Design of Polymer-Embedded Heterogeneous Fenton Catalysts for the Conversion of Organic Trace Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedures
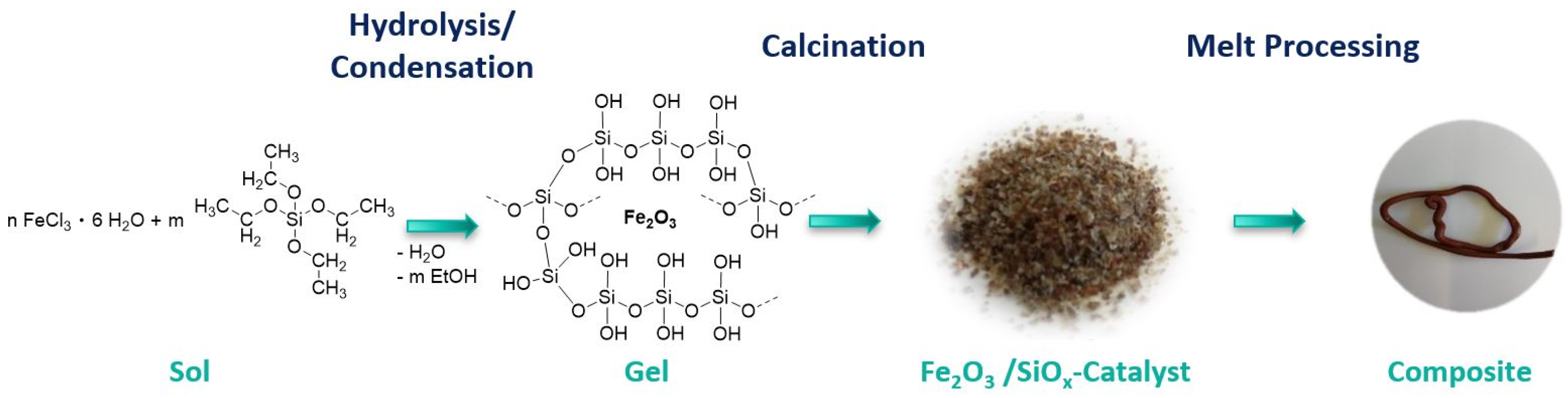
2.2.1. Synthesis of the Fe2O3/SiOx Fenton Catalysts F1 and F2
2.2.2. Fe2O3/SiOx Polymer Composites
2.3. Methods
2.3.1. X-ray Fluorescence Spectroscopy (XRF)
2.3.2. Wide-Angle X-ray Scattering (WAXS)
2.3.3. Nitrogen Sorption Measurements
2.3.4. Scanning Electron Microscope
2.3.5. Degradation Kinetics of Reactive Black 5 (RB5)
2.3.6. Analysis of the Degradation of Phenol
2.3.7. Iron Leaching Studies
2.3.8. Degradation of Organic Trace Compounds
3. Results and Discussion
3.1. Characterization of the Fenton Catalysts F1 and F2
3.2. Preparation and Characterization of the Fenton Polymer Composites
3.2.1. Preparation of the Fenton Polymer Composites
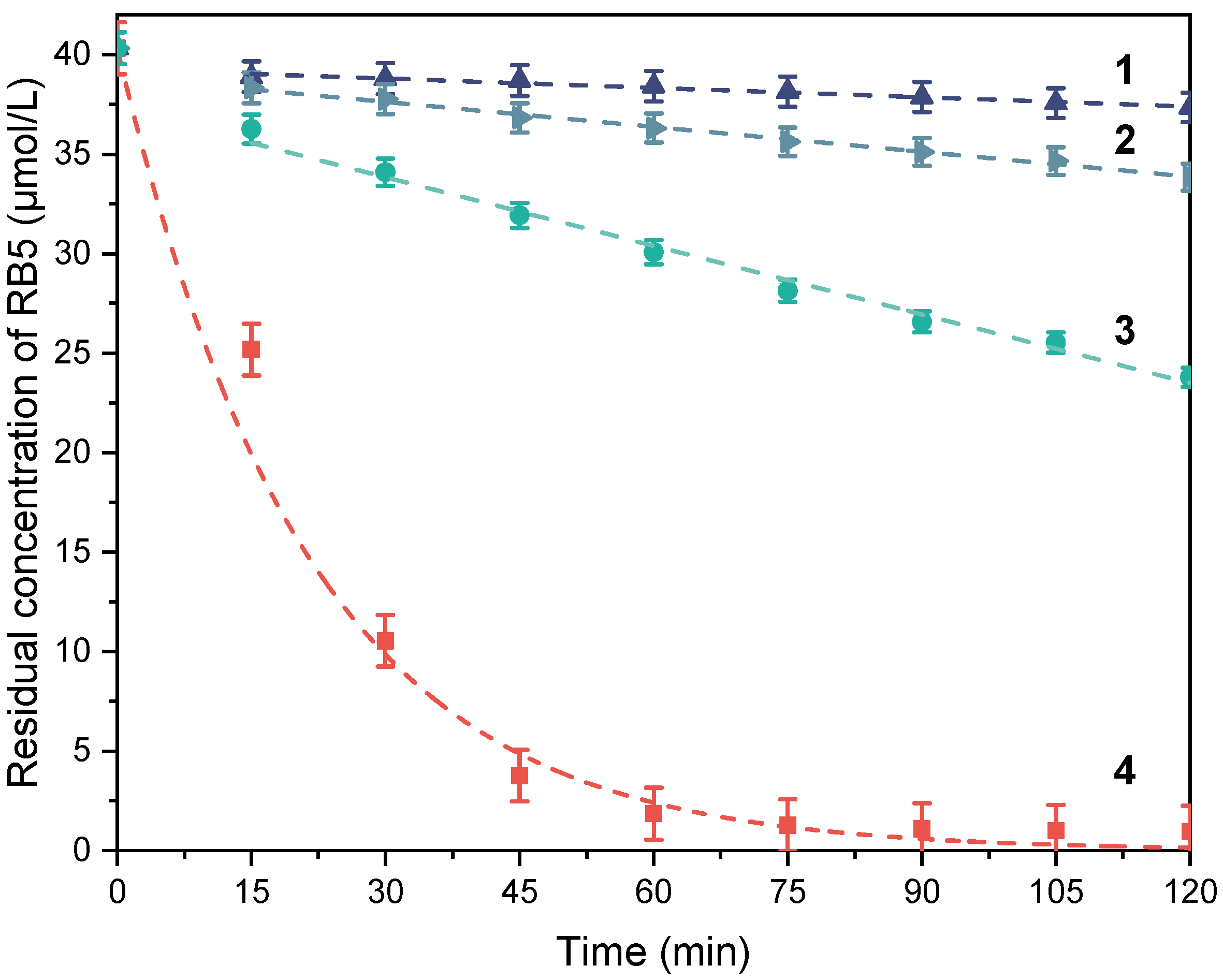
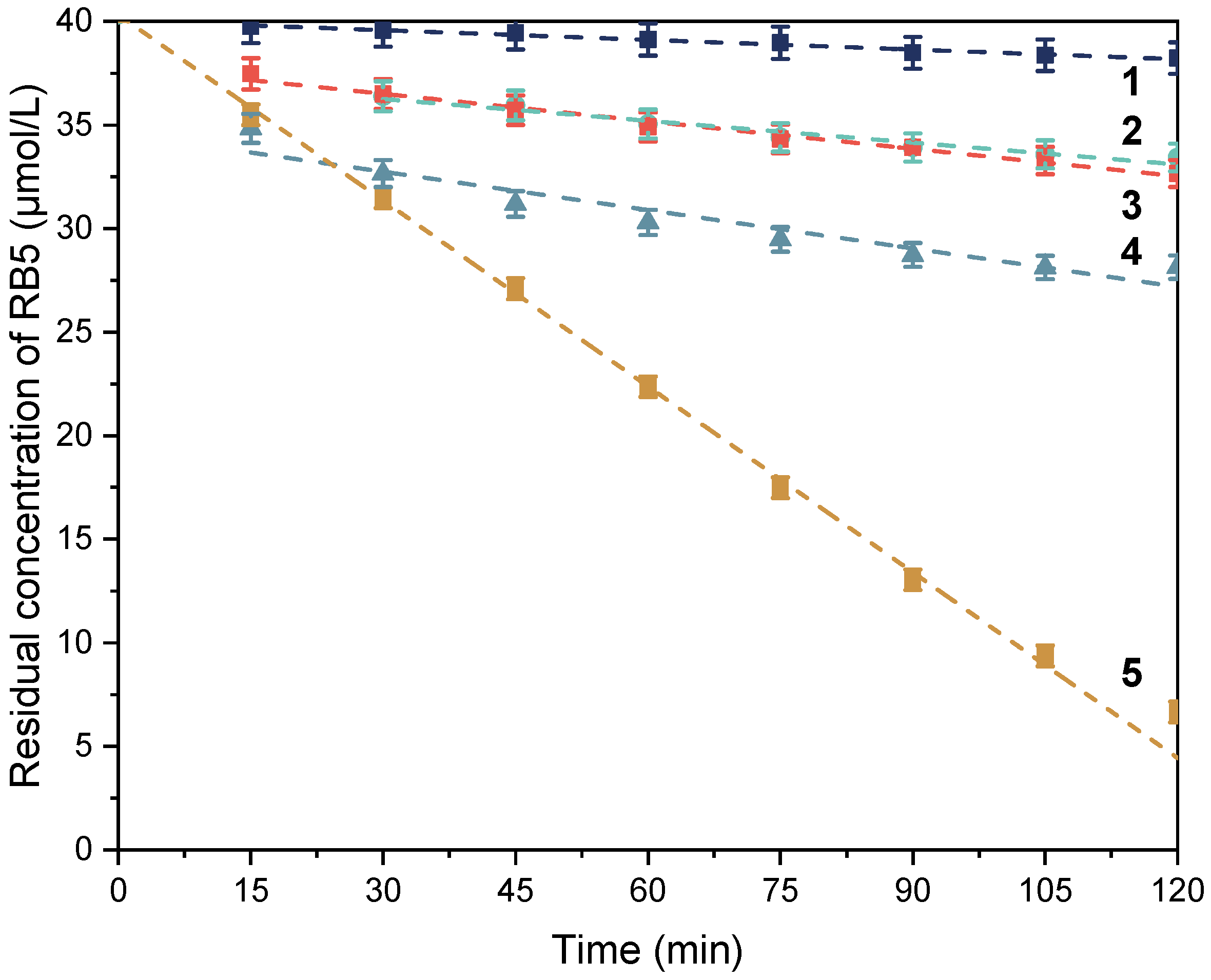
3.2.2. Catalytic Activity of the Polymer Composites as Studied by the RB5 Assay
3.2.3. Phenol Degradation Studies Catalyzed by the Fenton Polymer Composites
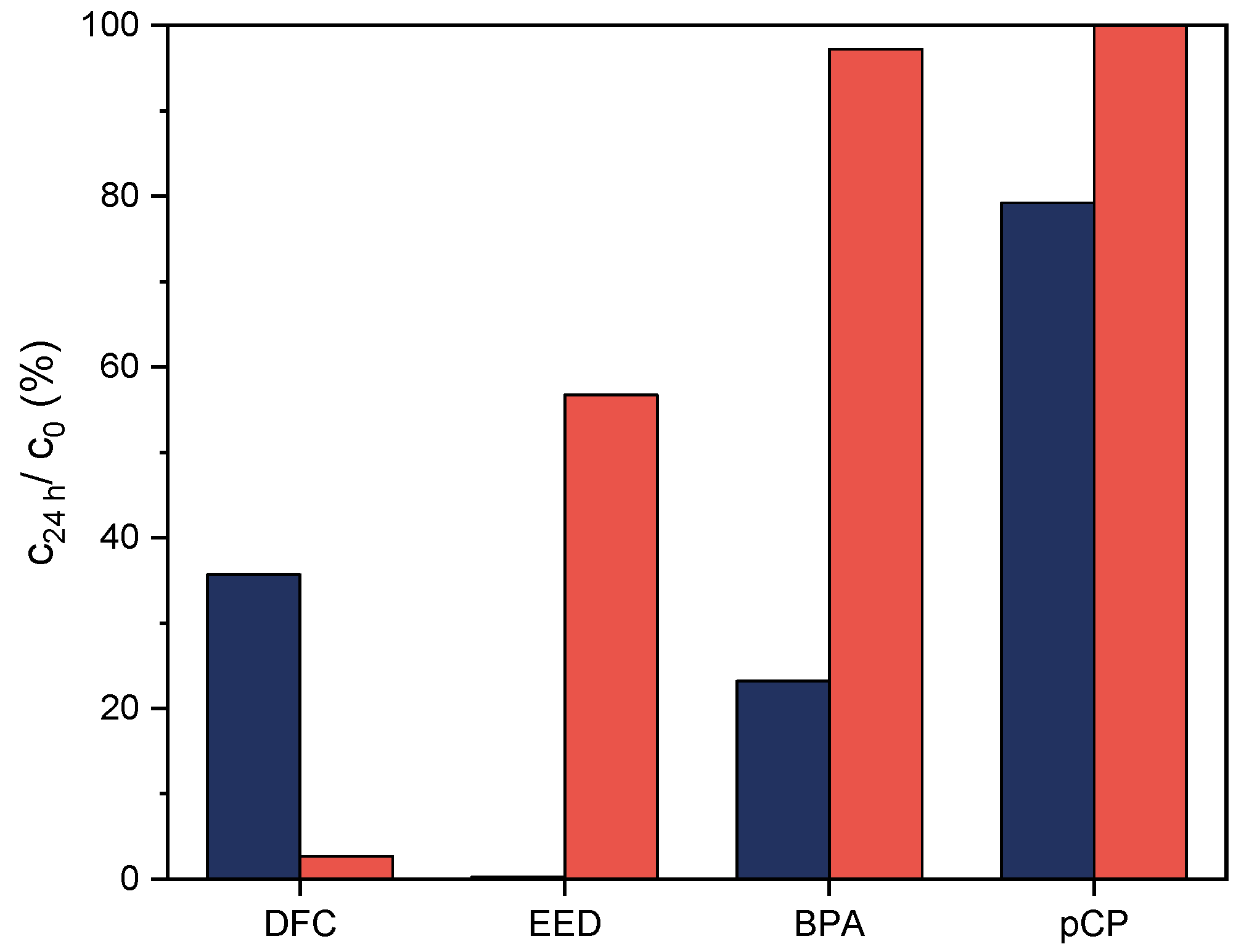
3.3. Degradation Studies of Model Substances for Trace Pollutants Catalyzed by F2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Underhill, R.; Lewis, R.J.; Freakley, S.J.; Douthwaite, M.; Miedziak, P.J.; Akdim, O.; Edwards, J.K.; Hutchings, G.J. Oxidative Degradation of Phenol using in situ Generated Hydrogen Peroxide Combined with Fenton’s Process. Johns. Matthey Technol. Rev. 2018, 62, 417–425. [Google Scholar] [CrossRef]
- Ni Soon, A.; Hameed, B. Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process. Desalination 2011, 269, 1–16. [Google Scholar] [CrossRef]
