A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals
Abstract
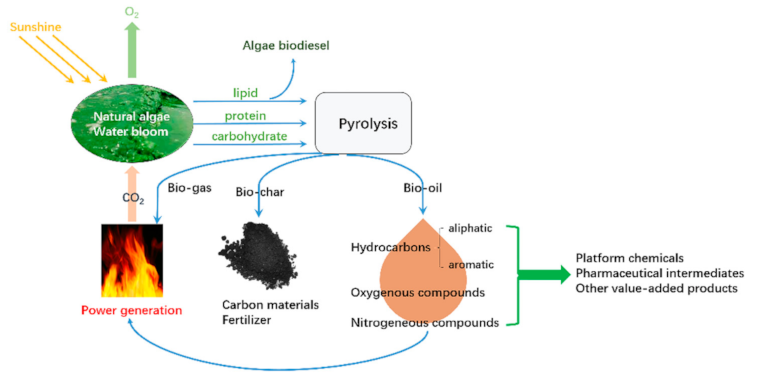
:1. Introduction
2. Chemical Composition of the Natural Algae
3. Pyrolysis Process of Natural Algae
3.1. Non-Catalytic Pyrolysis Process
3.1.1. Effects of Pyrolysis Temperature
3.1.2. Effects of Heating Rates
3.1.3. Effects of Sweep Gases
3.2. Catalytic Pyrolysis Process
3.2.1. Performance of Zeolite Catalysts
3.2.2. Performance of Catalysts Other Than Zeolites
3.3. Non-Catalytic Pyrolysis Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, X.; Lu, S.; Hu, X.; Jiang, X.; Wu, F. Control concept and countermeasures for shallow lakes’ eutrophication in China. Front. Environ. Sci. Eng. China 2008, 2, 257–266. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef]
- Jain, P.; Arora, N.; Mehtani, J.; Pruthi, V.; Majumder, C.B. Pretreated algal bloom as a substantial nutrient source for microalgae cultivation for biodiesel production. Bioresour. Technol. 2017, 242, 152–160. [Google Scholar] [CrossRef]
- Sarsekeyeva, F.; Zayadan, B.K.; Usserbaeva, A.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 2015, 125, 329–340. [Google Scholar] [CrossRef]
- Wei, L.Z.; Li, X.; Yi, J.; Yang, Z.; Wang, Q.X.; Ma, W.M. A simple approach for the efficient production of hydrogen from Taihu Lake Microcystis spp. blooms. Bioresour. Technol. 2013, 139, 136–140. [Google Scholar] [CrossRef]
- Benvenuti, G.; Bosma, R.; Cuaresma, M.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J. Appl. Phycol. 2015, 27, 1425–1431. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Ahmad, S.F.K.; Ali, U.F.M.; Isa, K.M. Compilation of liquefaction and pyrolysis method used for bio-oil production from various biomass: A review. Environ. Eng. Res. 2019, 25, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Silva, L.; López-González, D.; García-Minguillán, A.M.; Valverde, J.L. Pyrolysis, combustion and gasification characteristics of Nannochloropsis gaditana microalgae. Bioresour. Technol. 2013, 130, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Wei, C.; Ning, P.; Tian, S.; Gu, J. Catalytic Gasification of Algae Nannochloropsis sp. in Sub/Supercritical Water. Procedia Environ. Sci. 2013, 18, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Hu, Z.; Xiao, B. Bio-oil Production by Thermochemical Catalytic Liquefaction of Bloom-Forming Cyanobacteria: Optimization Using Response Surface Methodology (RSM). Energy Fuels 2017, 31, 13733–13742. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.C.; Batchelor, W.; Bhattacharya, S. A study on growth and pyrolysis characteristics of microalgae using Thermogravimetric Analysis-Infrared Spectroscopy and synchrotron Fourier Transform Infrared Spectroscopy. Bioresour. Technol. 2017, 229, 1–10. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.C.; Bhattacharya, S. A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygeneous and nitrogenous compounds. Renew. Sustain. Energy Rev. 2019, 108, 481–497. [Google Scholar] [CrossRef]
- Zainan, N.H.; Srivatsa, S.C.; Bhattacharya, S. Catalytic pyrolysis of microalgae Tetraselmis suecica and characterization study using in situ Synchrotron-based Infrared Microscopy. Fuel 2015, 161, 345–354. [Google Scholar] [CrossRef]
