2.1. Catalyst Preparation
Ni–Fe–Cu/LDH was prepared by coprecipitation from aqueous solutions at room temperature. Two solutions were prepared—solution A containing metal precursors, and prepared from their respective nitrates: Ni(NO3)2 6H2O, Cu(NO3)2 3H2O, Fe(NO3)3 9H2O, Mg(NO3)2 6 H2O, and Al(NO3)3 9 H2O. Solution A was 4M NO3−2, and was prepared to get the following molar cation concentration: Ni/Fe/Cu/Mg/Al: 30/5/5/40/20. Solution B contained Na2CO3 (0.22 M) and NaOH (3.56 M). Then, 100 mL of solution A and B was slowly dropped by HPLC pumps under vigorous stirring over 100 mL deionized water. To ensure a good mix and dispersion, we used a homogenizer (Model IKA magic, IKA-Werke GmbH & Co. KG, Staufen, Germany) as the reactor. The temperature of the solution was kept constant at 60 °C by means a thermostatic bath. The pH of the solution was also maintained constant during all test by means of a control system that added solution NaOH 1 M to obtain the pH at 10 to ensure the co-precipitation of the metallic salts.
The gel formed was aged for 20 h at 100 °C under reflux in order to improve its crystalline characteristics. The solid obtained was then filtered and washed with distilled water at 90 °C until the solution was a conductivity bellow 30 µS cm
−1 [
11]. The LDH formed was dried at 105 °C overnight, and then finally crushed and calcined using a heating rate of 5 °C min
−1, from room temperature to 800 °C and keeping at this temperature for 3 h.