- Baptisttella, A.M.S.; de Araujo, C.M.B.; da Silva, M.P.; Nascimento, G.F.O.D.; da Costa, G.R.B.; Nascimento, B.F.D.; Ghislandi, M.G.; Sobrinho, M.A.D.M. Magnetic Fe3O4-graphene oxide nanocomposite—Synthesis and practical application for the heterogeneous photo-Fenton degradation of different dyes in water. Sep. Sci. Technol. 2021, 56, 425–438. [Google Scholar] [CrossRef]
- Lumbaque, E.C.; Gomes, M.F.; Carvalho, V.D.S.; De Freitas, A.M.; Tiburtius, E.R.L. Degradation and ecotoxicity of dye Reactive Black 5 after reductive-oxidative process. Environ. Sci. Pollut. Res. 2016, 24, 6126–6134. [Google Scholar] [CrossRef]
- Kumar, V.; Mohapatra, T.; Dharmadhikari, S.; Ghosh, P. A Review Paper on Heterogeneous Fenton Catalyst: Types of Preparation, Modification Techniques, Factors Affecting the Synthesis, Characterization, and Application in the Wastewater Treatment. Bull. Chem. React. Eng. Catal. 2020, 15, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Buthiyappan, A.; Aziz, A.R.A.; Daud, W.M.A.W. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.-Y.; Wang, Y.; Shi, T.-N.; He, X.-L.; Qi, S.-Y. Process optimization on methyl orange discoloration in Fe3O4/RGO-H2O2 Fenton-like system. Water Sci. Technol. 2018, 77, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Firak, D.S.; Ribeiro, R.R.; De Liz, M.V.; Peralta-Zamora, P. Investigations on iron leaching from oxides and its relevance for radical generation during Fenton-like catalysis. Environ. Earth Sci. 2018, 77, 117. [Google Scholar] [CrossRef]
- Yu, T.; Breslin, C.B. Graphene-Modified Composites and Electrodes and Their Potential Applications in the Electro-Fenton Process. Materials 2020, 13, 2254. [Google Scholar] [CrossRef]
- Fana, X.; Caoa, Q.; Menga, F.; Songa, B.; Baia, Z.; Zhaoa, Y.; Chenab, D.; Zhouc, Y.; Songa, M. A Fenton-like system of biochar loading Fe–Al layered double hydroxides (FeAl-LDH@BC)/H2O2 for phenol removal. Chemosphere 2021, 266, 128992. [Google Scholar] [CrossRef]
