The Importance of Dose Intensity When Administering Cytotoxic Chemotherapy in NSCLC—A Matter as Actual Now as in the Past
Abstract
:1. Introduction
2. Materials and Methods
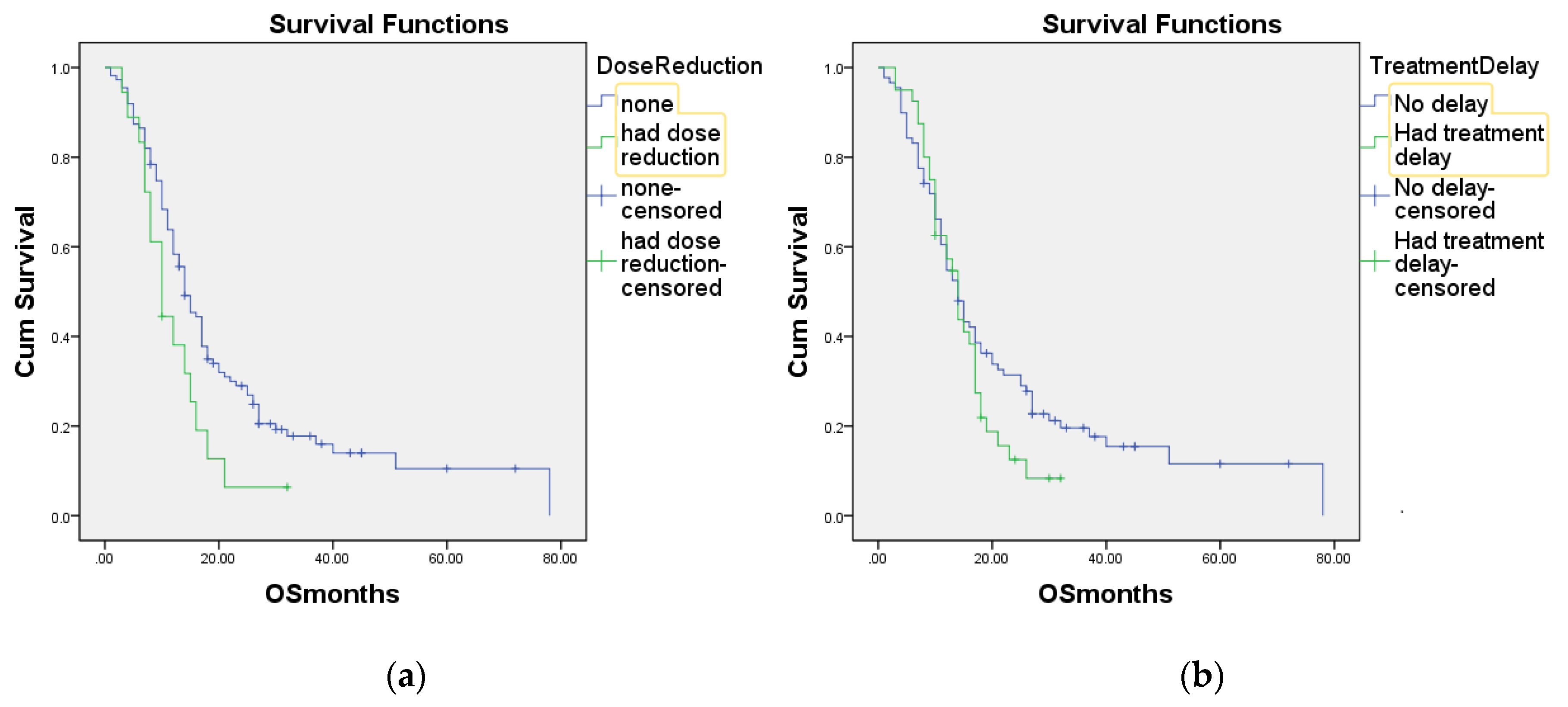
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyman, G.H. Impact of Chemotherapy Dose Intensity on Cancer Patient Ourcomes. J. Natl. Compr. Cancer Netw. 2009, 7, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4, published 28 May 2009 (v4.03: 14 June 2010) U.S. Department of Health and Human Services, NCI. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 5 May 2020).