- Casoni, A.I.; Zunino, J.; Piccolo, M.C.; Volpe, M.A. Valorization of Rhizoclonium sp. algae via pyrolysis and catalytic pyrolysis. Bioresour. Technol. 2016, 216, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Chagas, B.M.E.; Mullen, C.A.; Dorado, C.; Elkasabi, Y.; Boateng, A.A.; Melo, M.A.F.; Ataíde, C.H. Stable Bio-oil Production from Proteinaceous Cyanobacteria: Tail Gas Reactive Pyrolysis of Spirulina. Ind. Eng. Chem. Res. 2016, 55, 6734–6741. [Google Scholar] [CrossRef]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Campanella, A.; Harold, M.P. Fast pyrolysis of microalgae in a falling solids reactor: Effects of process variables and zeolite catalysts. Biomass Bioenergy 2012, 46, 218–232. [Google Scholar] [CrossRef]
- Gao, L.; Sun, J.; Xu, W.; Xiao, G. Catalytic pyrolysis of natural algae over Mg-Al layered double oxides/ZSM-5 (MgAl-LDO/ZSM-5) for producing bio-oil with low nitrogen content. Bioresour. Technol. 2017, 225, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Loe, R.; Santillan-Jimenez, E.; Morgan, T.; Sewell, L.; Ji, Y.; Jones, S.; Isaacs, M.A.; Lee, A.F.; Crocker, M. Effect of Cu and Sn promotion on the catalytic deoxygenation of model and algal lipids to fuel-like hydrocarbons over supported Ni catalysts. Appl. Catal. B Environ. 2016, 191, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Gao, L.; Bai, Q.; Xu, S.; Pan, D.; Wu, Y.; Xiao, G. Nitrogenous compounds produced by catalytic pyrolysis of cyanobacteria over metal loaded MCM-41 with vaporized methanol. New J. Chem. 2019, 43, 6569–6576. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Xiao, G.; Liu, F. Blooming-forming cyanobacteria pyrolysis over Ni-Al layered double oxides/MCM-41 for nitriles under nitrogen and methanol atmosphere. Biomass Convers. Biorefinery 2019, 10, 1063–1070. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, Y.; Fu, Y. Sustainable Production of Acetonitrile from Microalgae via Catalytic Fast Pyrolysis with Ammonia over Ga/HZSM-5 Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 16173–16181. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Zhang, R.; Tong, D.; Hu, C. Fractional pyrolysis of Cyanobacteria from water blooms over HZSM-5 for high quality bio-oil production. J. Energy Chem. 2014, 23, 732–741. [Google Scholar] [CrossRef]
- Maddi, B.; Viamajala, S.; Varanasi, S. Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresour. Technol. 2011, 102, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zheng, Y.; Hu, Z.-Q.; Xiao, B.; Cai, H.-Y.; He, P.-W.; Wang, J.-B. Kinetic Study on Pyrolysis of Blooming-forming Cyanobacteria. Energy Sources, Part A Recover. Util. Environ. Eff. 2015, 37, 625–632. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, B.; Zhu, L.; Tong, D.; Hu, C. Catalytic pyrolysis of natural algae from water blooms over nickel phosphide for high quality bio-oil production. RSC Adv. 2013, 3, 10806–10816. [Google Scholar] [CrossRef]