- Rubeena, K.; Reddy, P.H.P.; Laiju, A.; Nidheesh, P. Iron impregnated biochars as heterogeneous Fenton catalyst for the degradation of acid red 1 dye. J. Environ. Manag. 2018, 226, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.-J.; Chen, T.-C.; Young, W.-L. Competitive Removal of Two Contaminants in a Goethite-Catalyzed Fenton Process at Neutral pH. Environ. Eng. Sci. 2013, 30, 47–52. [Google Scholar] [CrossRef]
- Pradhan, G.K.; Sahu, N.; Parida, K.M. Fabrication of S, N co-doped α-Fe2O3 nanostructures: Effect of doping, OH radical formation, surface area, [110] plane and particle size on the photocatalytic activity. RSC Adv. 2013, 3, 7912–7920. [Google Scholar] [CrossRef]
- Jaramillo-Páez, C.; Navío, J.A.; Hidalgo, M.; Bouziani, A.; El Azzouzi, M. Mixed α-Fe2O3/Bi2WO6 oxides for photoassisted hetero-Fenton degradation of Methyl Orange and Phenol. J. Photochem. Photobiol. A Chem. 2017, 332, 521–533. [Google Scholar] [CrossRef]
- Nguyen, X.S.; Zhang, G.; Yang, X. Mesocrystalline Zn-Doped Fe3O4 Hollow Submicrospheres: Formation Mechanism and Enhanced Photo-Fenton Catalytic Performance. ACS Appl. Mater. Interfaces 2017, 9, 8900–8909. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Gómez, J.; Toncón-Leal, C.; Dos Santos, A.; Moreno, M.; Sapag, K.; Martínez-Huitle, C. Fe/SBA-15: Characterization and its application to a heterogeneous solar photo-Fenton process in order to decolorize and mineralize an azo dye. Mater. Lett. X 2020, 5, 100034. [Google Scholar] [CrossRef]
- Farhadian, N.; Liu, S.; Asadi, A.; Shahlaei, M.; Moradi, S. Enhanced heterogeneous Fenton oxidation of organic pollutant via Fe-containing mesoporous silica composites: A review. J. Mol. Liq. 2021, 321, 114896. [Google Scholar] [CrossRef]
