Synthesis and Characterization of Magnetic Xerogel Monolith as an Adsorbent for As(V) Removal from Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetic Xerogel Monoliths
2.3. Characterization of Magnetic Xerogel Monoliths
2.4. Batch Adsorption Experiments for As(V)
2.5. Desorption Study
3. Results
3.1. Behavior of Fe Contents in Magnetic Xerogel Monoliths on As(V) Removal
3.2. Characterization of Magnetic Xerogel Monoliths
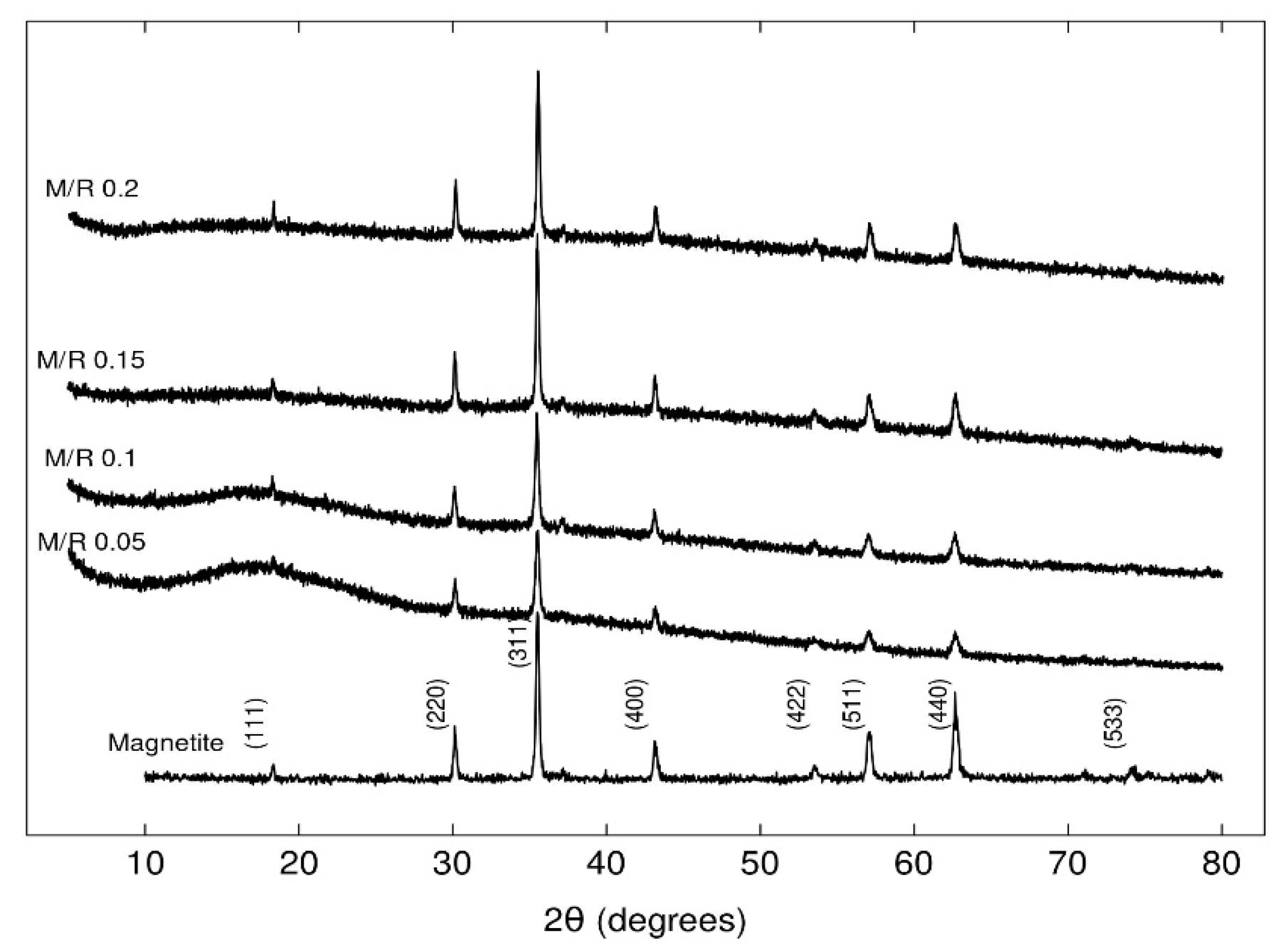
3.2.1. XRD Analysis
3.2.2. SEM Analysis
3.2.3. pHPZC and IEP Analysis
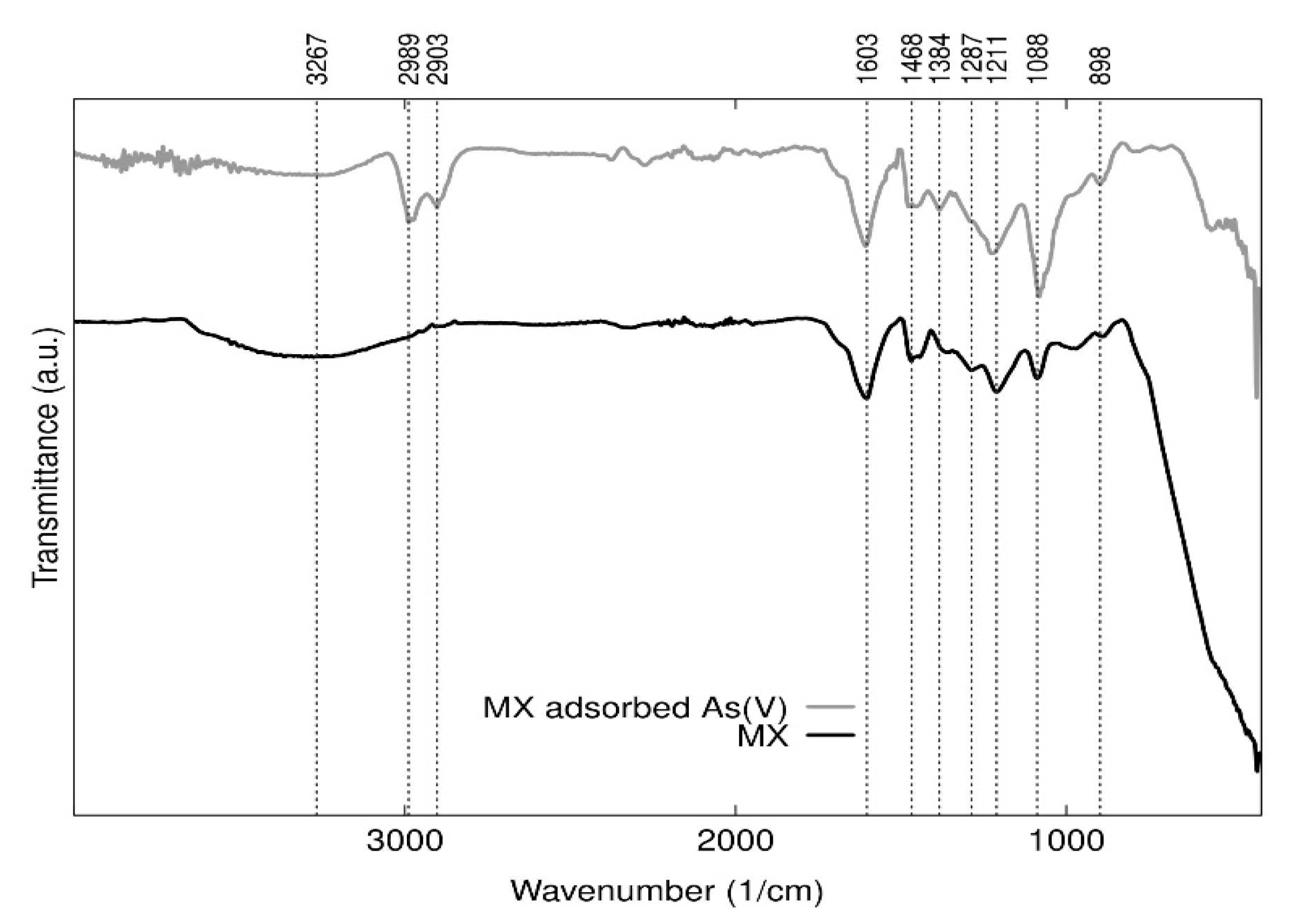
3.2.4. FTIR Analysis
3.3. Arsenic Adsorption Study
3.3.1. Effect of Initial pH on As(V) Removal
3.3.2. Effect of Adsorbent Dose on the Removal of As(V)
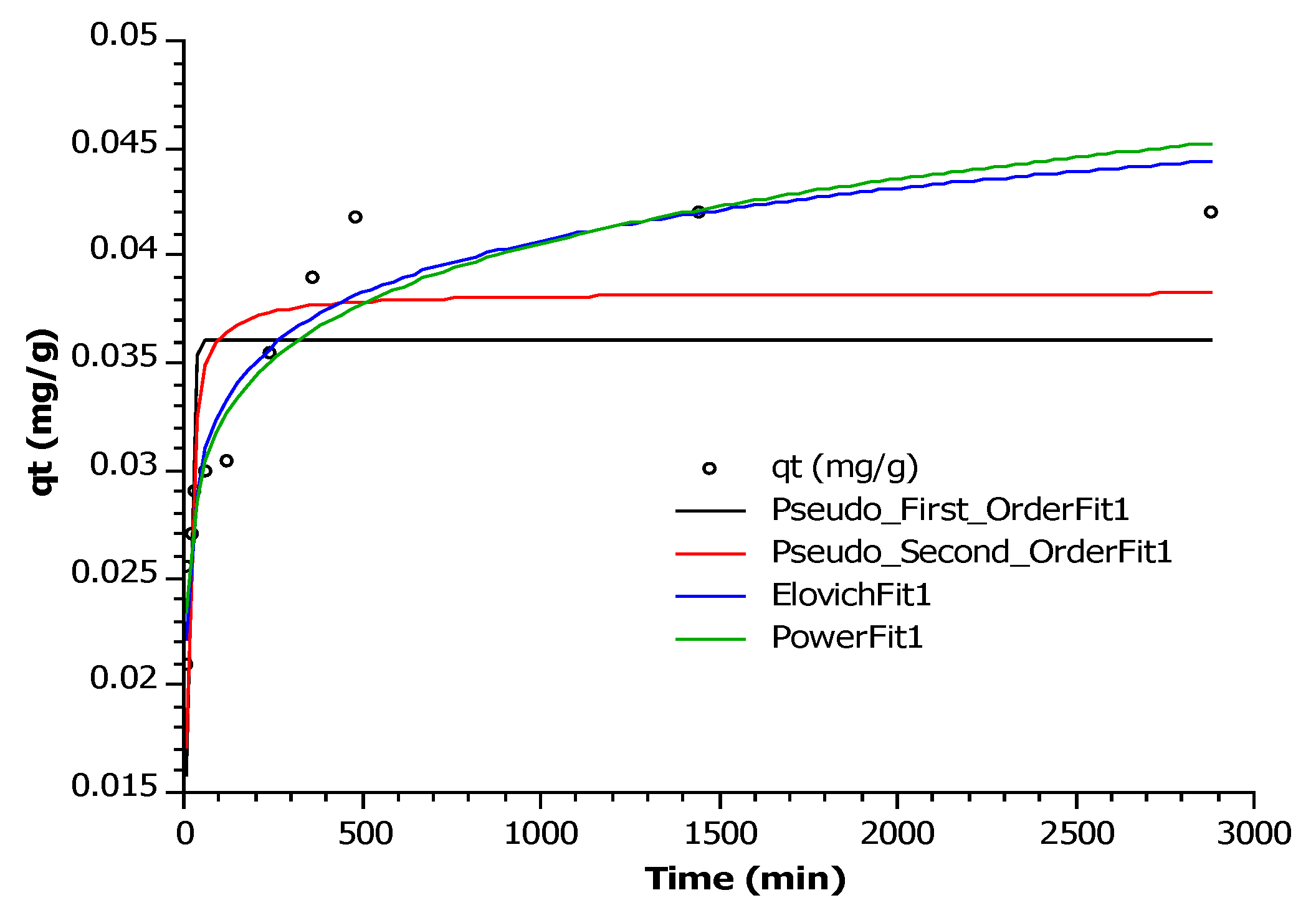
3.4. Kinetic Adsorption Models
3.5. Adsorption Isotherm
3.6. Adsorption Thermodynamics
3.7. Desorption Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic pollution sources. Rev. Environ. Contam. Toxicol. 2009, 197, 17–60. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Sen, M.; Manna, A.; Pal, J.; Pal, P.; Roy, S.; Roy, P. Contamination of groundwater by arsenic: A review of occurrence, causes, impacts, remedies and membrane-based purification. J. Integr. Environ. Sci. 2009, 6, 295–316. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U.; Shikha. Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Arbuckle, T.E.; Fisher, M.; Liang, C.L.; Davis, K.; Cirtiu, C.-M.; Bélanger, P.; Leblanc, A.; Fraser, W.D. Arsenic levels among pregnant women and newborns in Canada: Results from the Maternal-Infant Research on Environmental Chemicals (MIREC) cohort. Environ. Res. 2017, 153, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limón-Pacheco, J.H.; Jiménez-Córdova, M.I.; Cárdenas-González, M.; Retana, I.M.S.; Gonsebatt, M.E.; Del Razo, L.M. Potential co-exposure to arsenic and fluoride and biomonitoring equivalents for Mexican children. Ann. Glob. Health 2018, 84, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Hurtado-Jiménez, R.; Gardea-Torresdey, J.L. Arsenic in drinking water in the Los Altos de Jalisco region of Mexico. Rev. Panam. Salud Pública 2006, 20, 236–247. [Google Scholar] [CrossRef]
