Development of a Quantitative Method for Analysis of Compounds Found in Mondia whitei Using HPLC-DAD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Preparation of the Stock Solutions
2.4. Extraction of Compounds from the Samples
2.5. Optimisation of the HPLC-DAD Method
2.6. Method Validation
2.6.1. Preparation of Standards for External Calibration Method
2.6.2. Preparation of Standards for the Standard-Addition Method
2.6.3. Limit of Detection and Limit of Quantification
2.6.4. Method Precision
2.6.5. Accuracy and Recovery
2.6.6. Measurement Uncertainty
2.7. Quantification of the Compounds of Interest in Real Mondia whitei Samples
3. Results
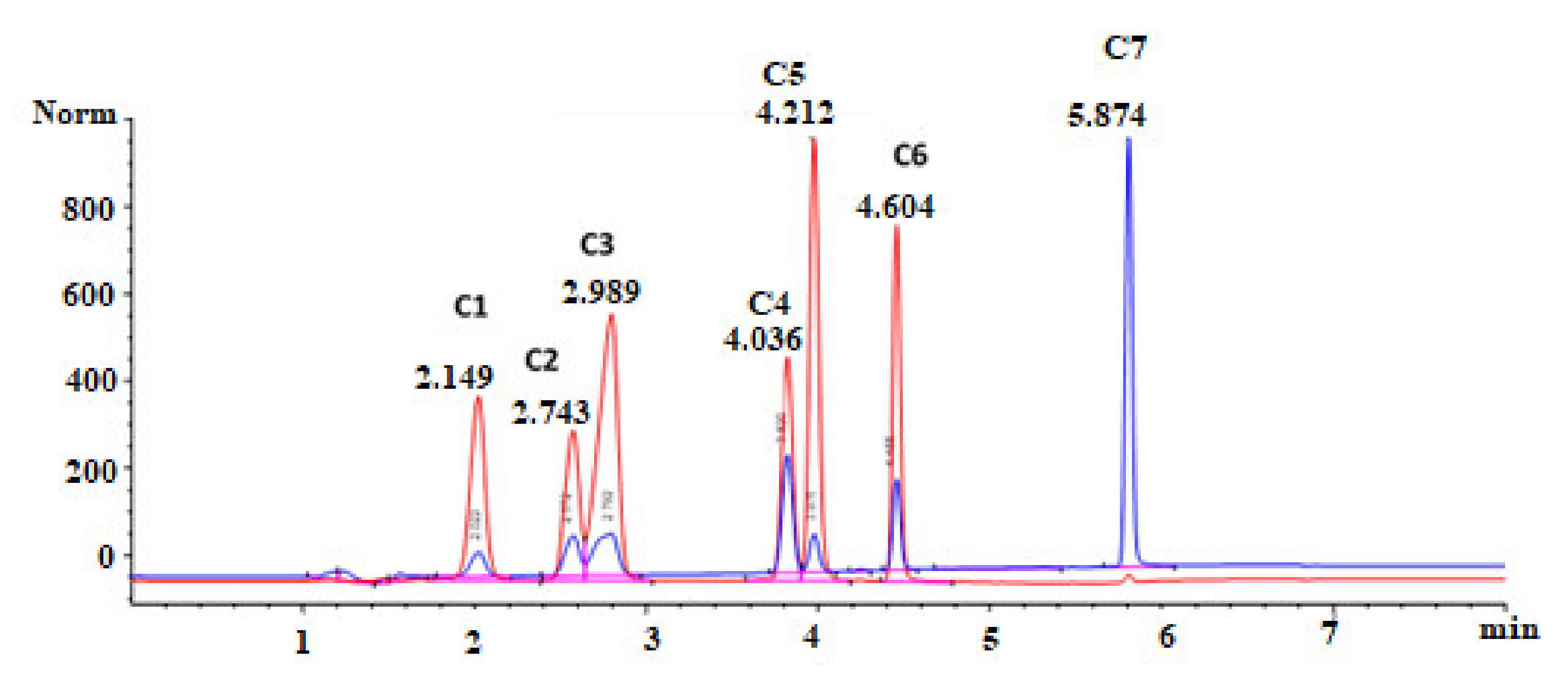
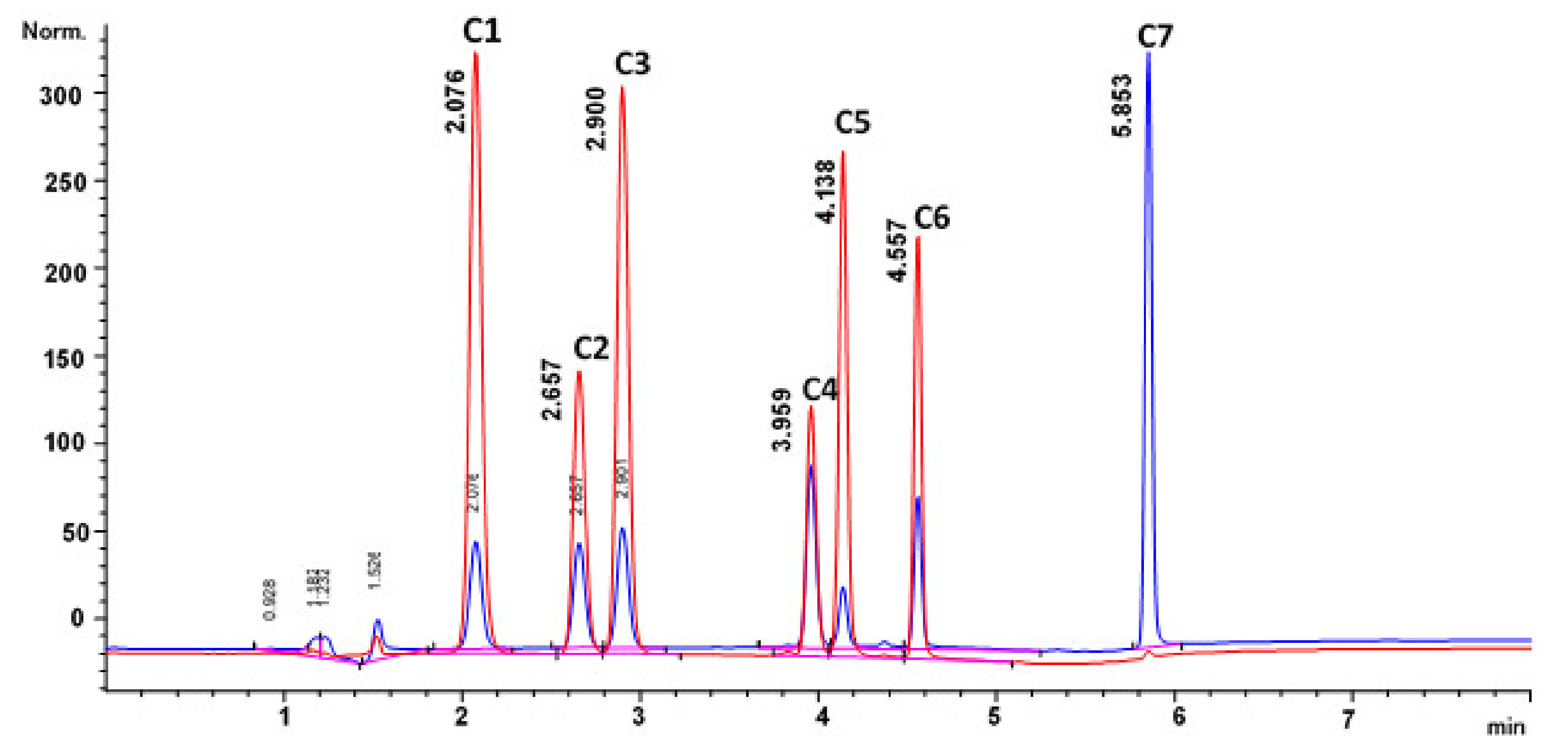
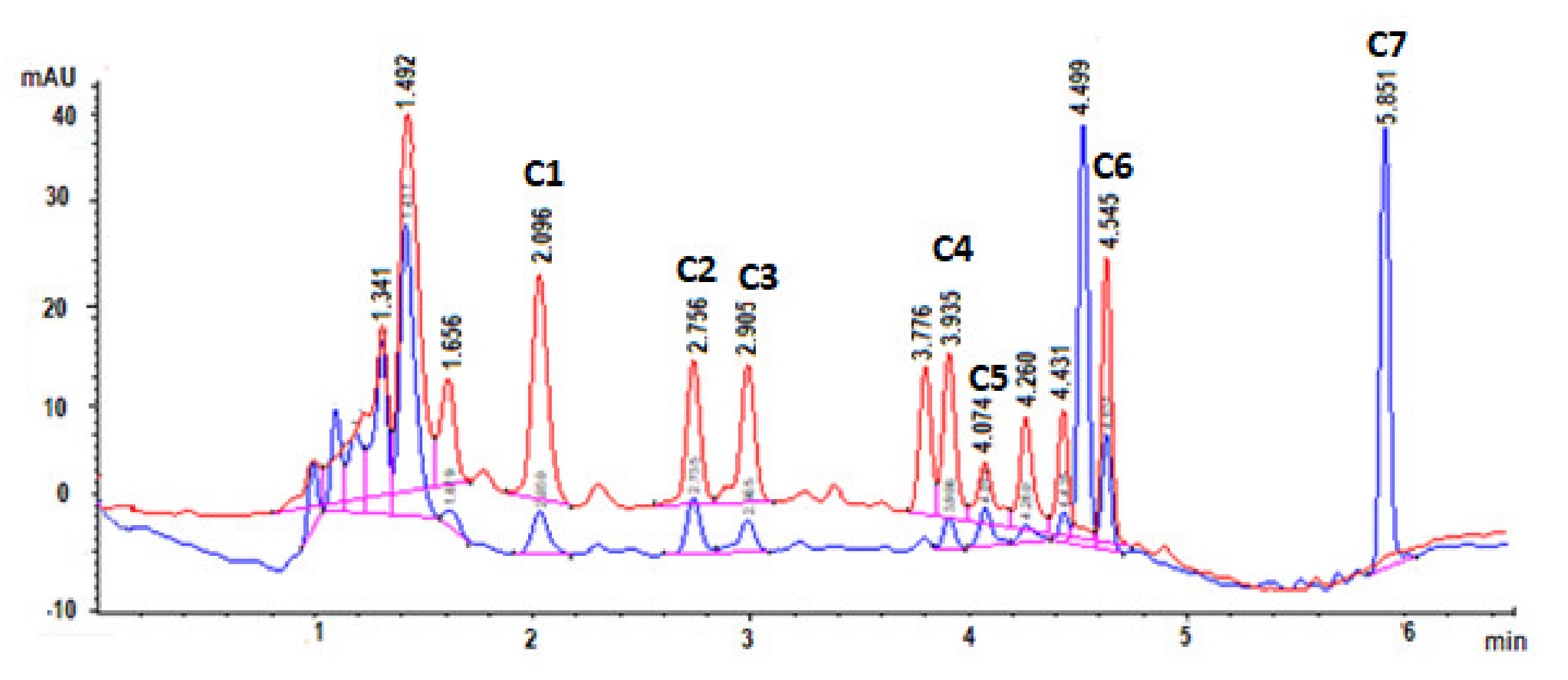
3.1. Optimisation of the HPLC-DAD Method Using One-Factor-at-a-Time
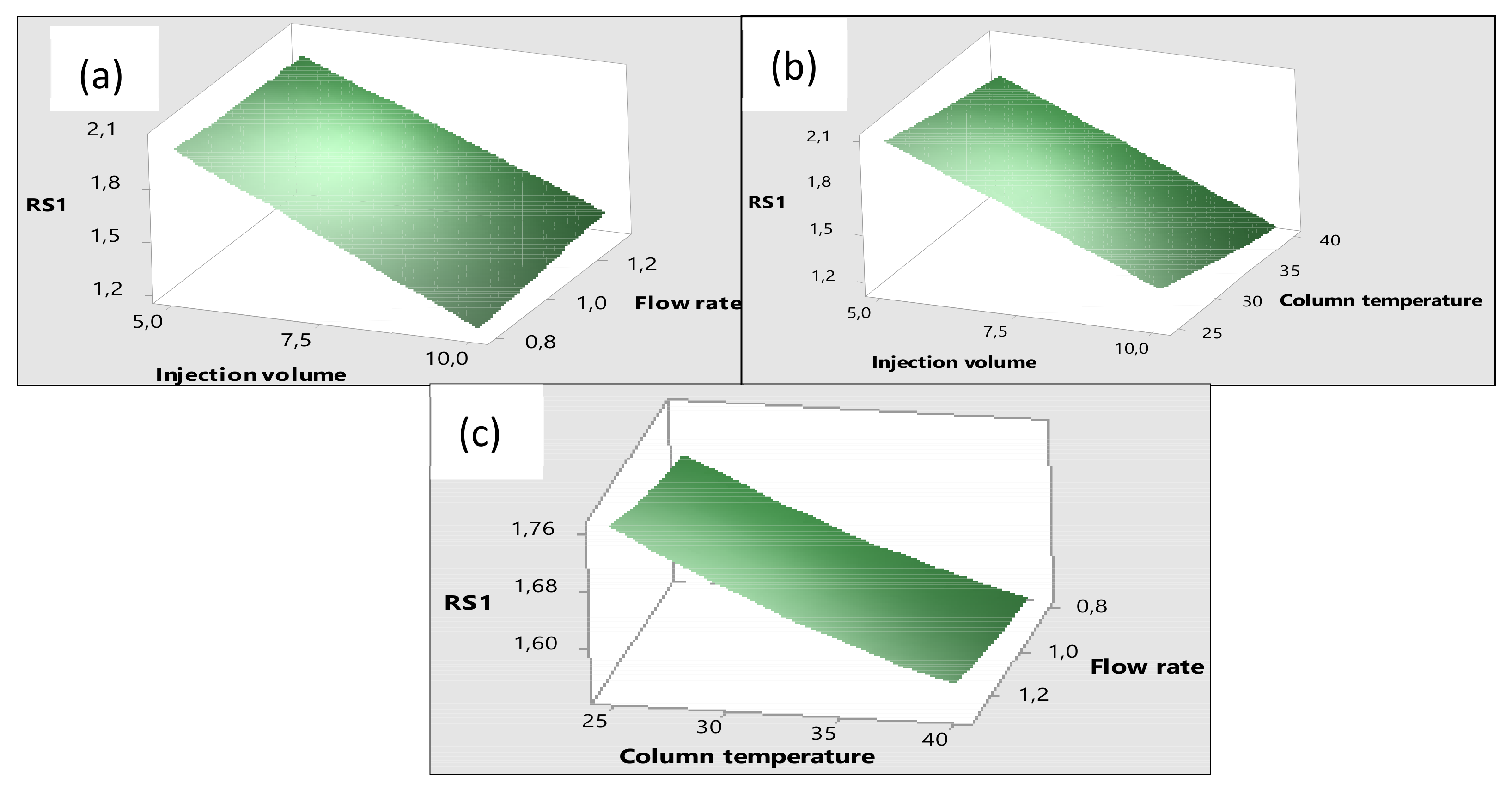
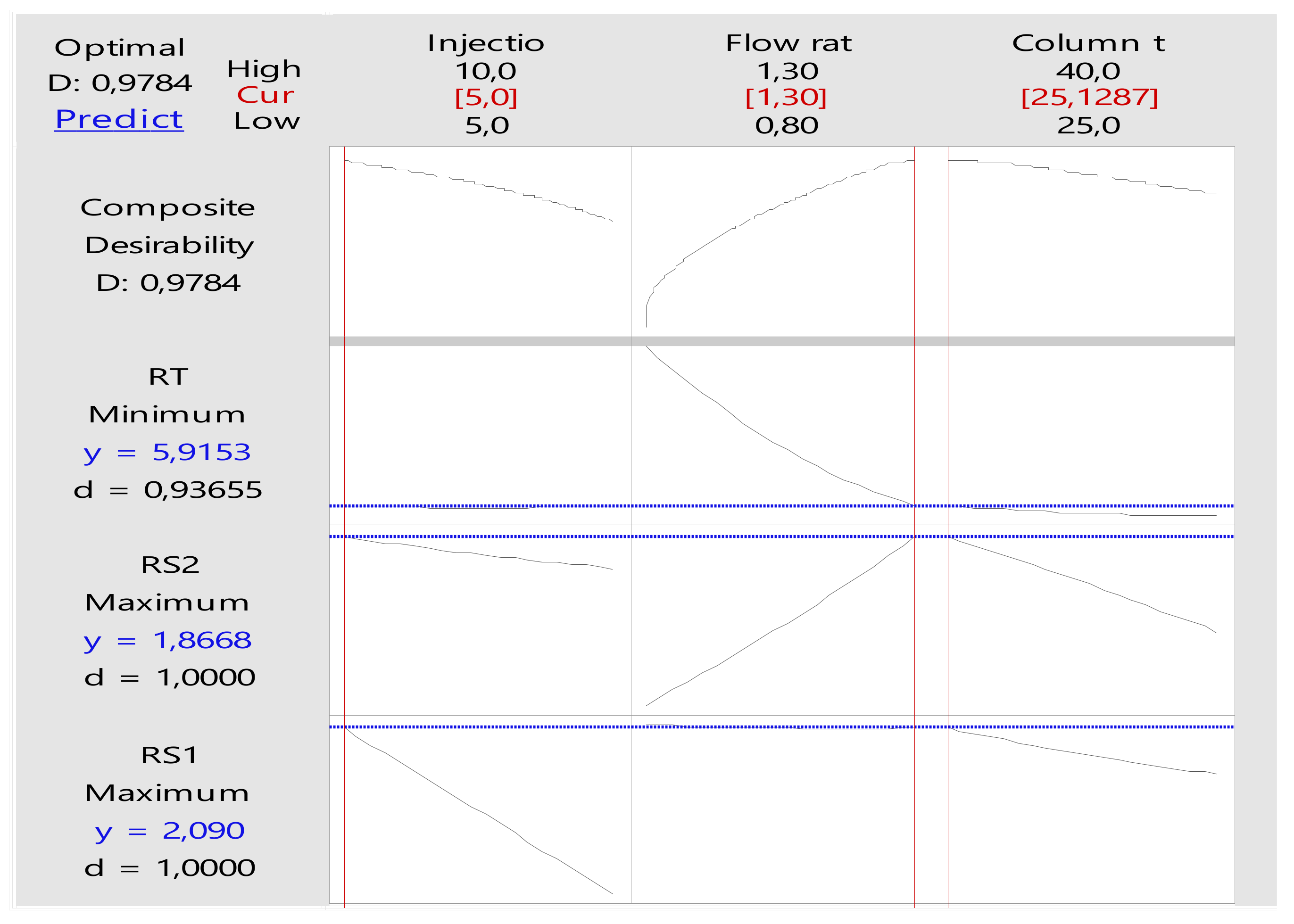
3.2. Optimisation of the HPLC-DAD Method Using Design of Experiment (DoE)
| Injection Volume | Flow Rate | Temperature | RS1 | RS2 | tR |
|---|---|---|---|---|---|
| 5.0 | 1.3 | 32.5 | 1.99 | 1.65 | 5.85 |
| 7.5 | 1.3 | 40 | 1.56 | 1.28 | 5.81 |
| 5.0 | 0.8 | 32.5 | 2.00 | 0.98 | 7.40 |
| 7.5 | 1.05 | 32.5 | 1.66 | 1.29 | 6.44 |
| 7.5 | 1.05 | 32.5 | 1.55 | 1.11 | 6.39 |
| 10.0 | 1.05 | 40.0 | 1.17 | 0.98 | 6.39 |
| 5.0 | 1.05 | 40 | 1.92 | 1.13 | 6.39 |
| 10.0 | 0.80 | 32.5 | 1.20 | 0.96 | 7.40 |
| 7.5 | 0.80 | 25.0 | 1.72 | 1.06 | 7.47 |
| 10.0 | 1.05 | 25.0 | 1.36 | 1.36 | 6.51 |
| 7.5 | 0.80 | 40.0 | 1.54 | 0.86 | 7.33 |
| 5.0 | 1.05 | 25.0 | 2.09 | 1.44 | 6.51 |
| 7.5 | 1.30 | 25.0 | 1.76 | 1.78 | 5.91 |
| 10.0 | 1.30 | 32.5 | 1.32 | 1.47 | 5.85 |
| 7.5 | 1.05 | 32.5 | 1.66 | 1.28 | 6.45 |