In order to obtain a greater understanding of the structure of the catalyst, we synthesized three additional LDHs following the same procedure.
Table 1 shows the composition of all synthesized LDHs.
2.2. Catalyst Characterization
2.2.1. Chemical Element Analysis
The chemical element analysis of as-synthesized catalysts was performed using the inductively coupled plasma-optical emission spectroscopy (ICP-OES) on the Optima 4300 DV (Perkin Elmer, Waltham, MA, USA). Prior to the analysis, the solid (0.05 g) was dissolved in 4 mL HNO3 and then was heated to 100 °C for 5 min, to 170 for 15 min, and to 240 °C for 10 min; kept in isothermal condition for 15 min; and finally filtered and diluted.
2.2.2. Characterization of the Calcination Process
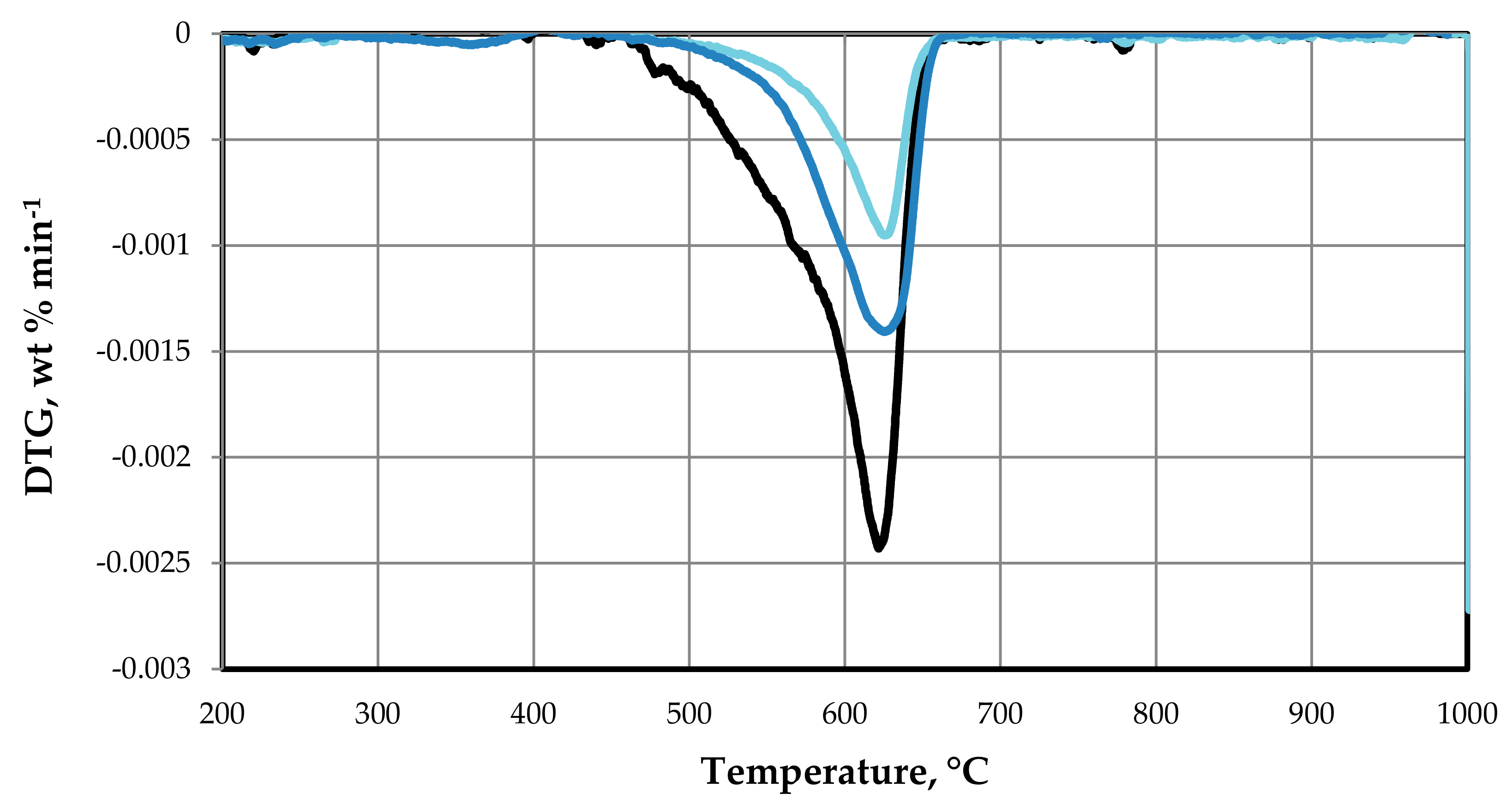
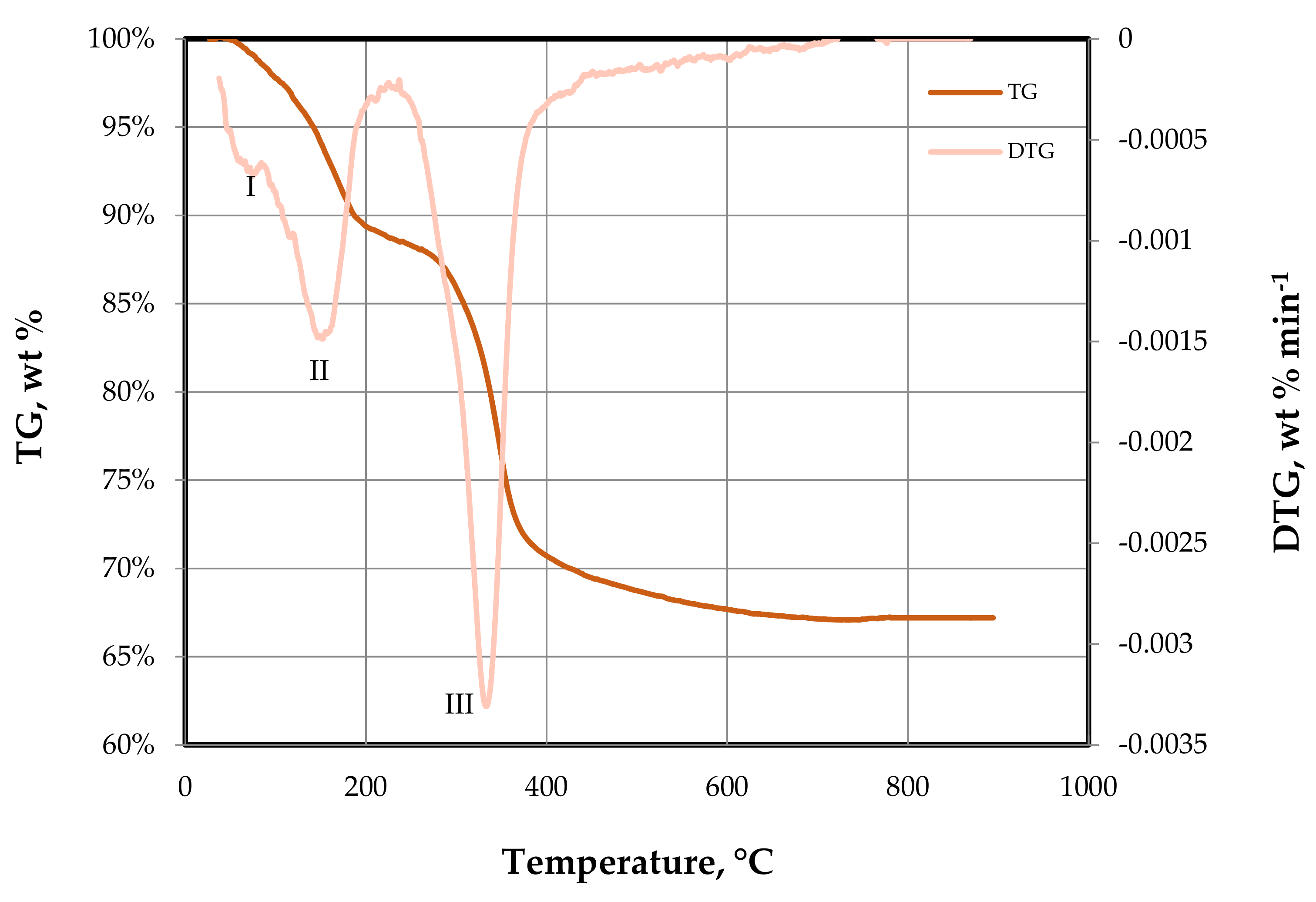
In order to analyze the calcination, process, we conducted a thermogravimetric analysis (TGA).
Thermogravimetric analysis was performed on a DTG-60H TG-DTA analyzer (Shimadzu Co. Ltd., Kyoto, Japan). The analyses were carried out using air with a flow rate of 50 mL min−1, and a heating rate of 10 °C min−1, from room temperature to 900 °C. The amount of sample analyzed was ≈3 mg. This analysis allowed us to determine the weight loss of a sample as a function of temperature and time (TG curve). In addition, the derivative of the TG signal (DTG curve) helped to determine the number of main thermal processes that took place, as well as their temperature intervals.
