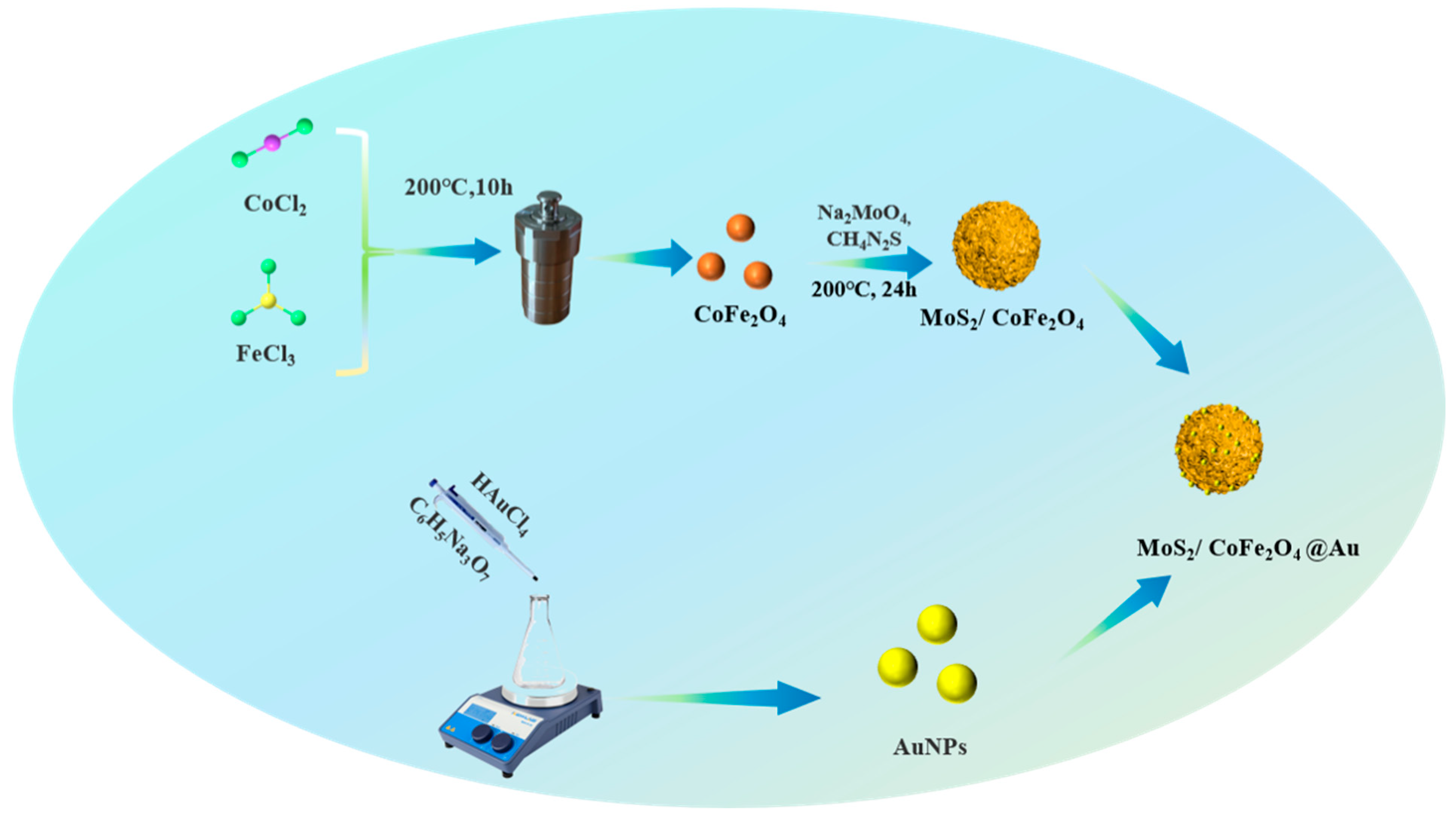
2.3. Synthesis of CoFe2O4/MoS2@Au
2.4. Photocatalytic Degradation
2.5. The Water Remediation Capability
2.6. Evaluation of SERS Activity and Analysis Procedures for Fungicides
2.7. In Situ Monitoring the Photodegradation Process
2.8. Application to Actual Samples
2.9. Statistical Analysis
3. Results and Discussion
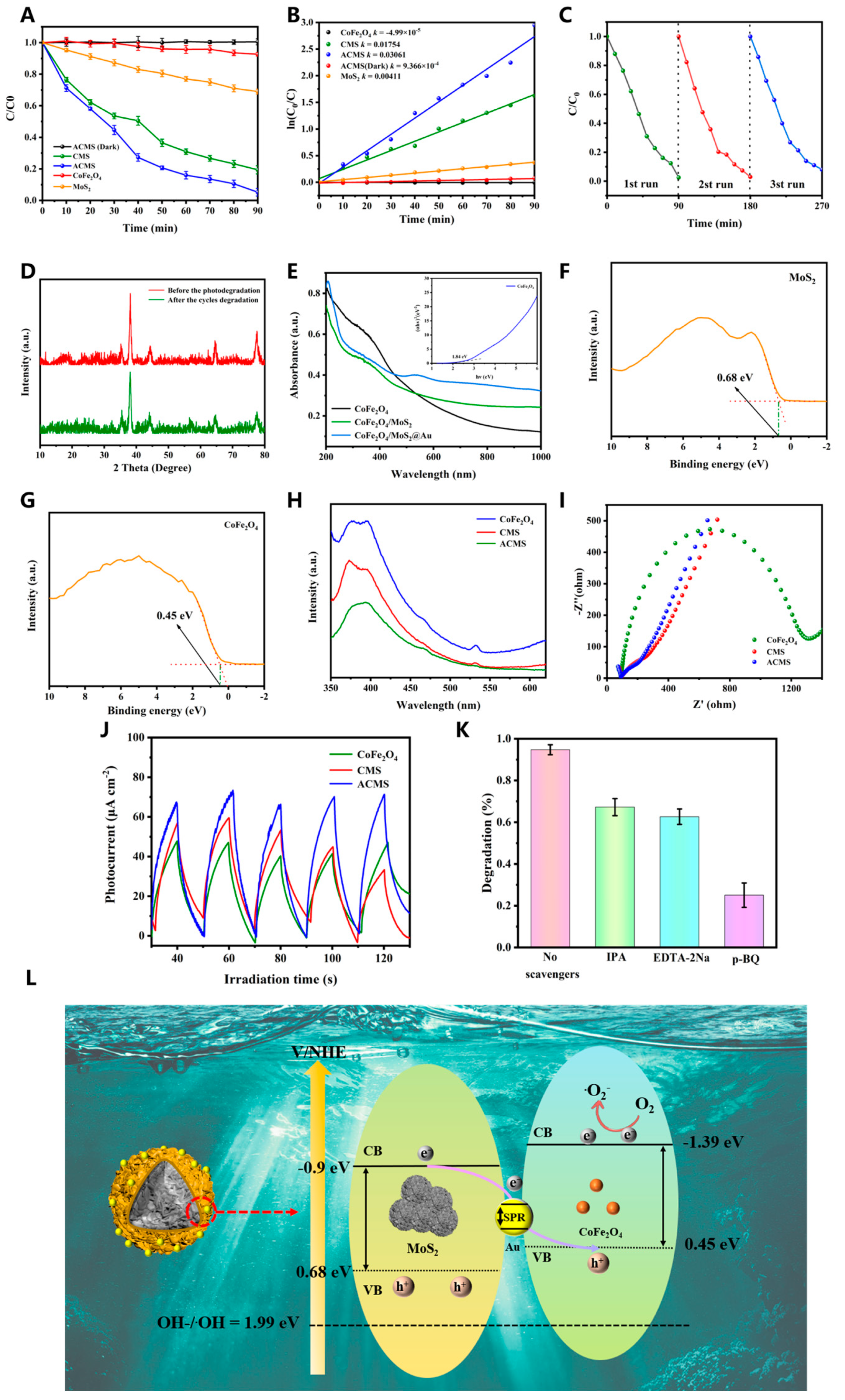
3.1. Characterization of As-Prepared Samples
3.2. Photocatalytic Activities
3.3. Photoelectric Properties of Samples
3.4. Water Remediation and Phytotoxicity Tests of ACMS in Water
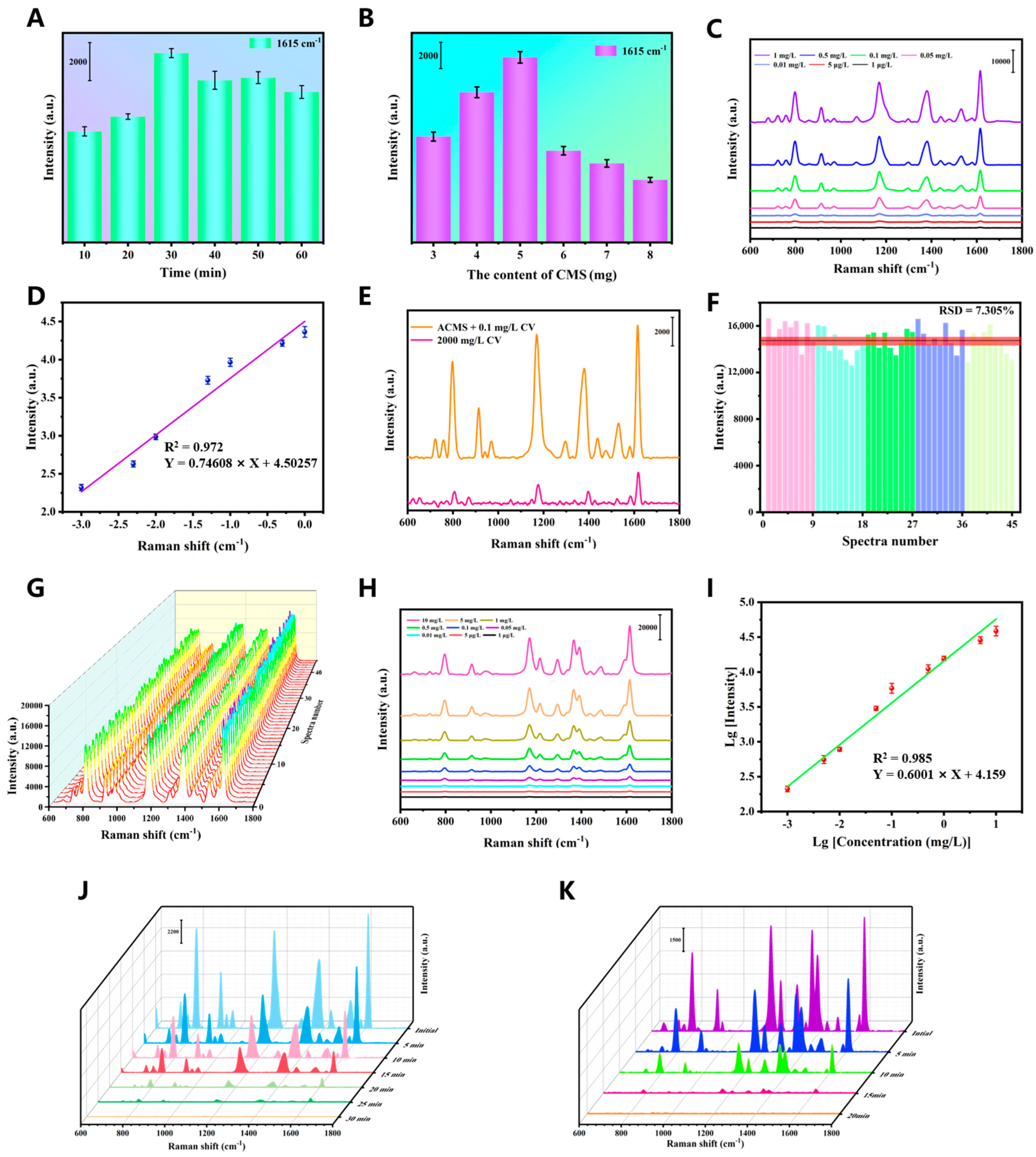
3.5. SERS Performance of ACMS
3.6. In Situ Photodegradation Monitoring
3.7. Detection of MG in Crucian Carp Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiang, T.; Chen, L.; Xia, Y.; Qin, X. Dual modified MoS2/SnS2 photocatalyst with Z-scheme heterojunction and vacancies defects to achieve a superior performance in Cr (VI) reduction and dyes degradation. J. Clean. Prod. 2021, 291, 125213. [Google Scholar] [CrossRef]
- Shen, K.; Cui, Y.; Zhang, D.; Liu, M.; Huang, H.; Sha, X.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Biomimetic preparation of MoS2-Fe3O4 MNPs as heterogeneous catalysts for the degradation of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104125. [Google Scholar] [CrossRef]
- Lai, H.; Ma, G.; Shang, W.; Chen, D.; Yun, Y.; Peng, X.; Xu, F. Multifunctional magnetic sphere-MoS2@Au hybrid for surface-enhanced Raman scattering detection and visible light photo-Fenton degradation of aromatic dyes. Chemosphere 2019, 223, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Tian, Y.; Du, Y.; Ma, Y.; Zeng, S.; Gu, C.; Jiang, T.; Zhou, J. In Situ Recyclable Surface-Enhanced Raman Scattering-Based Detection of Multicomponent Pesticide Residues on Fruits and Vegetables by the Flower-like MoS2@Ag Hybrid Substrate. ACS Appl. Mater. Interfaces 2020, 12, 14386–14399. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Chen, D.; Tachibana, Y.; Suzuki, H.; Abe, R.; Caruso, R.A. Developing sustainable, high-performance perovskites in photocatalysis: Design strategies and applications. Chem. Soc. Rev. 2021, 50, 13692–13729. [Google Scholar] [CrossRef]
- Jo, W.-K.; Lee, J.Y.; Selvam, N.C.S. Synthesis of MoS2 nanosheets loaded ZnO–g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 2016, 289, 306–318. [Google Scholar] [CrossRef]
- Jiang, Q.-Y.; Li, D.; Liu, Y.; Mao, Z.-S.; Yu, Y.; Zhu, P.; Xu, Q.; Sun, Y.; Hu, L.; Wang, J.; et al. Recyclable and green AuBPs@MoS2@tinfoil box for high throughput SERS tracking of diquat in complex compounds. Sens. Actuators B Chem. 2021, 344, 130290. [Google Scholar] [CrossRef]
- Zhou, H.; Lai, L.; Wan, Y.; He, Y.; Yao, G.; Lai, B. Molybdenum disulfide (MoS2): A versatile activator of both peroxymonosulfate and persulfate for the degradation of carbamazepine. Chem. Eng. J. 2020, 384, 123264. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Kim, W.; Alfarraj, S.; Alharbi, S.A. Facile fabrication of (2D/2D) MoS2@MIL-88(Fe) interface-driven catalyst for efficient degradation of organic pollutants under visible light irradiation. J. Hazard. Mater. 2021, 414, 125522. [Google Scholar] [CrossRef]
- Quan, Y.; Su, R.; Yang, S.; Chen, L.; Wei, M.; Liu, H.; Yang, J.; Gao, M.; Li, B. In-situ surface-enhanced Raman scattering based on MTi20 nanoflowers: Monitoring and degradation of contaminants. J. Hazard. Mater. 2021, 412, 125209. [Google Scholar] [CrossRef]
- Yang, Y.; Gong, W.; Li, X.; Liu, Y.; Liang, Y.; Chen, B.; Yang, Y.; Luo, X.; Xu, K.; Yuan, C. Light-assisted room temperature gas sensing performance and mechanism of direct Z-scheme MoS2/SnO2 crystal faceted heterojunctions. J. Hazard. Mater. 