- Sashkina, K.; Parkhomchuk, E.; Rudina, N.; Parmon, V. The role of zeolite Fe-ZSM-5 porous structure for heterogeneous Fenton catalyst activity and stability. Microporous Mesoporous Mater. 2014, 189, 181–188. [Google Scholar] [CrossRef]
- Sashkina, K.A.; Polukhin, A.V.; Labko, V.S.; Ayupov, A.B.; Lysikov, A.I.; Parkhomchuk, E.V. Fe-silicalites as heterogeneous Fenton-type catalysts for radiocobalt removal from EDTA chelates. Appl. Catal. B Environ. 2016, 185, 353–361. [Google Scholar] [CrossRef]
- Calleja, G.; Melero, J.; Martínez, F.; Molina, R. Activity and resistance of iron-containing amorphous, zeolitic and mesostructured materials for wet peroxide oxidation of phenol. Water Res. 2005, 39, 1741–1750. [Google Scholar] [CrossRef]
- Martínez, F.; Molina, R.; Pariente, M.I.; Siles, J.; Melero, J. Low-cost Fe/SiO2 catalysts for continuous Fenton processes. Catal. Today 2017, 280, 176–183. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dantas, T.; Mendonça, V.; José, H.; Rodrigues, A.; Moreira, R. Treatment of textile wastewater by heterogeneous Fenton process using a new composite Fe2O3/carbon. Chem. Eng. J. 2006, 118, 77–82. [Google Scholar] [CrossRef]
- Martins, R.C.; Lopes, R.J.; Quinta-Ferreira, R.M. Lumped kinetic models for single ozonation of phenolic effluents. Chem. Eng. J. 2010, 165, 678–685. [Google Scholar] [CrossRef]
- Covinich, L.G.; Felissia, F.; Massa, P.; Fenoglio, R.; Area, M.C. Kinetic modeling of a heterogeneous Fenton-type oxidative treatment of complex industrial effluent. Int. J. Ind. Chem. 2018, 9, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Gaya, U.I. Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Mitsika, E.E.; Christophoridis, C.; Fytianos, K. Fenton and Fenton-like oxidation of pesticide acetamiprid in water samples: Kinetic study of the degradation and optimization using response surface methodology. Chemosphere 2013, 93, 1818–1825. [Google Scholar] [CrossRef]