2.2.3. Temperature-Programmed Reduction (TPR) Analysis
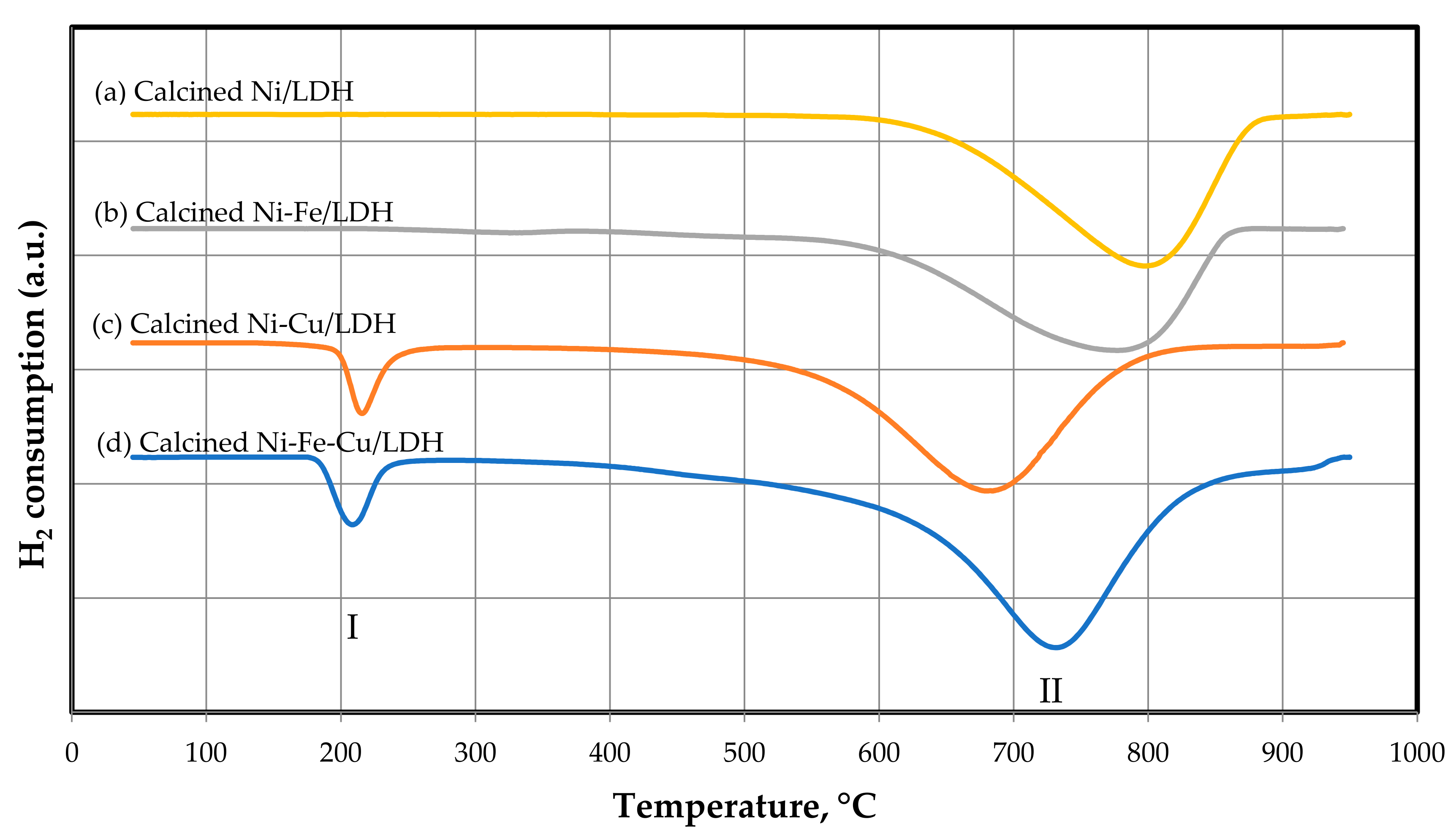
TPR analyses were performed on Auto Chem II 2920 analyzers (Micromeritics Instrument Corporation, Norcross, GA, USA) with a thermal conductivity detector (TCD) used to quantify the amount of H2 consumed during the analysis.
TPR was used to determine the reducible species and the corresponding reduction temperatures. Before measurement, a 0.20 g sample was dried by passing 50 mL min-1 of Ar up to 150 °C at a heating rate of 10 °C min−1, and holding time under these conditions 30 min. Then, the reduction of the sample was carried out by means of 50 mL min−1 of 5% H2/Ar to 950 °C min−1, heating rate 10 °C min−1, and holding time of 30 min.
2.2.4. X-Ray Diffraction (XRD) Analysis
Powder X-ray diffraction (XRD) measurements were performed on a D8 Discover (Bruker, Billerica, MA, USA) using Cu Kα radiation (λ = 0.154 nm) generated at 40 kV and 40 mA. The identification of the diffraction patterns was performed using the Joint Committee of Powder Diffraction Standards (JCPDS) database.
XRD analyses are suitable for determining lattice parameters and particle diameter. Thus, for example, LDHs are a hexagonal system whose most significant lattice parameters are
a and
c—
a represents the average cation-cation distance within the layers and
c represents the distance between the brucite-like layer and the inter-layer [
12,
13]. The relationship between lattice parameter and interplanar spacing
dhkl is given by
where (
h,
k,
l) are Miller indices,
a and
c are the lattice parameters, and
d is interplanar spacing [
14]. Lattice parameter
a and
c are calculated from the (110) and (003) plane, respectively obtained from Equations (4) and (5).
The interplanar spacing of the (110) and (003) crystal planes was estimated by Bragg’s law, which can be represented by Equation (6).
where
n,
λ, and
θ are the order, X-ray wavelength, and diffraction angle, respectively.
Using Equations (4)–(6) and the diffraction angle obtained from the XRD pattern, we can obtain the lattice parameters a and c.
The crystallite size (
D) was calculated by Scherrer Equation (7)
where
K is the shape factor (
K = 0.89),
β is the full width at half maximum (FWHM) of the analyzed diffraction peak in radians, and
θ is the Bragg angle.
A similar procedure was used to determine the lattice parameters of calcined and reduced LDHs, but using Equation (8), which is characteristic of a face-centered cubic (fcc) structure such as that of NiO and metallic Ni.
2.2.5. Textural Properties
Specific surface area (BET) pore volume and average pore diameter of both as-synthesized and calcined catalysts were measured via N2 adsorption at 198 °C using a surface area analyzer Autosorb-1C (Quantachrome Instruments, Boynton Beach, FL, USA). Surface area (SBET) was analyzed using Brunauer–Emmett–Teller (BET) theory, and average pore diameter by Barrett–Joiner–Halenda (BJH) procedure.