- Liu, G.; Wright, M.M.; Zhao, Q.; Brown, R.C.; Wang, K.; Xue, Y. Catalytic pyrolysis of amino acids: Comparison of aliphatic amino acid and cyclic amino acid. Energy Convers. Manag. 2016, 112, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Tahmasebi, A.; Maliutina, K.; Yu, J.L. Formation of nitrogen-containing compounds during microwave pyrolysis of microalgae: Product distribution and reaction pathways. Bioresour. Technol. 2017, 245, 1067–1074. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Zhang, R.; Hu, C. Fractional conversion of microalgae from water blooms. Faraday Discuss. 2017, 202, 197–212. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, Y.; Yan, F.; Xiao, B.; Liu, S. Bio-oil production through pyrolysis of blue-green algae blooms (BGAB): Product distribution and bio-oil characterization. Energy 2013, 52, 119–125. [Google Scholar] [CrossRef]
- Hu, M.; Chen, Z.; Guo, D.; Liu, C.; Xiao, B.; Hu, Z.; Liu, S. Thermogravimetric study on pyrolysis kinetics of Chlorella pyrenoidosa and bloom-forming cyanobacteria. Bioresour. Technol. 2014, 177, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Amin, N.S. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Li, R.; Zhong, Z.; Jin, B.; Zheng, A. Selection of Temperature for Bio-oil Production from Pyrolysis of Algae from Lake Blooms. Energy Fuels 2012, 26, 2996–3002. [Google Scholar] [CrossRef]
- Demirbaş, A. Oily Products from Mosses and Algae via Pyrolysis. Energy Sources Part A: Recover. Util. Environ. Eff. 2006, 28, 933–940. [Google Scholar] [CrossRef]
- Strezov, V.; Moghtaderi, B.; Lucas, J.A. Thermal study of decomposition of selected biomass samples. J. Therm. Anal. Calorim. 2003, 72, 1041–1048. [Google Scholar] [CrossRef]
- Supeng, L.; Guirong, B.; Hua, W.; Fashe, L.; Yizhe, L. TG-DSC-FTIR Analysis of Cyanobacteria Pyrolysis. Phys. Procedia 2012, 33, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Chen, J.J.; Liu, D.X.; Ning, X.N.; Liu, J.Y.; Wang, T.J. Consequence of replacing nitrogen with carbon dioxide as atmosphere on suppressing the formation of polycyclic aromatic hydrocarbons in catalytic pyrolysis of sawdust. Bioresour. Technol. 2019, 297, 122417. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Kim, K.-H.; Jeon, Y.J.; Kwon, E.E. Pyrolysis of microalgal biomass in carbon dioxide environment. Bioresour. Technol. 2015, 193, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-M.; Lee, J.; Kim, J.; Kim, K.-H.; Kim, H.-W.; Jeon, Y.J.; Kwon, E.E. Enhanced thermal destruction of toxic microalgal biomass by using CO2. Sci. Total. Environ. 2016, 566–567, 575–583. [Google Scholar] [CrossRef]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Chattanathan, S.A.; Gupta, R.B. Catalytic pyrolysis of green algae for hydrocarbon production using H+ZSM-5 catalyst. Bioresour. Technol. 2012, 118, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, X.; Shoemaker, C.A.; Wang, C.-H. Experimental and numerical study of biomass catalytic pyrolysis using Ni2P-loaded zeolite: Product distribution, characterization and overall benefit. Energy Convers. Manag. 2020, 208, 112581. [Google Scholar] [CrossRef]
- Bai, Q.; Gao, L.; Sun, J.; Xu, W.; Wei, R.; Xiao, G. Cyanobacteria pyrolysis with methanol catalyzed by Mg-Al hydrotalcite-derived oxides/ZSM-5. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 1273–1278. [Google Scholar] [CrossRef]