- Bae, S.; Kim, D.; Lee, W. Degradation of diclofenac by pyrite catalyzed Fenton oxidation. Appl. Catal. B Environ. 2013, 134–135, 93–102. [Google Scholar] [CrossRef]
- Sun, S.-P.; Lemley, A.T. p-Nitrophenol degradation by a heterogeneous Fenton-like reaction on nano-magnetite: Process optimization, kinetics, and degradation pathways. J. Mol. Catal. A Chem. 2011, 349, 71–79. [Google Scholar] [CrossRef]
- Teel, A.L.; Watts, R.J. Degradation of carbon tetrachloride by modified Fenton’s reagent. J. Hazard. Mater. 2002, 94, 179–189. [Google Scholar] [CrossRef]
- Xanthos, M. Interfacial agents for multiphase polymer systems: Recent advances. Polym. Eng. Sci. 1988, 28, 1392–1400. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol removal from industrial wastewaters: A short review. Desalination Water Treat. 2015, 53, 2215–2234. [Google Scholar] [CrossRef]
- Botas, J.; Melero, J.; Martínez, F.; Pariente, M.I. Assessment of Fe2O3/SiO2 catalysts for the continuous treatment of phenol aqueous solutions in a fixed bed reactor. Catal. Today 2010, 149, 334–340. [Google Scholar] [CrossRef]
- Horn, C.; Pospiech, D.; Allertz, P.J.; Müller, M.; Salchert, K.; Hommel, R. Chemical Design of Hydrogels with Immobilized Laccase for the Reduction of Persistent Trace Compounds in Wastewater. ACS Appl. Polym. Mater. 2021, 3, 2823–2834. [Google Scholar] [CrossRef]
- Solano, M.D.L.; Montagner, C.C.; Vaccari, C.; Jardim, W.F.; Anselmo-Franci, J.A.; Carolino, R.D.O.; Luvizutto, J.F.; De A Umbuzeiro, G.; De Camargo, J.L. Potential endocrine disruptor activity of drinking water samples. Endocr. Disruptors 2015, 3, e983384. [Google Scholar] [CrossRef] [Green Version]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Saal, F.S.V. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Igbinosa, E. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the marine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Crews, D.; Doan, L.L.; La Merrill, M.; Patisaul, H.; Zota, A. Introduction to Endocrine Disrupting Chemicals (EDCs) A Guide for Public Interest Organizations and Policy-Makers; Endocrine Society: Washington, DC, USA, 2014; pp. 1–76. [Google Scholar]
- Choubert, J.-M.; Ruel, S.M.; Esperanza, M.; Budzinski, H.; Miège, C.; Lagarrigue, C.; Coquery, M. Limiting the emissions of micro-pollutants: What efficiency can we expect from wastewater treatment plants? Water Sci. Technol. 2011, 63, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margot, J.; Maillard, J.; Rossi, L.; Barry, D.; Holliger, C. Influence of treatment conditions on the oxidation of micropollutants by Trametes versicolor laccase. New Biotechnol. 2013, 30, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Saracevic, E.; Kreuzinger, N. Adsorption of bisphenol-A, 17β-estradiole and 17α-ethinylestradiole to sewage sludge. Chemosphere 2004, 56, 843–851. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.; Wang, J.; Zhang, X.; Chen, C. Adsorption of chlorophenols from aqueous solutions by pristine and surface functionalized single-walled carbon nanotubes. J. Environ. Sci. 2016, 43, 187–198. [Google Scholar] [CrossRef]
- Wang, M.; Fang, G.; Liu, P.; Zhou, D.; Ma, C.; Zhang, D.; Zhan, J. Fe3O4@β-CD nanocomposite as heterogeneous Fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP). Appl. Catal. B Environ. 2016, 188, 113–122. [Google Scholar] [CrossRef]
- Huang, R.; Fang, Z.; Yan, X.; Cheng, W. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem. Eng. J. 2012, 197, 242–249. [Google Scholar] [CrossRef]