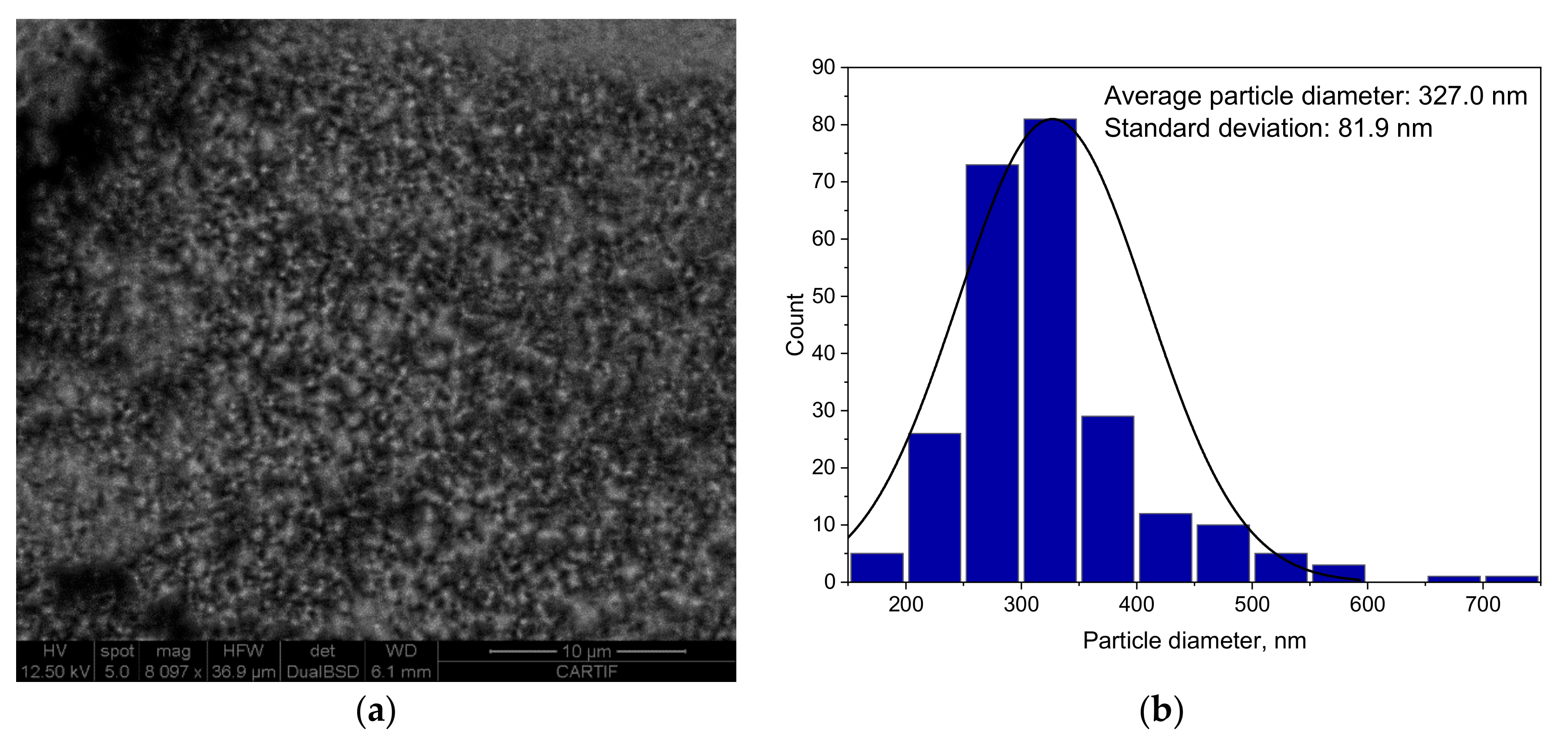
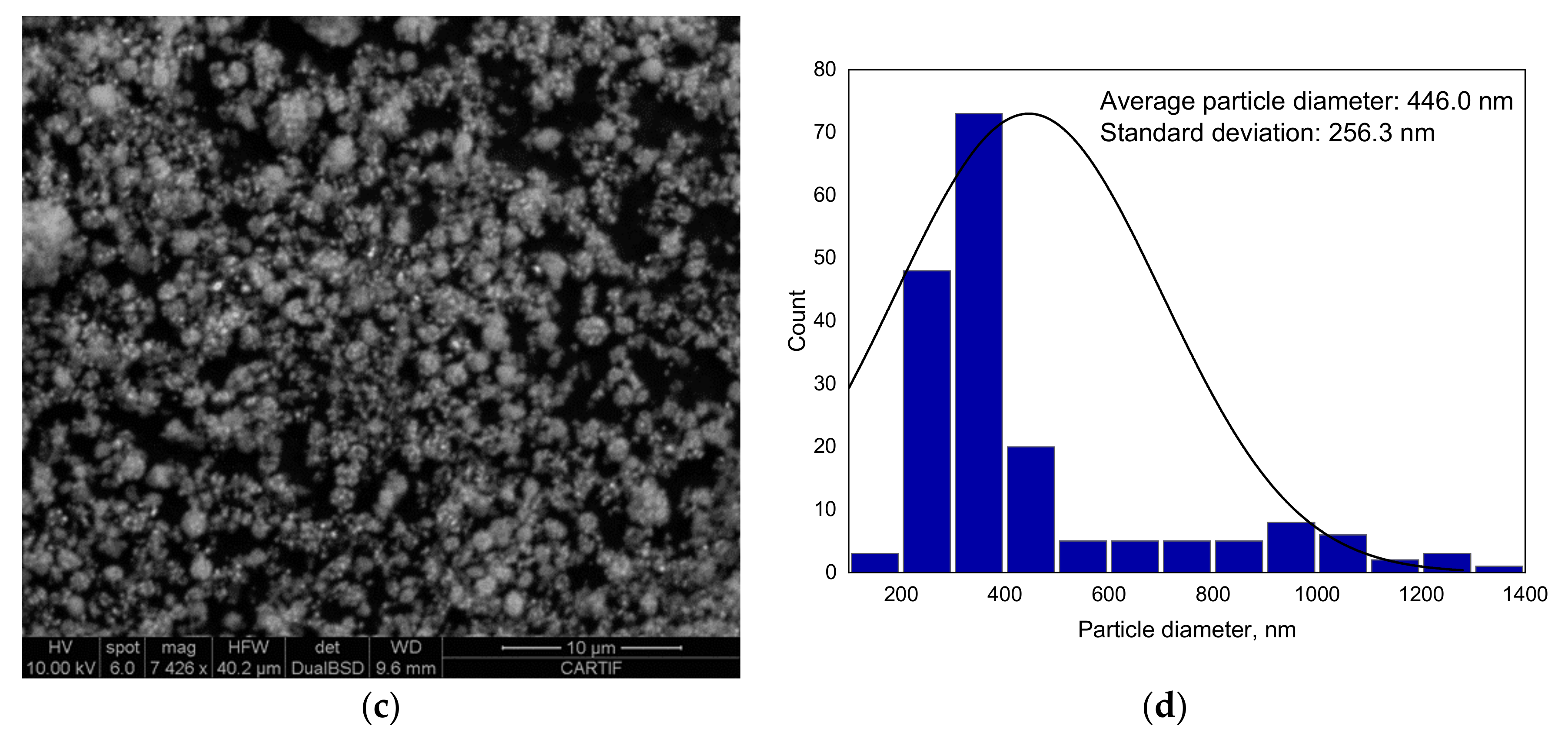
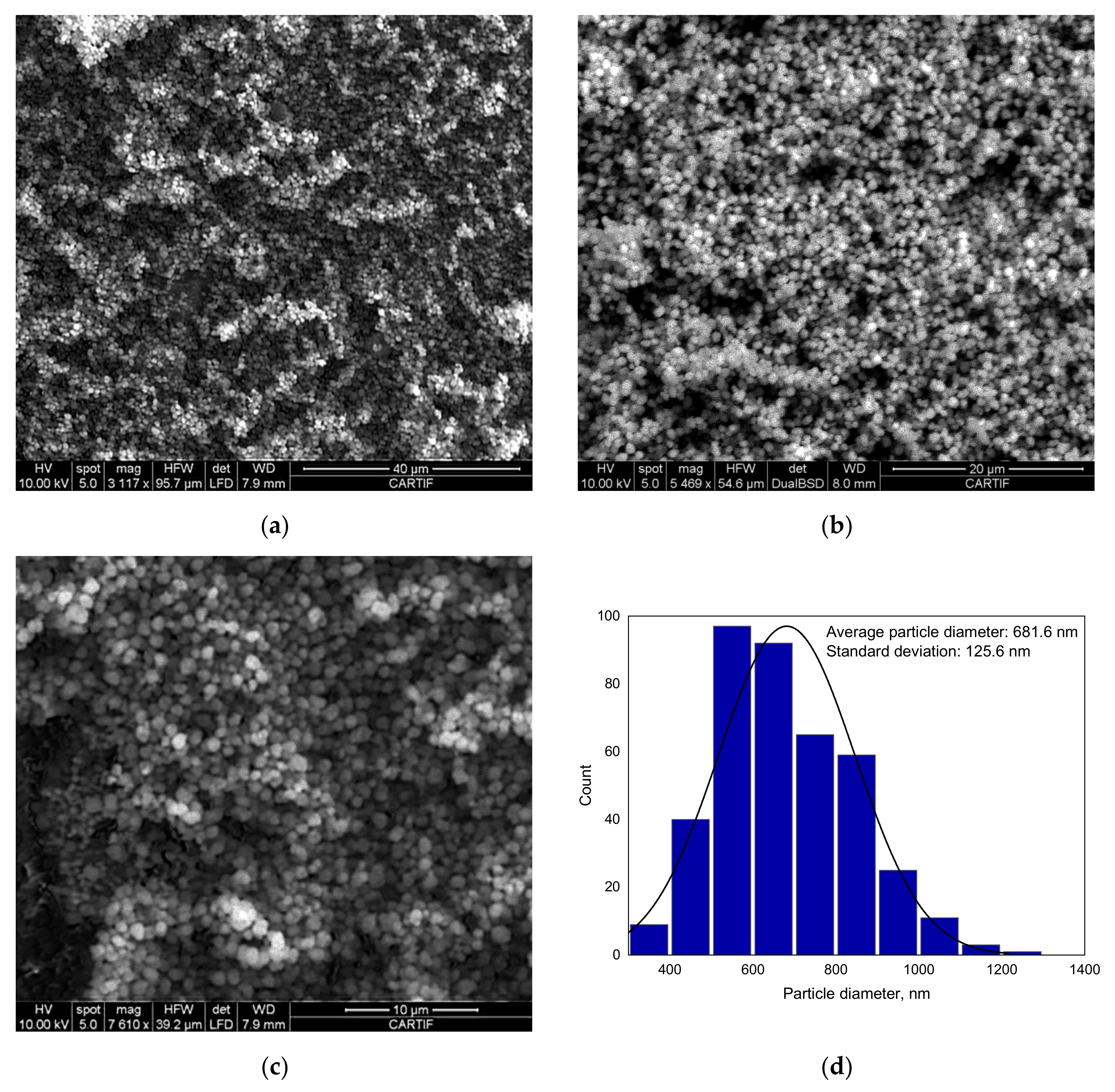
2.2.6. Scanning Electron Microscopy (SEM)
A high-resolution scanning electron microscope (SEM) FEI ESEM Quanta 200 (FELMI-ZFE, Graz, Austria) was used to analyze the surface morphology of the as-synthetized and used catalyst.