- Ansah, E.; Wang, L.; Zhang, B.; Shahbazi, A. Catalytic pyrolysis of raw and hydrothermally carbonized Chlamydomonas debaryana microalgae for denitrogenation and production of aromatic hydrocarbons. Fuel 2018, 228, 234–242. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Jeon, J.-K.; Kim, J.; Ryoo, R.; Jeong, K.-E.; Park, Y.-K. Highly valuable chemicals production from catalytic upgrading of radiata pine sawdust-derived pyrolytic vapors over mesoporous MFI zeolites. Appl. Catal. B Environ. 2010, 95, 365–373. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef]
- Anand, V.; Sunjeev, V.; Vinu, R. Catalytic fast pyrolysis of Arthrospira platensis (spirulina) algae using zeolites. J. Anal. Appl. Pyrolysis 2016, 118, 298–307. [Google Scholar] [CrossRef]
- Du, Z.; Ma, X.; Li, Y.; Chen, P.; Liu, Y.; Lin, X.; Lei, H.; Ruan, R. Production of aromatic hydrocarbons by catalytic pyrolysis of microalgae with zeolites: Catalyst screening in a pyroprobe. Bioresour. Technol. 2013, 139, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Brown, R.C. Catalytic pyrolysis of microalgae for production of aromatics and ammonia. Green Chem. 2013, 15, 675–681. [Google Scholar] [CrossRef]
- Jazrawi, C.; Biller, P.; He, Y.; Montoya, A.; Ross, A.B.; Maschmeyer, T.; Haynes, B.S. Two-stage hydrothermal liquefaction of a high-protein microalga. Algal Res. 2015, 8, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yang, J.; Shi, M. Review of Denitrogenation of Algae Biocrude Produced by Hydrothermal Liquefaction. Trans. Tianjin Univ. 2017, 23, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Lorenzetti, C.; Conti, R.; Fabbri, D.; Yanik, J. A comparative study on the catalytic effect of H-ZSM5 on upgrading of pyrolysis vapors derived from lignocellulosic and proteinaceous biomass. Fuel 2016, 166, 446–452. [Google Scholar] [CrossRef]
- Hanifzadeh, M.M.; Sarrafzadeh, M.H.; Tavakoli, O. Carbon dioxide biofixation and biomass production from flue gas of power plant using microalgae. In Proceedings of the 2012 Second Iranian Conference on Renewable Energy and Distributed Generation, Tehran, Iran, 6–8 March 2012; pp. 61–64. [Google Scholar]
- Sabegh, M.Y.; Norouzi, O.; Jafarian, S.; Khosh, A.G.; Tavasoli, A. Pyrolysis of marine biomass to produce bio-oil and its upgrading using a novel multi-metal catalyst prepared from the spent car catalytic converter. Bioresour. Technol. 2018, 249, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, B.; Liu, X.; Xu, L.; Hu, Y.; Afonaa-Mensah, S.; Abomohra, A.E.-F.; He, Z.; Wang, Q.; Xu, S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour. Technol. 2018, 256, 446–455. [Google Scholar] [CrossRef]
- Wang, S.; Cao, B.; Feng, Y.; Sun, C.; Wang, Q.; Abomohra, A.E.-F.; Afonaa-Mensah, S.; He, Z.; Zhang, B.; Qian, L.; et al. Co-pyrolysis and catalytic co-pyrolysis of Enteromorpha clathrata and rice husk. J. Therm. Anal. Calorim. 2018, 135, 2613–2623. [Google Scholar] [CrossRef]
- Zhang, R.; Li, L.; Tong, D.; Hu, C. Microwave-enhanced pyrolysis of natural algae from water blooms. Bioresour. Technol. 2016, 212, 311–317. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Ji, G.; Lei, H.; Qu, H.; Chen, J.; Wang, F.; Liu, J. Renewable production of nitrogen-containing compounds and hydrocarbons from catalytic microwave-assisted pyrolysis of chlorella over metal-doped HZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2020, 151, 104902. [Google Scholar] [CrossRef]