- Bokemeyer, C.F.; Aapro, M.S.; Courdi, A.; Foubert, J.; Link, H.; Österborg, A.; Repetto, L.; Soubeyran, P. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur. J. Cancer 2007, 43, 258–270. [Google Scholar] [CrossRef]
- Aapro, M.S.; Link, H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist 2008, 13 (Suppl. 3), 33–36. [Google Scholar] [CrossRef]
- Pujol, J.L.; Breton, J.L.; Gervais, R.; Rebattu, P.; Depierre, A.; Morère, J.F.; Milleron, B.; Debieuvre, D.; Castéra, D.; Souquet, P.J.; et al. Gemcitabine-docetaxel versus cisplatin-vinorelbine in advanced or metastaic non-small cell lung cancer: A phase III study adressing the case for cisplatin. Ann. Oncol. 2005, 16, 602–610. [Google Scholar] [CrossRef]
- Lymann, G.H.; Kunderer, N.M.; Crawford, J.; Wolff, D.A.; Culakova, E.; Poniewierski, M.S.; Dale, D.C. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 2011, 117, 1917–1927. [Google Scholar] [CrossRef] [Green Version]
- Mazilu, L.; Stanculeanu, D.L.; Gheorghe, A.D.; Suceveanu, A.P.; Parepa, I.R.; Stoian, A.P.; Pop, C.S.; Bratu, O.; Suceveanu, A.I. Chemotherapy and other Factors Affecting Quality of Life in Non-Small Cell Lung Cancer (NSCLC) Patients. Rev. Chim. 2019, 70, 33–35. [Google Scholar] [CrossRef]
- Aagaard, T.; Roen, A.; Reekie, J.; Daugaard, G.; Brown, P.D.; Specht, L.; Sengeløv, H.; Mocroft, A.; Lundgren, J.; Helleberg, M. Development and validation of a risk score for febrile neutropenia after chemotherapy in patients with cancer: The FENCS score. JNCI Cancer Spectr. 2018, 2, pky053. [Google Scholar] [CrossRef] [Green Version]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit. Rev. Oncol. Hematol. 2014, 90, 190–199. [Google Scholar] [CrossRef]
- BC Cancer Agency. Pharmacy Policy Number II-20: Guiding Principles for Chemotherapy Preparation Chart; Cancer Agency: Vancouver, BC, Canada, 2007. [Google Scholar]
- Grunberg, S.M.; Warr, D.; Gralla, R.J.; Rapoport, B.L.; Hesketh, P.J.; Jordan, K.; Espersen, B.T. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity- state of the art. Support Care Cancer 2011, 19, S43–S47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazilu, L.; Stanculeanu, D.L.; Gheorghe, A.D.; Voinea, F.; Suceveanu, A.P.; Pituru, S.; Diaconu, C.C.; Parepa, I.R.; Stoian, A.P.; Pop, C.S.; et al. Incidence of Chemotherapy-Induced Peripheral Neuropathy In Cancer Patients In Clinical Practice. FARMACIA 2019, 67, 472–476. [Google Scholar] [CrossRef]
- Adrian, T.; Alecu, L.; Poiana, C.; Tomescu, L.; Slavu, I.; Tulin, R.; Pituru, S.; Orlov, C.; Jecan, R.; Cristian, B.; et al. Functional radical cervical dissection for differentiated thyroid cancer: The experience of a single center. JMMS 2018, 5, 278–283. [Google Scholar] [CrossRef]
- Grønberg, B.H.; Bremnes, R.M.; Fløtten, Ø.; Amundsen, T.; Brunsvig, P.F.; Hjelde, H.H.; Kaasa, S.; von Plessen, C.; Stornes, F.; Tollåli, T.; et al. Phase III Study of the Norwegian Lung Cancer Study Group: Pemetrexed Plus carboplatin Compared With Gemcitabine Plus carboplatin As First Line Chemotherapy in Advanced NSCLC. J. Clin. Oncol. 2009, 27, 3217–3224. [Google Scholar] [CrossRef]
- Le Chevalier, T.; Scagliotti, G.; Natale, R.; Danson, S.; Rosell, R.; Stahel, R.; Thomas, P.; Rudd, R.M.; Vansteenkiste, J.; Thatcher, N.; et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: A meta-analysis of survival outcomes. Lung Cancer 2005, 47, 69–80. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Wang, W.; Schiller, J.H.; Langer, C.J.; Sandler, A.B.; Belani, C.P.; Johnson, D.H.; Eastern Cooperative Oncology Group. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J. Thorac. Oncol. 2006, 1, 441–446. [Google Scholar] [CrossRef]