2.2.7. Type and Amount of Carbonaceous Species Deposited on the Catalyst
The type of carbonaceous material deposited on the surface of the catalyst at the end of the steam reforming reaction was performed on a DTG-60H TG-DTA analyzer (Shimadzu Co. Ltd., Kyoto, Japan). The analyses were carried out using air with a flow rate of 50 mL min
−1, and a heating rate of 20 °C min
−1, from room temperature to 1000 °C. The amount of sample analyzed was ≈3 mg. This method was used to determine the type of carbon deposited by its relationship to its oxidation temperature [
15].
The amount of carbon formed on the surface of the catalyst after reaction was measured using an elemental TruSpec CHN analyzer (Leco, St. Joseph, MI, USA).
2.3. Catalytic Tests
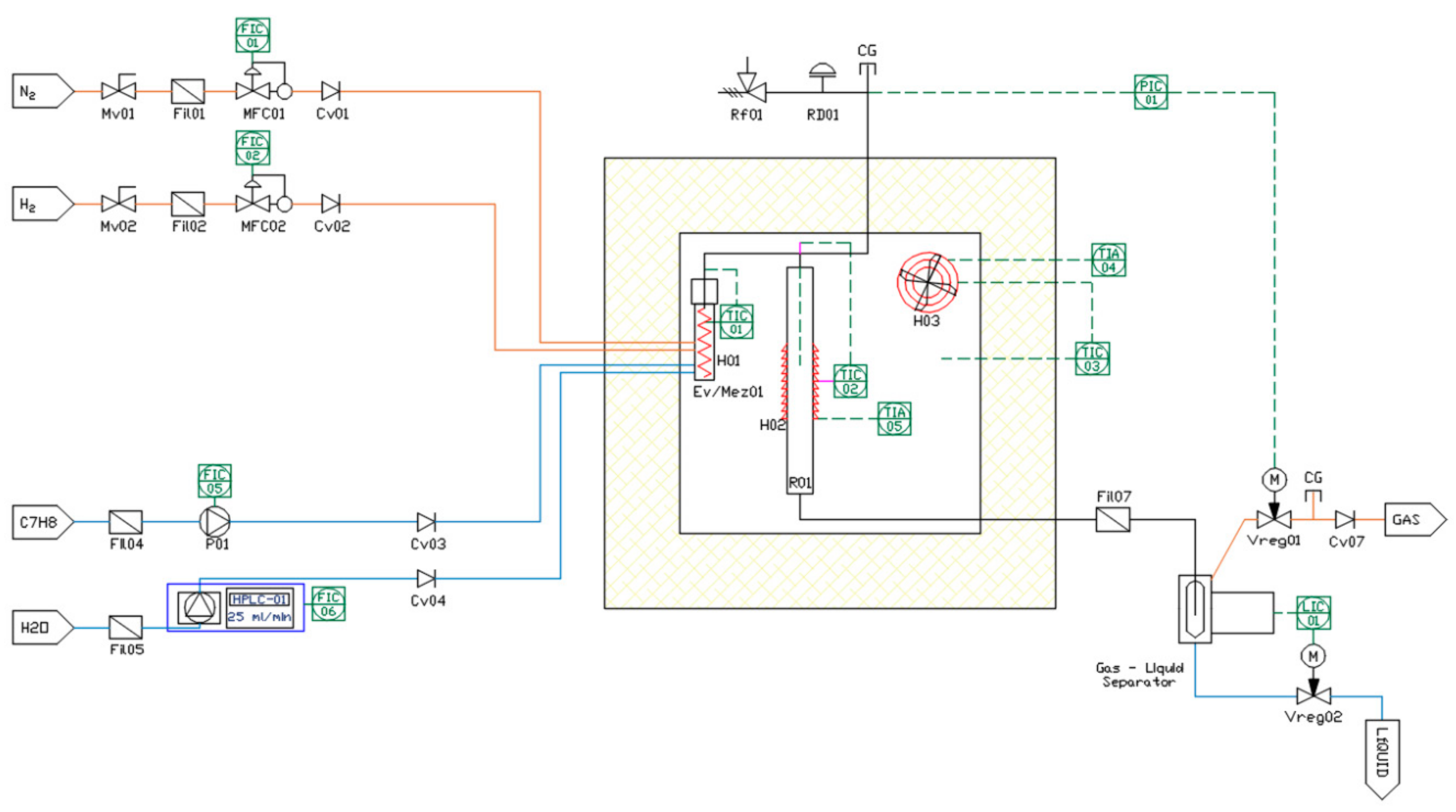
Steam reforming of toluene used as a model compound of tar was carried out using a fixed-bed reactor made of Hastelloy alloy steel, with an inner diameter of 10 mm and a length of 500 mm (
Figure 1). The reactor was heated by a tubular electric furnace coupled to the reactor. The process temperature was measured by a K-type thermocouple placed in the center of the bed and controlled using a controller in closed loop (DEMEDE, Spain).
In each test, 250 mg of calcined catalyst was placed in the middle of the reactor on a 316 stainless steel sintered porous metal filter disc with an internal pore size of 40 µm. Quartz wool was used above and below the bed to help catalyst inside the reactor.
Prior to each test, the system was purged with 50 mL min
−1 N
2 for 15 min, and then the catalyst was reduced with a flow of H
2 and N
2 (H
2/N
2 = 50/20 mL min
−1) at 900 °C for 2 h to transform Mg(Ni,Al)O periclase species of Ni
2+ into well-dispersed Ni particles [
16]. After reduction, the system was purged again with 50 mL min
−1 N
2 for 15 min while the temperature was kept at the desired temperature of the steam reforming 500–900 °C.
Once the system was purged, the steam reforming reaction was started, with the duration of each test being 1 h. To do this, the water and the toluene were fed into a preheater by means of a HPLC pump and syringe pump, respectively. Moreover, to ensure a concentration of toluene in the gas phase of 1.50 vol %, we fed a current of N2 into the preheater through a mass flow controller. This preheater was maintained at 250 °C in order to ensure a complete vaporization of the feedstock before entering the fixed bed reactor containing the catalyst. To prevent condensation and to ensure that feedstock was maintained in a homogeneous vapor phase, we placed the preheater and the reactor in a chamber that was kept warm at 200 °C.