2022, 436, 129246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Sun, X.; Ji, H.; Liu, W.; Cai, Z. Construction of Z-scheme Ag/AgVO3/carbon-rich g-C3N4 heterojunction for enhanced photocatalytic degradation of sulfamethiadiazole: DFT calculation and mechanism study. Chem. Eng. J. 2022, 433, 133604. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.-Y.A.; Ghanbari, F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Feng, S.; Yu, M.; Xie, T.; Li, T.; Kong, D.; Yang, J.; Cheng, C.; Chen, H.; Wang, J. MoS2/CoFe2O4 heterojunction for boosting photogenerated carrier separation and the dominant role in enhancing peroxymonosulfate activation. Chem. Eng. J. 2022, 433, 134467. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, K.; Yu, H.; Sun, Z. Synergistic effect of Ag/MoS2/TiO2 heterostructure arrays on enhancement of photoelectrochemical and photocatalytic performance. Int. J. Energy Res. 2020, 45, 6850–6862. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, N.; Song, Y.; Zhao, Y.; Li, H.; Xu, X.; Qiu, J.; Yu, H. Fabrication of Z-scheme magnetic MoS2/CoFe2O4 nanocomposites with highly efficient photocatalytic activity. J. Colloid Interface Sci. 2018, 514, 664–674. [Google Scholar] [CrossRef]
- Ren, B.; Shen, W.; Li, L.; Wu, S.; Wang, W. 3D CoFe2O4 nanorod/flower-like MoS2 nanosheet heterojunctions as recyclable visible light-driven photocatalysts for the degradation of organic dyes. Appl. Surf. Sci. 2018, 447, 711–723. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, Q.; Sun, D.-W.; Pu, H. Synthesis of recyclable SERS platform based on MoS2@TiO2@Au heterojunction for photodegradation and identification of fungicides. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 285, 121895. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Al-Saeedi, S.I.; Almotairi, S.; Alshehri, S.M. Fabrication of MoS2/ZnS embedded in N/S doped carbon for the photocatalytic degradation of pesticide. Mater. Lett. 2020, 263, 127271. [Google Scholar] [CrossRef]