Effects of the Factors on the Responses
3.3. HPLC-DAD Method Validation
3.3.1. Linearity
3.3.2. Limit of Detection and Limit of Quantification
3.3.3. Precision
3.3.4. Accuracy and Recovery
3.3.5. Measurement Uncertainty
3.3.6. Specificity of the Method
3.4. Comparison of the External Standard-Calibration and Standard-Addition Methods
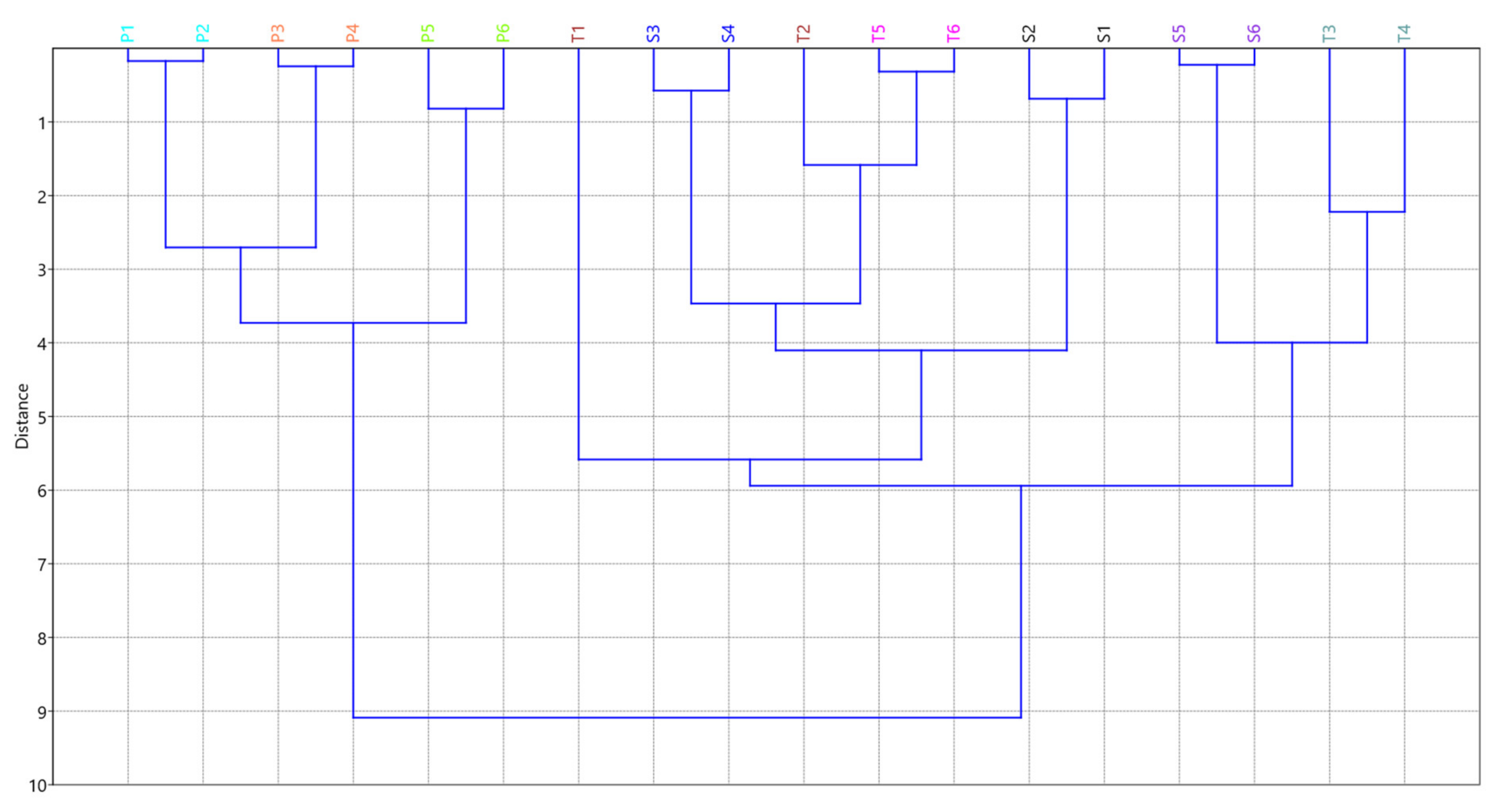
3.5. Quantification of the Compounds of Interest in Real Mondia whitei Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mbamalu, O.N.; Antunes, E.; Silosini, N.; Samsodien, H.; Syce, J. HPLC determination of selected flavonoid glycosides and their corresponding aglycones in Sutherlandia frutescens materials. Med. Aromat. Plants 2016, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, A.; Yan-Hong, W.; Smillie, T.J.; Fu, X.; Li, X.C.; Mabusela, W.; Syce, J.; Johnson, Q.; Folk, W.; Khan, I.A. Quantitative determination of flavonoids and cycloartanol glycosides from aerial parts of Sutherlandia frutescens (L.) R. BR. by using LC-UV/ELSD methods and confirmation by using LC-MS method. J. Pharm. Biomed. Anal. 2010, 52, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Engsuwan, J.; Waranuch, N.; Limpeanchob, N.; Ingkaninan, K. HPLC methods for quality control of Moringa oleifera extract using isothiocyanates and astragalin as bioactive markers. ScienceAsia 2017, 34, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Chokwe, R.C.; Dube, S.; Nindi, M.M. Development of an HPLC-DAD method for the quantification of ten compounds from Moringa oleifera Lam. and its application in quality control of commercial products. Molecules 2020, 25, 4451. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Cheesman, L.; Finnie, J.F.; Van Staden, J. Mondia whitei (Apocynaceae): A review of its biological activities, conservation strategies and economic potential. S. Afr. J. Bot. 2011, 77, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Stafford, G.I.; Pedersen, M.E.; Van Staden, J.; Jäger, A.K. Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 2008, 119, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Odugbemi, T.O.; Akinsulire, O.R.; Aibinu, I.E.; Fabeku, P.O. Medicinal plants useful for malaria therapy in Okeigbo, Ondo State, Southwest Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewani-Rusike, C.R.; Iputo, J.E.; Ndebia, E.J.; Gondwe, M.; Kamadyaapa, D.R. A comparative study on the aphrodisiac activity of food plants Mondia whitei, Chenopodium album, Cucurbita pepo and Sclerocarya birrea extracts in male wistar rats. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Watcho, P.; Zelefack, F.; Ngouela, S.; Nguelefack, T.B.; Kamtchouing, P.; Tsamo, E. Enhancement of erectile function of sexually naïve rats by β -sitosterol and α- β -amyrin acetate isolated from the hexane extract of Mondia whitei. Asian Pac. J. Trop. Biomed. 2012, 2, S1266–S1269. [Google Scholar] [CrossRef]
- Watcho, P.; Donfack, M.M.; Zelefack, F.; Nguelefack, T.B.; Wansi, S.; Ngoula, F.; Kamtchouing, P.; Tsamo, E.; Kamanyi, A. Effects of the hexane extract of Mondia whitei on the reproductive organs of male rats. Afr. J. Tradit. Complementary Altern. Med. 2005, 2, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Martey, O.N.K.; He, X. Possible mode of action of Mondia whitei: An aphrodisiac used in the management of erectile dysfunction. J. Pharmacol. Toxicol. 