- Elcik, H.; Celik, S.O.; Cakmakci, M.; Özkaya, B. Performance of nanofiltration and reverse osmosis membranes for arsenic removal from drinking water. Desalination Water Treat. 2015, 57, 20422–20429. [Google Scholar] [CrossRef]
- Vithanage, M.; Dabrowska, B.B.; Mukherjee, A.B.; Sandhi, A.; Bhattacharya, P. Arsenic uptake by plants and possible phytoremediation applications: A brief overview. Environ. Chem. Lett. 2011, 10, 217–224. [Google Scholar] [CrossRef]
- Hoyos, S.E.G.; Flores, M.A.; Gonzalez, A.R.; Fajardo, C.G.; Zoloeta, S.C.; Orozco, H.V. Comparing two operating configurations in a full-scale arsenic removal plant. Case study: Guatemala. Water 2013, 5, 834–851. [Google Scholar] [CrossRef] [Green Version]
- Ozkula, G.; Urbano, B.F.; Rivas, B.L.; Kabay, N.; Bryjak, M. Arsenic sorption using mixtures of ion exchange resins containing n-methyl-d-glucamine and quaternary ammonium groups. J. Chil. Chem. Soc. 2016, 61, 2752–2756. [Google Scholar] [CrossRef] [Green Version]
- Urbano, B.F.; Rivas, B.L.; Martínez, F.; Alexandratos, S.D. Water-insoluble polymer–clay nanocomposite ion exchange resin based on N-methyl-d-glucamine ligand groups for arsenic removal. React. Funct. Polym. 2012, 72, 642–649. [Google Scholar] [CrossRef]
- Karimi, P.; Javanshir, S.; Sayadi, M.H.; Arabyarmohammadi, H. Arsenic removal from mining effluents using plant-mediated, green-synthesized iron nanoparticles. Processes 2019, 7, 759. [Google Scholar] [CrossRef] [Green Version]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. J. Environ. Health Sci. Eng. 2014, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. Adsorption Processes for Water Treatment and Purification, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319581361. [Google Scholar]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.; Ritter, J. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Khamkure, S.; Treesatayapun, C.; Garrido-Hoyos, S.E.; Gamero-Melo, P.; Reyes-Rosas, A. Prediction of the pH effect on arsenic (V) removal by varying catalyst of magnetic xerogel monoliths based on FREN model. Water Supply 2020, 20, 2747–2761. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J. The control of porosity at nano scale in resorcinol formaldehyde carbon aerogels. J. Mater. Sci. 2009, 44, 2705–2713. [Google Scholar] [CrossRef] [Green Version]
- Oyedoh, E.A.; Albadarin, A.B.; Walker, G.M.; Mirzaeian, M.; Ahmad, M.N.M. Preparation of controlled porosity resorcinol formaldehyde xerogels for adsorption applications. Chem. Eng. Trans. 2013, 32, 1651–1656. [Google Scholar] [CrossRef]
- Prostredný, M.; Abduljalil, M.G.M.; Mulheran, P.A.; Fletcher, A.J. Process variable optimization in the manufacture of resorcinol–formaldehyde gel materials. Gels 2018, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Castilla, C.; Maldonado-Hódar, A.F.J.; Pérez-Cadenas, A. Physicochemical surface properties of Fe, Co, Ni, and Cu-doped monolithic organic aerogels. Langmuir 2003, 19, 5650–5655. [Google Scholar] [CrossRef]
- Łuzny, R.; Ignasiak, M.; Walendziewski, J.; Stolarski, M. Heavy metal ions removal from aqueous solutions using carbon aerogels and xerogels. Chemik 2014, 68, 544–553. [Google Scholar]
- Huang, G.; Li, W.; Song, Y. Preparation of SiO2–ZrO2 xerogel and its application for the removal of organic dye. J. Sol-Gel Sci. Technol. 2018, 86, 175–186. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marin, F.; Regufe, M.J.; Ribeiro, A.M.; Ferreira, A.F.P.; Rodrigues, A.E. Resorcinol–formaldehyde carbon xerogel as selective adsorbent of carbon dioxide present on biogas. Adsorption 2018, 24, 169–177. [Google Scholar] [CrossRef]
- Haro, M.; Rasines, G.; Macias, C.; Ania, C. Stability of a carbon gel electrode when used for the electro-assisted removal of ions from brackish water. Carbon 2011, 49, 3723–3730. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.K.; Khare, P.; Verma, N. Synthesis of iron-doped resorcinol formaldehyde-based aerogels for the removal of Cr(VI) from water. Green Process. Synth. 2015, 4, 37–46. [Google Scholar] [CrossRef]