- Li, G.; Wang, R.; Wang, B.; Zhang, J. Sm-doped mesoporous g-C3N4 as efficient catalyst for degradation of tylosin: Influencing factors and toxicity assessment. Appl. Surf. Sci. 2020, 517, 146212. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. A dynamically optical and highly stable pNIPAM@Au NRs nanohybrid substrate for sensitive SERS detection of malachite green in fish fillet. Talanta 2020, 218, 121188. [Google Scholar] [CrossRef]
- Ibrahim, I.; Kaltzoglou, A.; Athanasekou, C.; Katsaros, F.; Devlin, E.; Kontos, A.G.; Ioannidis, N.; Perraki, M.; Tsakiridis, P.; Sygellou, L.; et al. Magnetically separable TiO2/CoFe2O4/Ag nanocomposites for the photocatalytic reduction of hexavalent chromium pollutant under UV and artificial solar light. Chem. Eng. J. 2020, 381, 122730. [Google Scholar] [CrossRef]
- He, Z.; Siddique, M.S.; Yang, H.; Xia, Y.; Su, J.; Tang, B.; Wang, L.; Kang, L.; Huang, Z. Novel Z-scheme In2S3/Bi2WO6 core-shell heterojunctions with synergistic enhanced photocatalytic degradation of tetracycline hydrochloride. J. Clean. Prod. 2022, 339, 130634. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Zhang, H.; Yao, H.; Chai, J.; Wang, J.; Fang, D.; Zhang, Z.; Tie, M. Construction of high-proportion dual bismuth-based Z-scheme Bi3O4Cl/Bi2MoO6 photocatalytic system via in-situ growth of Bi2MoO6 on Bi3O4Cl for enhanced photocatalytic degradation of organic pollutants. J. Alloys Compd. 2023, 956, 170375. [Google Scholar] [CrossRef]
- Yu, C.; Wang, K.; Yang, P.; Yang, S.; Lu, C.; Song, Y.; Dong, S.; Sun, J.; Sun, J. One-pot facile synthesis of Bi2S3/SnS2/Bi2O3 ternary heterojunction as advanced double Z-scheme photocatalytic system for efficient dye removal under sunlight irradiation. Appl. Surf. Sci. 2017, 420, 233–242. [Google Scholar] [CrossRef]
- Mohamed, H.H. Rationally designed Fe2O3/GO/WO3 Z-Scheme photocatalyst for enhanced solar light photocatalytic water remediation. J. Photochem. Photobiol. A Chem. 2019, 378, 74–84. [Google Scholar] [CrossRef]