| Proximate Analysis (wt %) | Ultimate Analysis (wt %) | HHV 2 (MJ/kg) | Chemical Composition (wt %) | Source of the Algae | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | O | N | S | Lipid | Protein | Carbohydrate | |||
| 3.38 | 5.62 | 75.04 | 15.96 | 46.37 | 6.73 | 37.39 | 8.71 | 0.8 | 19.77 | 5.12 | 60.02 | 21.08 | Dianchi Lake, China | [11] |
| 2.5 | 12.9 | - 3 | - | 43.7 | 6.4 | 28.9 | 8.1 | - | 18.3 | 8.4 | 28.9 | 15.4 | Taihu Lake, China | [24] |
| 9.78 | 12.12 | 53.94 | 24.16 | 42.26 | 6.27 | 43.07 | 7.88 | 0.52 | 16.0 | - | - | - | Dianchi Lake, China | [26] |
| 11.2 | 16.5 | 75.5 | 8.0 | 38.1 | 5.9 | 51.8 | 4.2 | - | - | - | - | - | Puan shallow Lake, Argentina | [15] |
| 3.5 | 28.2 | - | - | 43.3 | 6.2 | - | 8.7 | - | 18.3 | 13.6 | - | 16.0 | Taihu Lake, China | [27] |
| 2.4 | 25.7 | 55.6 | 16.3 | 37.3 | 5.8 | 26.2 | 4.2 | - | 15.6 | 1.39 | 29.9 | 13.25 | Lake Erie, USA 4 | [25] |
| 5.91 | 13.3 | 64.1 | 16.7 | 38.9 | 6.1 | 38.1 | 2.7 | - | 15.8 | 5.8 | 24.6 | 24.8 | ||
| Species | Catalyst | Conditions | Elemental Composition (wt%) | HHV 1 (MJ/kg) | Reference | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | |||||
| Blue-green algae blooms | None | final pyrolysis temperature: 500 °C, particle size: <0.25 mm, N2 flow rate: 100 mL/min, heating rate: 40 °C/min | 67.58 | 8.95 | 7.75 | 14.48 | 31.9 | [32] |
| Algae from lake blooms | None | final pyrolysis temperature: 700 °C, particle size: <0.18 mm, N2 flow rate: 300 mL/min | 61.87 | 8.07 | 3.7 | 26.36 | 21.57 | [36] |
| Cyanobacteria from lake blooms | HZSM-5 (mass ratio to algae: 0.5:1) | final pyrolysis temperature: 400 °C, particle size: <0.42mm, N2 flow rate: 50 mL/min | 73.6 | 8.3 | 7.4 | 10.7 | 36.6 | [24] |
| Algal biomass (C. vulgaris) | H+ZSM-5 (mass ratio to algae: 1:1) | final pyrolysis temperature: 800 °C, N2 flow rate: 30 mL/min | 51.4 ± 2.3 | 10.4 ± 0.4 | 12.4 ± 1.1 | 24.8 ± 1.6 | 18.6 ± 0.3 | [45] |
| Algae (mainly cyanobacteria), | silica-supported nickel phosphide(mass ratio to algae: 1:1) | final pyrolysis temperature: 450 °C, H2 flow rate: 100 mL/min | 69.2 | 9.1 | 10.8 | 10.8 | 35.0 | [27] |
| Natural algae (mainly cyanobacteria) | MgAl4-LDO/ZSM-5 (mass ratio to algae: 0.75:1) | final pyrolysis temperature: 550 °C, N2 flow rate: 40 mL/min | 71.19 | 9.683 | 10.597 | 8.530 | 37.164 | [19] |
| Algae bloom | Ni2P loaded zeolite (mass ratio to bi0mass: 0.8:1) | co-pyrolysis with water hyacinth (0.4:1), final pyrolysis temperature: 450 °C, N2 flow rate: 100 mL/min, | - 2 | - | - | - | 32.00 ± 0.08 | [46] |
| Cyanobacteria | MgAl3-LDO/ZSM-5 (mass ratio to algae: 0.75:1) | final pyrolysis temperature: 550 °C, vaporized methanol flow rate: 66 mL/min, heating rate: 10 °C/min | - | - | - | - | 37.47 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Ding, K.; Hu, L. A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals. Processes 2021, 9, 2042. https://doi.org/10.3390/pr9112042
Xu W, Ding K, Hu L. A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals. Processes. 2021; 9(11):2042. https://doi.org/10.3390/pr9112042
Chicago/Turabian StyleXu, Wei, Keqiang Ding, and Lihua Hu. 2021. "A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals" Processes 9, no. 11: 2042. https://doi.org/10.3390/pr9112042