- Minovic-Arsic, T.; Kalijadis, A.; Matovic, B.; Stoiljkovic, M.; Pantic, J.; Jovanovic, J.; Petrovic, R.; Jokic, B.; Babic, B. Arsenic(III) adsorption from aqueous solutions on novel carbon cryogel/ceria nanocomposite. Process. Appl. Ceram. 2016, 10, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Hódar, F.J. Advances in the development of nanostructured catalysts based on carbon gels. Catal. Today 2013, 218–219, 43–50. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Venieri, D.; Antonopoulou, M.; Konstantinou, I.; Silva, A.M.; Faria, J.L.; Gomes, H.T. Magnetic carbon xerogels for the catalytic wet peroxide oxidation of sulfamethoxazole in environmentally relevant water matrices. Appl. Catal. B Environ. 2016, 199, 170–186. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Jirglová, H.; Pastrana-Martínez, L.M.; Maldonado-Hódar, F.J. Influence of electrostatic interactions during the resorcinol-formaldehyde polymerization on the characteristics of mo-doped carbon gels. Processes 2020, 8, 746. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Perdigoto, M.; Martins, R.; Rocha, N.; Quina, M.; Gando-Ferreira, L.; Patrício, R.; Durães, L. Application of hydrophobic silica based aerogels and xerogels for removal of toxic organic compounds from aqueous solutions. J. Colloid Interface Sci. 2012, 380, 134–140. [Google Scholar] [CrossRef]
- Fathy, N.A.; Rizk, M.S.; Awad, R.M. Pore structure and adsorption properties of carbon xerogels derived from carbonization of tannic acid-resorcinol-formaldehyde resin. J. Anal. Appl. Pyrolysis 2016, 119, 60–68. [Google Scholar] [CrossRef]
- Gore, P.; Khraisheh, M.; Kandasubramanian, B. Nanofibers of resorcinol–formaldehyde for effective adsorption of As (III) ions from mimicked effluents. Environ. Sci. Pollut. Res. 2018, 25, 11729–11745. [Google Scholar] [CrossRef] [PubMed]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.-P. Synthesis of transition metal-doped carbon xerogels by solubilization of metal salts in resorcinol–formaldehyde aqueous solution. Carbon 2004, 42, 3217–3227. [Google Scholar] [CrossRef]