| Sample | Composition | Calcination | Iron Content (wt.%) (a) | Shape, Ø (mm) |
|---|---|---|---|---|
| Fe2O3 | Fe2O3 | - | - | Powder, 0.2 |
| F1 | Fe2O3/SiOx | 550 °C | 6.9 | Powder, 0.2 |
| F2 | Fe2O3/SiOx | 2 × 550 °C | 7.4 | Powder, 0.2 |
| Sample | Polymer | Catalyst | Composition Polymer/Catalyst (wt.%/wt.%) | Shape, Ø (mm) | Pore Diameter Ø (µm) (a) |
|---|---|---|---|---|---|
| PP-g-MA/F1 (80/20)-G | PP-g-MA | F1 | 80/20 | Granules, 3.1 | 3.1 |
| PP-g-MA/F1 (80/20)-C | PP-g-MA | F1 | 80/20 | Crushed, 0.9 | not det. |
| PP-g-MA/F1 (80/20) | PP-g-MA | F1 | 80/20 | Powder 0.2 | not det. |
| PP-g-MA/F2 (65/35) | PP-g-MA | F2 | 65/35 | Powder 0.2 | 3.2 |
| PP-g-MA/APTES/F2 (65/35) | PP-g-MA + APTES | F2 | 65/35 + 1.17 APTES | Powder 0.2 | 1.9 |
| PP-g-MA-g-PEO600/APTES/F2 (65/35) | PP-g-MA-g-PEO600 + APTES | F2 | 65/35 + 1.17 APTES | Powder 0.2 | 2.0 |
| PP-g-MA-g-PEO1000/APTES/F2 (65/35) | PP-g-MA-g-PEO1000 + APTES | F2 | 65/35 + 1.17 APTES | Powder 0.2 | 0.9 |
| Sample | SBET (m2 g−1) (a) | PV (cm3 g−1) (b) | MPV (cm3 g−1) (c) |
|---|---|---|---|
| F2O3 | 5 | <0.01 | n.a. |
| F1 | 272 | 0.46 | 0.10 |
| F2 | 264 | 0.69 | 0.10 |
| Sample | Conversion (%) (a,b) | Iron Leaching After 2 h (a,c) (mg/L) | Pseudo-Reaction Order (a) | Reaction Rate Coefficient (a) | R2 |
|---|---|---|---|---|---|
| Fe2O3 | 12 | 0.01 | First | 2.12 × 10−05 s−1 | 0.937 |
| F1 | 98 | 0.66 | First | 7.81 × 10−04 s−1 | 0.983 |
| F2 | 84 | 0.09 | Zeroth | 4.99 × 10−09 mol·L−1·s−1 | 0.999 |
| Sample | RB5 Conversion (%) (a,b) | Iron Leaching after 2 h (mg/L) (a,c) | Reaction Order (a) | Reaction Rate Coefficient (a) | R2 |
|---|---|---|---|---|---|
| Fe2O3 | 12 | 0.01 | First | 2.12 × 10−05 s−1 | 0.937 |
| F1 | 98 | 0.66 | First | 7.81 × 10−04 s−1 | 0.983 |
| F2 | 85 | 0.09 | Zeroth | 4.99 × 10−09 mol·L−1·s−1 | 0.999 |