- Ferdosi, E.; Bahiraei, H.; Ghanbari, D. Investigation the photocatalytic activity of CoFe2O4/ZnO and CoFe2O4/ZnO/Ag nanocomposites for purification of dye pollutants. Sep. Purif. Technol. 2019, 211, 35–39. [Google Scholar] [CrossRef]
- Chen, L.; Tsai, M.-L.; Chuang, Y.; Chen, C.-W.; Dong, C.-D. Construction of carbon nanotubes bridged MoS2/ZnO Z-scheme nanohybrid towards enhanced visible light driven photocatalytic water disinfection and antibacterial activity. Carbon 2022, 196, 877–889. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Z.; Zhao, H.; Song, S.; Liu, M.; Li, Z.; Zhou, W. MoS2@In2S3/Bi2S3 Core-shell dual Z-scheme tandem heterojunctions with Broad-spectrum response and enhanced Photothermal-photocatalytic performance. Chem. Eng. J. 2022, 431, 133355. [Google Scholar] [CrossRef]
- Mandal, S.; Adhikari, S.; Choi, S.; Lee, Y.; Kim, D.-H. Fabrication of a novel Z-scheme Bi2MoO6/GQDs/MoS2 hierarchical nanocomposite for the photo-oxidation of ofloxacin and photoreduction of Cr(VI) as aqueous pollutants. Chem. Eng. J. 2022, 444, 136609. [Google Scholar] [CrossRef]
- Ma, E.; Sun, G.; Duan, F.; Wang, H.; Wang, H. Visible-light-responsive Z-scheme heterojunction MoS2 NTs/CuInS2 QDs photoanode for enhanced photoelectrocatalytic degradation of tetracycline. Appl. Mater. Today 2022, 28, 101504. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, S.; Nie, M.; Yan, C.; Qiu, L.; Wu, L.; Ding, M. Remediation of sulfathiazole contaminated soil by peroxymonosulfate: Performance, mechanism and phytotoxicity. Sci. Total Environ. 2022, 830, 154839. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Yang, H.; Zhang, S.; Li, J.; Shi, K.; Jin, F. Controllable preparation of the Au–MoS2 nano-array composite: Optical properties study and SERS application. J. Mater. Chem. C 2021, 9, 6823–6833. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. Polymer multilayers enabled stable and flexible Au@Ag nanoparticle array for nondestructive SERS detection of pesticide residues. Talanta 2021, 223, 121782. [Google Scholar] [CrossRef] [PubMed]