2010, 5, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Quasie, O.; Martey, O.N.K.; Nyarko, A.K.; Gbewonyo, W.S.K.; Okine, L.K.N. Modulation of penile erection in rabbits by Mondia whitei: Possible mechanism of action. Afr. J. Tradit. Complementary Altern. Med. 2010, 7, 241–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampiao, F. The role of Mondia whitei in reproduction: A review of current evidence. Internet J. Third World Med. 2008, 8, 1–3. [Google Scholar]
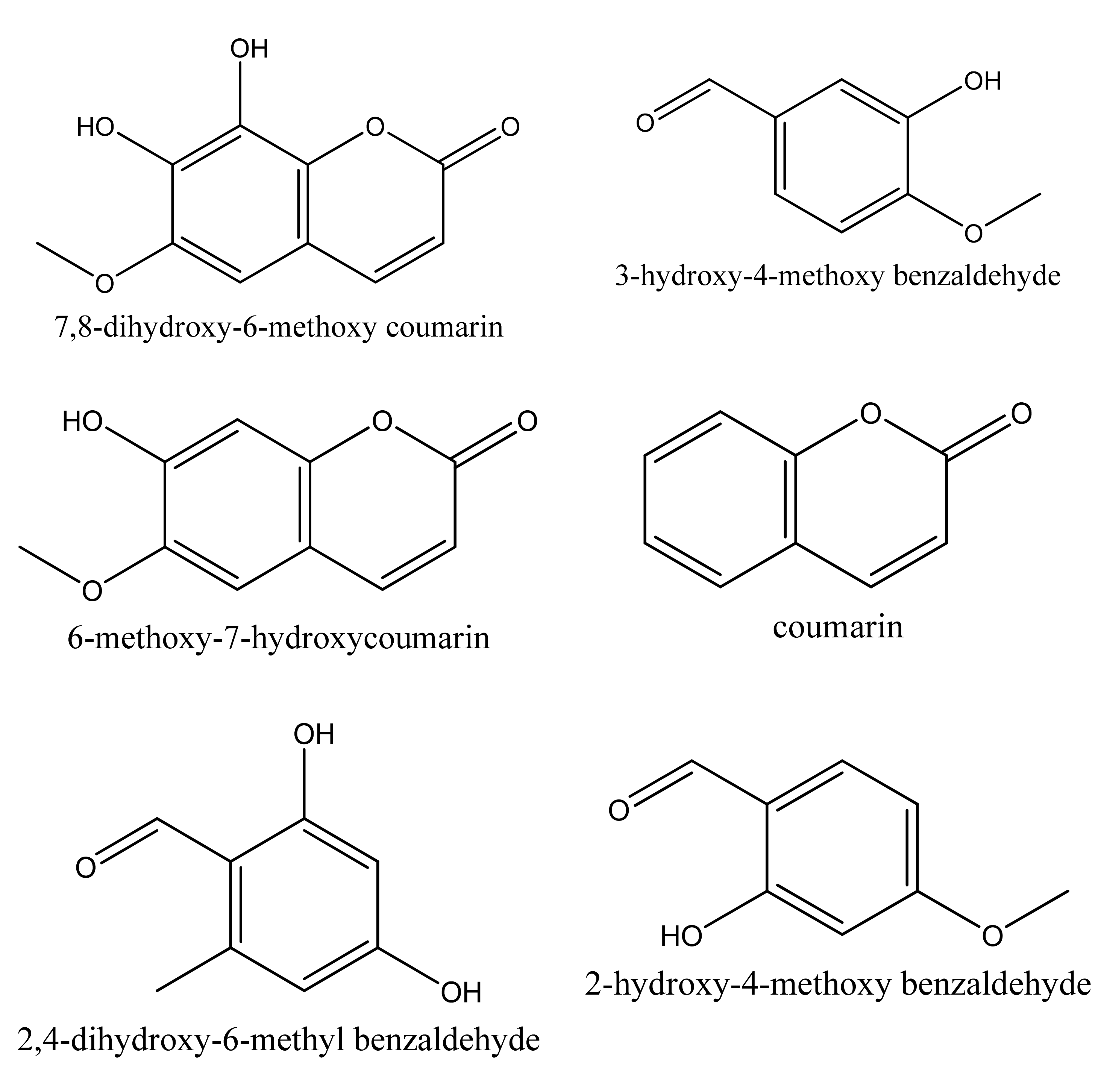
- Patnam, R.; Kadali, S.S.; Koumaglo, K.H.; Roy, R. A chlorinated coumarinolignan from the African medicinal plant. Mondia Whitei. Phytochem. 2005, 66, 683–686. [Google Scholar] [CrossRef]
- Mukonyi, K.W.; Ndiege, I.O. 2-hydroxy-4-methoxybenzaldehyde: Aromatic taste modifying compound from Mondia whytei skeels. Bull. Chem. Soc. Ethiop. 2001, 15, 137–141. [Google Scholar]
- Kundu, A.; Mitra, A. Methoxybenzaldehydes in plants: Insight to the natural resources, isolation, application and biosynthesis. Curr. Sci. 2016, 111, 1325–1334. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Zhao, J.; Gao, H.; Zhou, L.; Liu, Z.; Chen, Y.; Sui, P. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component 2-hydroxy-4-methoxybenzaldehyde. Molecules 2010, 15, 5807–5817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harohally, N.V.; Cherita, C.; Bhatt, P.; Anu Appaiah, K.A. Antiaflatoxigenic and antimicrobial activities of Schiff bases of 2-hydroxy-4-methoxybenzaldehyde, cinnamaldehyde, and similar aldehydes. J. Agric. Food Chem. 2017, 65, 8773–8778. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, X.-Y.; Wu, Y.-L.; Zhang, W. The study on biological and pharmacological activity of coumarins. In Proceedings of the Asia-Pacific Energy Equipment Engineering Research Conference, Zhuhai, China, 13–14 June 2015; Volume 9, pp. 135–138. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.S.; Paul, S.; Dutta, S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Anti-oncogenic potentials of a plant coumarin (7-hydroxy-6-methoxy coumarin) against 7,12-dimethylbenz[a]anthracene-induced skin papilloma in mice: The possible role of several key sihnal proteins. J. Chin. Integr. Med. 2010, 7, 645–654. [Google Scholar] [CrossRef]



| RS1 | RS2 | tR | ||||
|---|---|---|---|---|---|---|
| R2 | 99.28 | 97.90 | 99.96 | |||
| R2(adj) | 97.99 | 94.13 | 99.88 | |||
| R2(pred) | 97.94 | 94.83 | 99.89 | |||
| P-value | Coefficient | P-value | Coefficient | P-value | Coefficient | |
| Linear | 0.00 | 0.00 | 0.00 | |||
| Second | 0.90 | 0.83 | 0.00 | |||
| Interaction | 0.51 | 0.18 | 0.82 | |||
| Constant | 0.00 | 1.6233 | 0.00 | 1.2267 | 0.00 | 6.4267 |
| Injection volume | 0.00 | −0.3688 | 0.065 | −0.0537 | 1.00 | 0.000 |
| Flow rate | 0.20 | 0.0213 | 0.00 | 0.2900 | 0.00 | −0.7725 |
| Column temperature | 0.00 | −0.0925 | 0.00 | −0.1738 | 0.00 | −0.0600 |
| Injection volume2 | 0.90 | −0.0029 | 0.77 | 0.0104 | 0.43 | 0.0092 |
| Flow rate2 | 0.75 | 0.0071 | 0.44 | 0.0279 | 0.00 | 0.1892 |
| Column temperature2 | 0.53 | 0.0146 | 0.49 | −0.0096 | 0.24 | 0.0142 |
| Injection volume * flow rate | 0.18 | 0.0325 | 0.27 | −0.0400 | 1.00 | 0.0000 |
| Injection volume * column temp | 0.82 | −0.0050 | 0.61 | −0.0175 | 1.00 | 0.0000 |
| Flow rate * column temperature | 0.82 | −0.0050 | 0.07 | −0.0750 | 0.38 | 0.0100 |
| Lack-of-fit | 0.99 | 1.00 | 1.00 | |||
| Compound Name | Solvent (mg/L) | Root Powder (mg/kg) | Teabag (mg/kg) | Syrup (mg/L) |
|---|---|---|---|---|