- Amaringo, F.A.; Anaguano, A. Determinación del punto de carga cero y punto isoeléctrico de dos residuos agrícolas y su aplicación en la remoción de colorantes. Rev. Investig. Agrar. Ambient. 2013, 4, 27–36. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.D.C.A.; Carrillo-González, R.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.D.C.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Van Dang, S.; Kawasaki, J.; Abella, L.C.; Auresenia, J.; Habaki, H.; Gaspillo, P.-A.D.; Kosuge, H.; Doan, H.T. Removal of arsenic from simulated groundwater by adsorption using iron-modified rice husk carbon. J. Water Environ. Technol. 2009, 7, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L. Arsenic adsorption from aqueous solutions on an Fe(III)-Si binary oxide adsorbent. Water Qual. Res. J. 2004, 39, 267–275. [Google Scholar] [CrossRef]
- Hanbali, M.; Holail, H.; Hammud, H. Remediation of lead by pretreated red algae: Adsorption isotherm, kinetic, column modeling and simulation studies. Green Chem. Lett. Rev. 2014, 7, 342–358. [Google Scholar] [CrossRef]
- Tipsawat, P.; Wongpratat, U.; Phumying, S.; Chanlek, N.; Chokprasombat, K.; Maensiri, S. Magnetite (Fe3O4) nanoparticles: Synthesis, characterization and electrochemical properties. Appl. Surf. Sci. 2018, 446, 287–292. [Google Scholar] [CrossRef]
- Attia, S.M.; Abdelfatah, M.S.; Mossad, M.M. Conduction mechanism and dielectric properties of pure and composite resorcinol formaldehyde aerogels doped with silver. J. Phys. Conf. Ser. 2017, 869, 12035. [Google Scholar] [CrossRef] [Green Version]
- Morales-Torres, S.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.; Carrasco-Marín, F. Textural and mechanical characteristics of carbon aerogels synthesized by polymerization of resorcinol and formaldehyde using alkali carbonates as basification agents. Phys. Chem. Chem. Phys. 2010, 12, 10365–10372. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Veličković, Z.; Rusmirović, J.; Rančić, M.; Pavlović, V.; Marinković, A. Arsenic removal by magnetite-loaded amino modified nano/microcellulose adsorbents: Effect of functionalization and media size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef] [Green Version]