The reaction products were then passed through a cold trap to condense unreacted toluene and water in the effluent stream. All the non-condensed gases (H2, N2, CO, CO2, and CH4) were analyzed online by gas chromatography using a chromatograph MicroGC CP4900 (Varian Inc., Palo Alto, CA, USA) with two channels, one equipped with a molecular sieve column and another one with a Polar Plot Q column, and both coupled to a thermal conductivity detector (TCD). The unreacted toluene and benzene produced were collected at the end of each test and analyzed offline by gas chromatography, using a 6890 N gas chromatograph coupled with mass spectrometer 5973 Network (Agilent, Santa Clara, CA, USA).
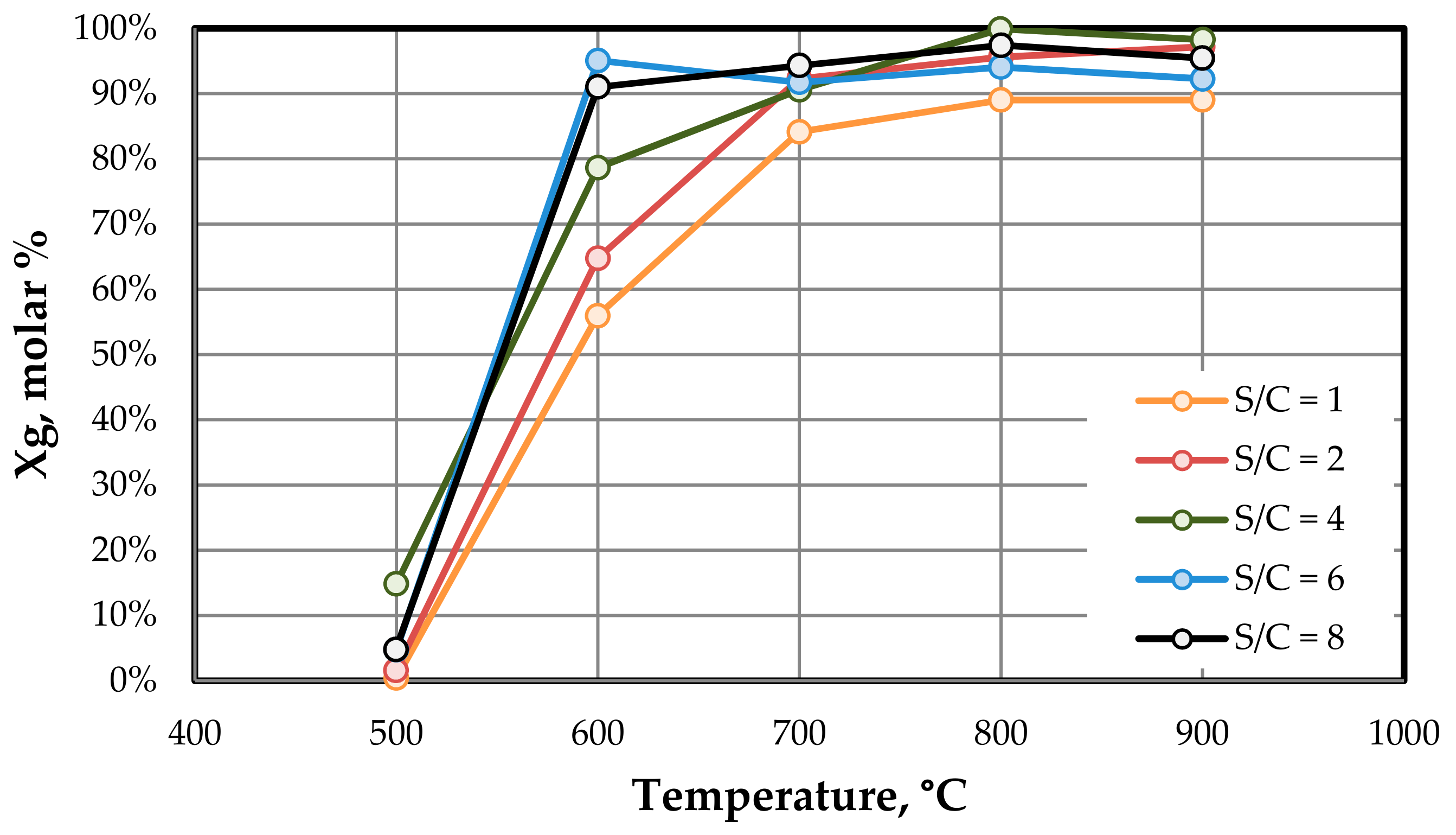
Different tests were developed using reaction temperatures of 500, 600, 700, 800, and 900 °C and steam/carbon molar ratios (S/C ratios) of 1, 2, 4, 6, and 8. The total flow rate of the product gases was calculated by means of a mass balance to the nitrogen stream. The contact time in the steam reforming reaction was calculated in all tests to be W/F = 0.32 g h mol−1. F is the total molar flow of the feedstock (toluene, water, and nitrogen) and W represents the weight of the catalyst.
Table 2 summarizes the operating conditions for each S/C ratio. As can be seen in
Table 2, the toluene flow rate was kept at 0.2 mmol min
−1, the water flow rate was adjusted to obtain the desired S/C, and the nitrogen flow rate was adjusted to ensure the same concentration of toluene in the feedstock and the same contact time.
Benzene yield was evaluated by a carbon balance between the carbon from the benzene formed and the carbon from the toluene at the feed, as shown in Equation (9).
In the same way, the conversion of toluene to gases was calculated by a carbon balance between the carbon from the gaseous products formed (CO, CO
2, and CH
4), and the carbon from the toluene at the feed.
where n
CO, n
CO2, n
CH4, and n
C7H8 represent the molar flow rate of CO, CO
2, CH
4, and toluene, respectively.