| Substrate | Targets/Concentration | Catalytic Conditions | Recovery Time | Ref. |
|---|---|---|---|---|
| 30% In2S3/Bi2WO6 | Tetracycline hydrochloride, 20 mg/L | Visible light | 96.0%, 120 min | [23] |
| Bi3O4Cl/Bi2MoO6 | Norfloxacin, 10 mg/L | Visible light | 87.34%, 150 min | [24] |
| Bi2S3/SnS2/Bi2O3 | CV, 5 mg/L | Visible light | 90 min, 99.6% | [25] |
| Fe2O3/GO/WO3 | CV, 20 mg/L | Solar light | 95.4%, 120 min | [26] |
| ACMS | CV, 37.5 mg/L | Solar light | 94.76%, b90 min | This work |
| Samples | Average Root Length (cm) | Germination Rate | Germination Index |
|---|---|---|---|
| Water | 8.7125 | 100 | 1 |
| Treated wastewater | 8.4 | 100 | 0.9641 |
| CV solution | 2.456 | 93.75 | 0.2819 |
| Sample | Added Mount (mg/kg) | Detected a ± SD b (mg/kg) | Recovery (%) |
|---|---|---|---|
| 1 | 0.5 | 0.528 ± 0.039 | 105.60 |
| 2 | 0.1 | 0.116 ± 0.051 | 116.00 |
| 3 | 0.05 | 0.048 ± 0.009 | 96.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Wei, Q.; Pu, H. CoFe2O4/MoS2@Au: Multifunction Z-Scheme Heterojunction for SERS Monitoring and Photocatalytic Degradation of Fungicides. Separations 2023, 10, 526. https://doi.org/10.3390/separations10100526
Dong Q, Wei Q, Pu H. CoFe2O4/MoS2@Au: Multifunction Z-Scheme Heterojunction for SERS Monitoring and Photocatalytic Degradation of Fungicides. Separations. 2023; 10(10):526. https://doi.org/10.3390/separations10100526
Chicago/Turabian StyleDong, Qirong, Qingyi Wei, and Hongbin Pu. 2023. "CoFe2O4/MoS2@Au: Multifunction Z-Scheme Heterojunction for SERS Monitoring and Photocatalytic Degradation of Fungicides" Separations 10, no. 10: 526. https://doi.org/10.3390/separations10100526