- Myneni, S.C.; Traina, S.J.; Waychunas, G.A.; Logan, T.J. Vibrational spectroscopy of functional group chemistry and arsenate coordination in ettringite. Geochim. Cosmochim. Acta 1998, 62, 3499–3514. [Google Scholar] [CrossRef]
- Yu, X.; Wei, Y.; Liu, C.; Ma, J.; Liu, H.; Wei, S.; Deng, W.; Xiang, J.; Luo, S. Ultrafast and deep removal of arsenic in high-concentration wastewater: A superior bulk adsorbent of porous Fe2O3 nanocubes-impregnated graphene aerogel. Chemosphere 2019, 222, 258–266. [Google Scholar] [CrossRef]
- Mandal, S.; Sahu, M.K.; Patel, R.K. Adsorption studies of arsenic(III) removal from water by zirconium polyacrylamide hybrid material (ZrPACM-43). Water Resour. Ind. 2013, 4, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Khamkure, S.; Treesatayapun, C.; Garrido-Hoyos, S.E.; Gamero-Melo, P.; Reyes-Rosas, A. Textural properties of Magnetic Xerogel monoliths and its Prediction of the Effect of pH on Arsenic (V) adsorption. In Proceedings of the 2019 International Conference on Engineering, Science, and Industrial Applications, ICESI 2019, Tokyo, Japan, 22–24 August 2019; pp. 111–116. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, K.; Wu, Y.; Zou, Y.; Zuo, J.; Arriagada, D.C.; Pan, Z.; Hu, G. The effect of pH on the adsorption of arsenic(III) and arsenic(V) at the TiO2 anatase [101] surface. J. Colloid Interface Sci. 2016, 462, 252–259. [Google Scholar] [CrossRef]
- Zhang, J.; Stanforth, R. Slow adsorption reaction between arsenic species and goethite (α-FeOOH): Diffusion or heterogeneous surface reaction control. Langmuir 2005, 21, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Yean, S.; Cong, L.; Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Kan, A.T.; Colvin, V.L.; Tomson, M.B. Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. J. Mater. Res. 2005, 20, 3255–3264. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and equilibrium models of adsorption. Carbon Nanostruct. 2015, 33–69. [Google Scholar] [CrossRef]