| C1 | 6.5811x − 0.0106 (0.9995), 0.1 #, 0.5 * | Y = 6.5895x 0.2918 (0.9940), 0.8 #, 2.7 * | Y = 6.5174x + 0.832 (0.9980), 0.6 #, 1.9 * | Y = 6.5183x + 0.7573 (0.9976), 0.6 #, 2.1 * |
| C2 | 3.3517x + 0.2585 (0.9959), 0.3 #, 1.0 * | Y = 3.313x + 0.8092 (0.9930), 1.1 #, 3.8 * | Y = 6.5183x + 0.7573 (0.9976), 0.6 #, 2.1 * | Y = 3.1779x + 1.4611 (0.9949), 0.9 #, 3.0 * |
| C3 | Y = 6.7035x + 0.3257 (0.9962), 0.5 #, 1.8 * | Y = 6.827x − 0.3864 (0.9956), 0.9 #, 3.0 * | Y = 6.7576x − 0.4369 (0.9965), 0.8 #, 2.5 * | Y = 6.7626x − 0.265 (0.9950), 0.9 #, 3.0 * |
| C4 | 2.1177x + 0.299 (0.9980), 0.5 #, 1.7 * | Y = 2.1324x 0.4067 (0.9949), 1.0 #, 3.4 * | Y = 2.1196x + 0.1178 (0.9926), 1.1 #, 3.7 * | Y = 2.1167x − 0.1433 (0.9915), 1.2 #, 3.9 * |
| C5 | Y = 3.8625x − 0.348 (0.9942), 0.7 #, 2.2 * | Y = 3.8673 − 0.0456 (0.9943), 0.9 #, 3.1 * | Y = 3.8354x + 0.0541 (0.9907), 1.2 #, 4.1 * | Y = 3.7524x + 0.451 (0.9936), 1.0 #, 3.4 * |
| C6 | Y = 3.5614x − 0.2993 (0.9960), 0.9 #, 3.0 * | Y = 3.5878 − 0.3009 (0.9949), 0.9 #, 3.1 * | Y = 3.5938x − 0.7325 (0.9931), 1.1 #, 3.5 * | Y = 3.489x + 0.1656 (0.9926), 1.1 #, 3.7 * |
| C7 | Y = 30.135x − 1.0792 (0.9993), 0.4 #, 1.3 * | Y = 30.115x − 0.6412 (0.9993), 0.4 #, 1.4 * | Y = 30.168x − 1.5828 (0.9986), 0.5 #, 1.6 * | Y = 30.2x − 1.9401 (0.9987), 0.5 #, 1.5 * |
| Concentration | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| Solvent | |||||||
| 1 mg/L | 1.6+ 3.8 * | 1.5+ 4.5 * | 1.1+ 1.7 * | 1.2+ 4.5 * | 1.4+ 3.8 * | 1.6+ 3.8 * | 1.7+ 4.8 * |
| 7 mg/L | 0.6+ 2.2 * | 1.2+ 1.1 * | 0.8+ 0.5 * | 1.2+ 3.8 * | 0.8+ 3.0 * | 1.2+ 2.9 * | 1.6+ 0.6 * |
| 14 mg/L | 0.3+ 2.8 * | 0.4+ 1.1 * | 1.0+ 0.3 * | 0.6+ 3.6 * | 0.4+ 2.4 * | 0.3+ 3.4 * | 0.7+ 0.5 * |
| Root powder | |||||||