The molar gas composition of each gas (H
2, CO, CO
2, and CH
4) was calculated as Equation (11).

 ) hydrotalcite, (
) hydrotalcite, (  ) MgO-like phase, (•) NiO, (
) MgO-like phase, (•) NiO, ( 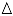 ) Ni(Fe)Ox, (
) Ni(Fe)Ox, (  ) Ni metal, (
) Ni metal, ( 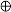 ) Ni–Fe alloy.
) Ni–Fe alloy.
 ) hydrotalcite, (
) hydrotalcite, ( 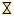 ) MgO-like phase, (•) NiO, (
) MgO-like phase, (•) NiO, ( 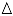 ) Ni(Fe)Ox, (
) Ni(Fe)Ox, (  ) Ni metal, (
) Ni metal, ( 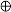 ) Ni–Fe alloy.
) Ni–Fe alloy.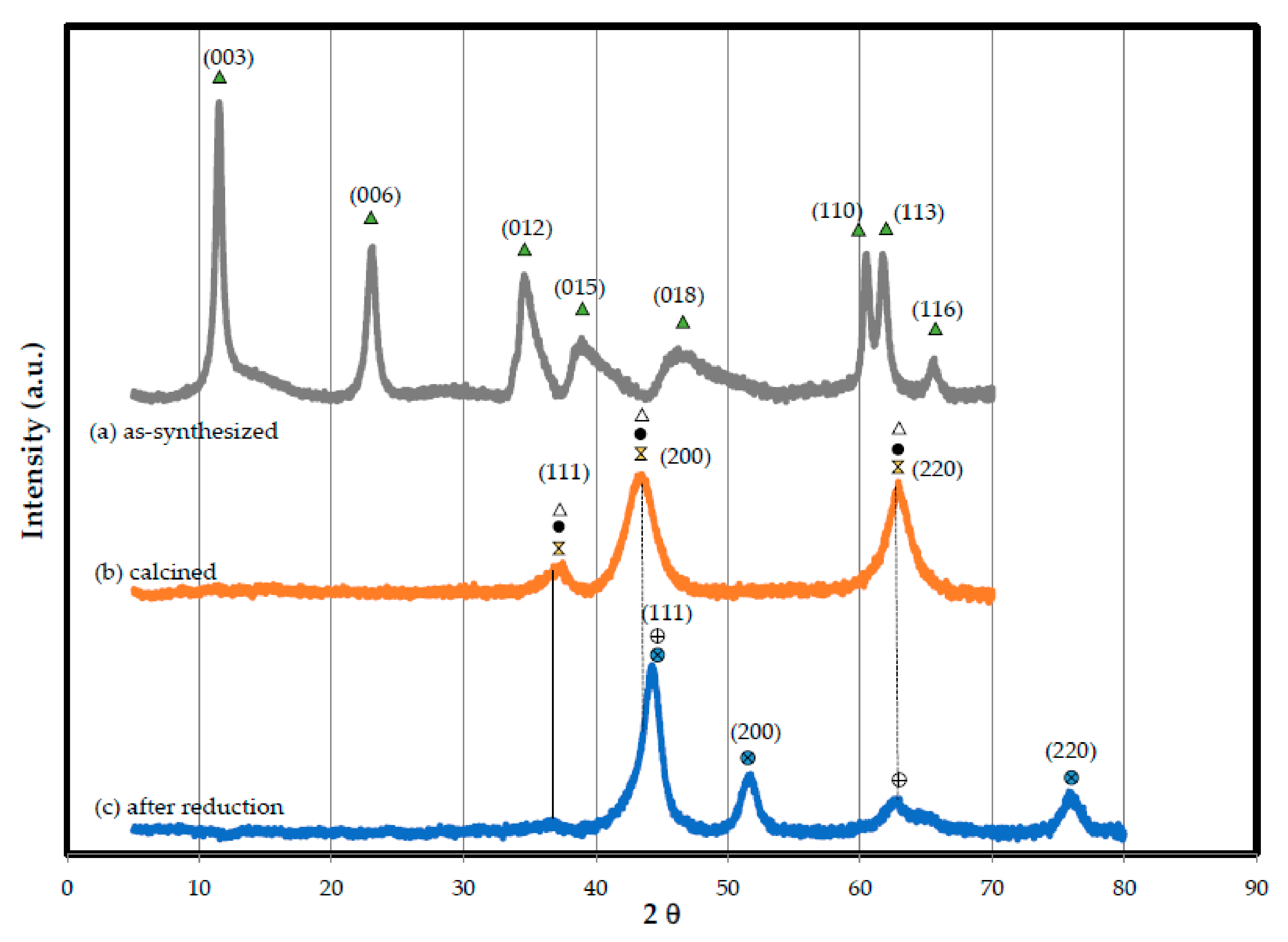
 ) as-synthesized; (
) as-synthesized; (  ) calcined.
) calcined.
 ), S/C = 2 (
), S/C = 2 (  ), S/C = 4(
), S/C = 4(  ), S/C = 6 (
), S/C = 6 (  ), S/C = 8 (
), S/C = 8 (  ).
).
 ), S/C = 2 (
), S/C = 2 (  ), S/C = 4(
), S/C = 4(  ), S/C = 6 (
), S/C = 6 (  ), S/C = 8 (
), S/C = 8 (  ).
).

 ) Ni metal, (
) Ni metal, (  ) Ni–Fe alloy, (
) Ni–Fe alloy, (  ) MgO-like phase, (•) NiO.
) MgO-like phase, (•) NiO.
 ) Ni metal, (
) Ni metal, (  ) Ni–Fe alloy, (
) Ni–Fe alloy, ( 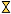 ) MgO-like phase, (•) NiO.
) MgO-like phase, (•) NiO.

 ), 4 (
), 4 (  ), and 6 (
), and 6 (  ).
).