- Malekian, R.; Abedi-Koupai, J.; Eslamian, S.S.; Mousavi, S.F.; Abbaspour, K.C.; Afyuni, M. Ion-exchange process for ammonium removal and release using natural Iranian zeolite. Appl. Clay Sci. 2011, 51, 323–329. [Google Scholar] [CrossRef]






| Parameters | pH | TDSa | Cl- | Fl- | As | Mn | Fe | ||
|---|---|---|---|---|---|---|---|---|---|
| Analysis results | 7.0 ± 0.5 | 173 ± 4 | 10.4 ± 0.5 | 0.25 ± 0.01 | 0 | 0.01 ± 0 | 0.11 ± 0.09 | 4.83 ± 0.84 | 34 ± 2.64 |
| NOM-127-SSA1-1994 | 6.5–8.5 | 1000 | 250 | 1.5 | 0.025 | 0.15 | 0.3 | 10 | 400 |
| Pseudo first-order equation | k1 | qt (µg/g) | R2 | RMSE | RSS |
| 0.1152 | 36.12 | 0.5043 | 5.50 × 10−3 | 2.72 × 10−4 | |
| Pseudo second-order equation | k2 | qt (µg/g) | R2 | RMSE | RSS |
| 4.1868 | 38.32 | 0.7477 | 3.92 × 10−3 | 1.38 × 10−4 | |
| Elovich equation | α(mg/g min) | β (g/mg) | R2 | RMSE | RSS |
| 0.38 | 284.6 | 0.9391 | 1.93 × 10−3 | 3.34 × 10−5 | |
| Power function equation | a | b | R2 | RMSE | RSS |
| 0.0199 | 0.1032 | 0.9171 | 2.25 × 10−3 | 4.55 × 10−5 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qm (µg/g) | b (L/mg) | R2 | K | n | R2 |
| 62.82 | 135.6 | 0.9404 | 0.1409 | 3.25 | 0.9528 |
| Temperature (K) | DG (KJ/mol) | DH (KJ/mol) | DS (J/mol) |
|---|---|---|---|
| 298 | −6.051 | 17.949 | 79.902 |
| 308 | −6.255 | ||
| 318 | −7.676 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamkure, S.; Garrido-Hoyos, S.E.; Gamero-Melo, P.; Reyes-Rosas, A. Synthesis and Characterization of Magnetic Xerogel Monolith as an Adsorbent for As(V) Removal from Groundwater. Processes 2021, 9, 386. https://doi.org/10.3390/pr9020386
Khamkure S, Garrido-Hoyos SE, Gamero-Melo P, Reyes-Rosas A. Synthesis and Characterization of Magnetic Xerogel Monolith as an Adsorbent for As(V) Removal from Groundwater. Processes. 2021; 9(2):386. https://doi.org/10.3390/pr9020386
Chicago/Turabian StyleKhamkure, Sasirot, Sofía Esperanza Garrido-Hoyos, Prócoro Gamero-Melo, and Audberto Reyes-Rosas. 2021. "Synthesis and Characterization of Magnetic Xerogel Monolith as an Adsorbent for As(V) Removal from Groundwater" Processes 9, no. 2: 386. https://doi.org/10.3390/pr9020386