| 3 mg/kg | 66.1 6.9+ 8.6 * | 82.9 6.5+ 11.7 * | 94.5 7.3+ 9.8 * | 96.7 6.4+ 8.2 * | 97.9 5.5+ 6.9 * | 89.8 5.0+ 4.2 * | 92.4 4.1+ 3.6 * |
| 9 mg/kg | 92.8 3.7+ 4.2 * | 94.5 4.2+ 6.8 * | 96.2 3.2+ 4.4 * | 99.3 3.9+ 5.4 * | 100.5 2.6+ 2.9 * | 94.6 3.4+ 3.6 | 99.6 1.4+ 4.4 |
| 14 mg/kg | 101.6 1.3+ 3.6 * | 98.0 4.8+ 4.0 * | 99.5 2.9+ 3.9 * | 101.5 3.4+ 3.2 * | 103.2 2.8+ 2.3 * | 99.7 3.2+ 2.5 * | 103.5 1.3+ 2.3 * |
| U (%) | 24.1 | 19.0 | 16.0 | 14.6 | 5.9 | 5.8 | 6.3 |
| Teabag | |||||||
| 3 mg/kg | 89.9 3.9+ 5.1 * | 74.5 4.5+ 6.8 * | 90.0 3.9+ 4.7 * | 80.2 6.0+ 9.4 * | 90.2 4.8+ 9.3 | 74.4 4.8+ 8.2 * | 94.5 5.5+ 4.3 * |
| 9 mg/kg | 90.2 2.6+ 1.7 * | 94.3 2.3+ 4.9 * | 94.8 2.9+ 3.1 * | 99.4 2.3+ 2.3 * | 96.4 3.0+ 3.9 * | 89.8 3.0+ 4.0 * | 98.3 1.8+ 4.9 * |
| 14 mg/kg | 99.4 1.3+ 2.4 * | 105.6 1.3+ 1.5 * | 98.7 2.1+ 3.6 * | 101.5 4.7+ 2.6 * | 99.1 2.4+ 3.6 * | 95.7 1.8+ 2.1 * | 102.4 1.4+ 2.2 * |
| U (%) | 10.1 | 17.8 | 10.6 | 19.1 | 12.1 | 18.2 | 11.9 |
| Syrup | |||||||
| 3 mg/L | 77.6 2.8+ 5.7 * | 80.1 3.9+ 7.9 * | 94.3 3.2+ 4.3 * | 90.4 5.0+ 6.4 * | 89.8 5.3+ 6.3 * | 90.4 3.1+ 7.0 * | 95.6 2.7+ 3.3 * |
| 9 mg/L | 89.9 3.0+ 4.9 * | 88.3 5.0+ 2.5 * | 95.0 1.9+ 3.0 * | 96.7 2.4+ 3.0 * | 93.4 31.6+ 2.0 * | 92.0 1.7+ 1.8 * | 98.4 1.1+ 1.9 * |
| 14 mg/L | 92.3 2.4+ 1.7 * | 96.7 2.6+ 1.2 * | 101.6 2.4+ 1.1 * | 98.3 2.1+ 2.9 * | 100.8 1.7+ 1.1 * | 98.6 1.4+ 1.5 * | 105.7 0.4+ 0.8 * |
| U (%) | 15.5 | 15.0 | 8.7 | 12.2 | 12.6 | 8.8 | 6.92 |
| External Standard-Calibration Method vs. the Standard-Addition Method | |||
|---|---|---|---|
| Root Powder | Teabag | Syrup | |
| t-value | −0.00 | −0.00 | 0.01 |
| p-value | 0.997 | 0.932 | 0.994 |
| REGION | C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
|---|---|---|---|---|---|---|---|---|