| PP-g-MA/F1 (80/20)-G | 7 | 0.04 | Zeroth | 3.39 × 10−10 mol·L−1·s−1 | 0.885 |
| PP-g-MA/F1 (80/20)-C | 16 | 0.04 | Zeroth | 8.05 × 10−10 mol·L−1·s−1 | 0.961 |
| PP-g-MA/F1 (80/20) | 41 | 0.18 | Zeroth | 2.17 × 10−10 mol·L−1·s−1 | 0.973 |
| PP-g-MA/F2 (65/35) | 19 | n.d. | Zeroth | 7.37 × 10−10 mol·L−1·s−1 | 0.987 |
| PP-g-MA/APTES/F2 (65/35) | 5 | n.d. | Zeroth | 2.60 × 10−10 mol·L−1·s−1 | 0.978 |
| PP-g-MA-g-PEO600/APTES/F2 (65/35) | 17 | n.d. | Zeroth | 5.84 × 10−10 mol·L−1·s−1 | 0.959 |
| PP-g-MA-g-PEO1000/APTES/F2 (65/35) | 30 | 0.02 | Zeroth | 1.03 × 10−09 mol·L−1·s−1 | 0.915 |
| Sample | Conversion (%) (a,b) | Iron Leaching after 2 h (mg/L) (a,c) |
|---|---|---|
| F1 | 100 | 3.70 |
| F2 | 15 | 0.04 |
| PP-g-MA/F1 (80/20) | 44 | 0.19 |
| PP-g-MA/F2 (65/35) | 17 | 0.04 |
| PP-g-MA-g-PEO600/APTES/F2 (65/35) | 7 | 0.01 |
| PP-g-MA-g-PEO1000/APTES/F2 (65/35) | 12 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horn, C.; Ihmann, S.; Müller, F.; Pospiech, D.; Borchert, K.B.L.; Hommel, R.; Qin, K.; Licha, K.; Allertz, P.J.; Drache, M. Design of Polymer-Embedded Heterogeneous Fenton Catalysts for the Conversion of Organic Trace Compounds. Processes 2021, 9, 942. https://doi.org/10.3390/pr9060942
Horn C, Ihmann S, Müller F, Pospiech D, Borchert KBL, Hommel R, Qin K, Licha K, Allertz PJ, Drache M. Design of Polymer-Embedded Heterogeneous Fenton Catalysts for the Conversion of Organic Trace Compounds. Processes. 2021; 9(6):942. https://doi.org/10.3390/pr9060942
Chicago/Turabian StyleHorn, Christoph, Stephanie Ihmann, Felix Müller, Doris Pospiech, Konstantin B. L. Borchert, Rolf Hommel, Kaite Qin, Kai Licha, Peter J. Allertz, and Marco Drache. 2021. "Design of Polymer-Embedded Heterogeneous Fenton Catalysts for the Conversion of Organic Trace Compounds" Processes 9, no. 6: 942. https://doi.org/10.3390/pr9060942