- Ohe, Y.; Ichinose, Y.; Nakagawa, K.; Tamura, T.; Kubota, K.; Yamamoto, N.; Adachi, S.; Nambu, Y.; Fujimoto, T.; Nishiwaki, Y.; et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 4206–4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scagliotti, G.V.; Parikh, P.; Von Pawel, J.; Biesma, B.; Vansteenkiste, J.; Manegold, C.; Serwatowski, P.; Gatzemeier, U.; Digumarti, R.; Zukin, M.; et al. Phase III Study Comparing Cisplatin Plus Gemcitabine with Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients With Advanced-Stage Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2008, 26, 3543–3551. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [Green Version]
- Skipper, H. Data and Analysis Having to do with the Influence of Dose Intensity and Duration of Treatment (Single Drugs and Combination) on Lethal Toxicity and the Therapeutic Response of Experimental Neoplasm; Southern Research Institute: Birmingam, UK, 1987. [Google Scholar]
- Mazilu, L.; Stanculeanu, D.L.; Gheorghe, A.D.; Suceveanu, A.P.; Parepa, I.R.; Stoian Pantea, A.; Suceveanu, A.I. Clinical Impact of Association between Diabetes and Lung Cancer. Rev. Chim. 2019, 70, 1149–1151. [Google Scholar] [CrossRef]
- Luciani, A.; Bertuzzi, C.; Ascione, G.; Di Gennaro, E.; Bozzoni, S.; Zonato, S.; Ferrari, D.; Foa, P. Dose intensity correlate with survival in elderly patients treated with chemotherapy for advanced non-small cell lung cancer. Lung Cancer 2009, 66, 94–96. [Google Scholar] [CrossRef] [PubMed]

| Regimen | Administration |
|---|---|
| paclitaxel 200 mg/m2 + carboplatin AUC = 6 | q3w |
| paclitaxel 200 mg/m2 + carboplatin AUC = 6 + bevacizumab 15 mg/m2 | q3w |
| pemetrexed 500 mg/m2 + carboplatin AUC = 6 | q3w |
| gemcitabine 1000 mg/m2 +carboplatin AUC = 4.5 | gemcitabine days 1, 8, 15, whole regimen q4w |
| gemcitabine 1250 mg/m2 day 1, 8 + cisplatin 80 mg/2 | gemcitabine days 1, 8 whole regimen q3w |
| docetaxel 75 mg/m2+ cisplatin 75 mg/m2 | q3w |
| Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 62 (48.07%) |
| Female | 67 (51.93%) |
| Age | |
| Mean | 62 |
| Range | (29–80) |
| Histology | |
| Squamous cell carcinoma | 37 (28.7%) |
| ADK | 88 (68.2%) |
| Other | 4 (3.1%) |
| Proportion of chemotherapy regimens | |
| paclitaxel + carboplatin | 54 (41.9%) |
| paclitaxel + carboplatin + bevacizumab | 16 (12.4%) |
| pemetrexed + carboplatin | 16 (12.4%) |
| gemcitabine + carboplatin | 34 (26.4%) |
| Other | 9 (7%) |
| Toxicity | |
| No toxicity | 21 (16.27%) |
| Grade 1 | 26 (20.15%) |
| Grade 2 | 34 (26.35%) |
| Over Grade 2 | 48 (37.20%) |
| Type of toxicity over grade 2 | |
| Hematological | 40 (31.8%) |
| Non-hematological (deafness, peripheral neuropathy, pain) | 8 (6.2%) |
| Dose reduction | |
| 75% dose reduction | 7 (5.42%) |
| dose reduction between 85% and 75% | 11 (8.57%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitipir, C.; Orlov-Slavu, C.; Olaru, M.; Parosanu, A.; Popa, A.-M.; Iaciu, C.; Popescu, B.C.; Barbu, M.A.; Pirlog, C.; Calu, V.; et al. The Importance of Dose Intensity When Administering Cytotoxic Chemotherapy in NSCLC—A Matter as Actual Now as in the Past. Processes 2020, 8, 936. https://doi.org/10.3390/pr8080936
Nitipir C, Orlov-Slavu C, Olaru M, Parosanu A, Popa A-M, Iaciu C, Popescu BC, Barbu MA, Pirlog C, Calu V, et al. The Importance of Dose Intensity When Administering Cytotoxic Chemotherapy in NSCLC—A Matter as Actual Now as in the Past. Processes. 2020; 8(8):936. https://doi.org/10.3390/pr8080936
Chicago/Turabian StyleNitipir, Cornelia, Cristina Orlov-Slavu, Mihaela Olaru, Andreea Parosanu, Ana-Maria Popa, Cristian Iaciu, Bogdan Catalin Popescu, Maria Alexandra Barbu, Cristina Pirlog, Valentin Calu, and et al. 2020. "The Importance of Dose Intensity When Administering Cytotoxic Chemotherapy in NSCLC—A Matter as Actual Now as in the Past" Processes 8, no. 8: 936. https://doi.org/10.3390/pr8080936