| S1 | M1 | <LOD | 8.5 | 6.5 | 4.5 | <LOD | 10.6 | <LOD |
| S2 | M1 | <LOD | 8.3 | 6.8 | 4.0 | <LOD | 10.3 | <LOD |
| S3 | M2 | 2.4 | 8.6 | 4.4 | 6.1 | <LOD | 9.5 | <LOD |
| S4 | M2 | 2.6 | 8.4 | 4.1 | 6.1 | <LOD | 9.9 | <LOD |
| S5 | M3 | <LOD | 8.7 | <LOQ | 7.0 | <LOQ | 9.6 | <LOD |
| S6 | M3 | <LOD | 8.6 | <LOQ | 7.0 | <LOQ | 9.4 | <LOD |
| T1 | M4 | <LOQ | 5.9 | 5.6 | <LOQ | <LOQ | 10.6 | <LOD |
| T2 | M4 | 2.4 | 5.7 | 5.6 | 3.8 | <LOQ | 10.6 | <LOD |
| T3 | M5 | 2.7 | 7.2 | 2.3 | 5.0 | <LOD | 9.8 | <LOD |
| T4 | M5 | 2.7 | 7.4 | <LOQ | 4.8 | <LOD | 9.7 | <LOD |
| T5 | M6 | 3.3 | 6.1 | 5.0 | 4.1 | <LOD | 9.6 | <LOD |
| T6 | M6 | 3.2 | 6.4 | 5.0 | 4.1 | <LOD | 9.6 | <LOD |
| P1 | KZN | 3.0 | 9.6 | 6.0 | 6.9 | <LOQ | 11.8 | 5.6 |
| P2 | KZN | 3.1 | 9.6 | 6.1 | 6.8 | <LOQ | 11.8 | 5.6 |
| P3 | KZN | 4.4 | 9.4 | 6.9 | 7.2 | <LOQ | 9.7 | 6.0 |
| P4 | KZN | 4.5 | 9.4 | 6.9 | 7.2 | <LOQ | 9.8 | 6.2 |
| P5 | KZN | 5.0 | 10.9 | 7.1 | 9.7 | <LOQ | 11.5 | 6.9 |
| P6 | KZN | 5.1 | 10.9 | 7.1 | 9.6 | <LOQ | 11.6 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokwe, R.C.; Dube, S.; Nindi, M.M. Development of a Quantitative Method for Analysis of Compounds Found in Mondia whitei Using HPLC-DAD. Processes 2021, 9, 1864. https://doi.org/10.3390/pr9111864
Chokwe RC, Dube S, Nindi MM. Development of a Quantitative Method for Analysis of Compounds Found in Mondia whitei Using HPLC-DAD. Processes. 2021; 9(11):1864. https://doi.org/10.3390/pr9111864
Chicago/Turabian StyleChokwe, Ramakwala Christinah, Simiso Dube, and Mathew Muzi Nindi. 2021. "Development of a Quantitative Method for Analysis of Compounds Found in Mondia whitei Using HPLC-DAD" Processes 9, no. 11: 1864. https://doi.org/10.3390/pr9111864






