1. Introduction
Sanitation processes (i.e., cleaning and disinfection) are mandatory in the food industry to ensure that the production of safe food is always maintained. Due to this, daily sanitation is a common procedure in food production areas. However, this practice has several drawbacks such as high cost, high water usage, hazardous chemical effluents, and chemical residues in processing equipment which may affect food safety and quality. Hence, nowadays food producers and consumers demand green sanitation chemicals. Green sanitation is, in essence, using cleaning detergents and disinfection chemicals that are eco-friendly and do not emit pollutants.
Green cleaning detergents may seem weaker or less potent compared with available commercial cleaning chemicals in the market. However, green sanitizers such as electrolyzed water (EW) have shown significant potential in cleaning and sanitation for different types of food processing surfaces such as stainless steel [
1,
2,
3,
4,
5,
6,
7], bamboo and wood [
8], rubbers [
9], and tiles [
8,
10]. Moreover, EW has several advantages that are favorable for a food manufacturer, especially small and medium companies [
11]. Their budget allocation for sanitation and wastewater treatment are often at a minimum due to financial barriers. EW is reported to be inexpensive, uses on-site generation, and does not require a considerable amount of detergent storage space [
1,
4,
12]. Moreover, in contact with organic matter or when diluted by tap water, osmosis water, and distilled water, acidic electrolyzed water (AcEW) will revert to its original form [
1,
4,
13,
14]. Thus, wastewater treatment costs can be reduced.
There are three main types of chemicals used for the cleaning and disinfection process: alkaline (e.g., sodium hydroxide), acidic (e.g., nitric acid), and disinfectants (e.g., hydrogen peroxide, sodium hypochlorite) [
15]. Carbohydrate-based fouling deposits such as pink guava puree fouling deposit [
16,
17,
18] and chili sauce fouling deposit [
19] are alkaline soluble. In contrast, mineral-based fouling deposits such as calcium are developed mostly from beer [
20,
21] and dairy [
22,
23,
24] and are acid soluble [
17]. Various types of fouling deposits or food soils have different characteristics which require different cleaning processes and cleaning chemicals [
15,
16]. Alkaline electrolyzed water (AlEW) and AcEW are potential clean-label alternatives to the food industry for alkaline wash and acidic wash, respectively. Moreover, AcEW can also be an alternative disinfectant due to its antimicrobial properties for foodborne pathogens such as
Escherichia coli [
25,
26,
27],
Salmonella [
28,
29,
30] and
Listeria monocytogenes [
10,
25,
26].
Liquids at oxidation-reduction potential (ORP) ranges of 200 to 800 mV and −700 to 200 mV, respectively, are conditions in which aerobic bacteria and anaerobic bacteria can grow optimally [
31]. Huang et al. [
31] reported that at pH of 4 to 9, bacteria can also grow optimally. In this research, we target AcEW with a low pH range of 2.3 to 2.7, high ORP of more than 800 mV, and high chlorine content. The target for AlEW is set at a high pH of 11 to 12 and low ORP at below −700 mV. Hypochlorous acid (HOCl) [
32], hypochlorite ion [
32,
33,
34], and chlorine gas [
32,
33,
34] are chlorine species in AcEW, and are responsible for the antimicrobial properties against different foodborne pathogens. At low pH of 2.3 to 2.7, AcEW sensitizes the bacterial cell’s outer membrane and allows HOCl to penetrate the cells of the bacteria [
35].
Several works in the literature have reported the effect of different electrolyzing parameters, which are the electrode types [
36,
37,
38,
39], electrode electrical conductivity [
12,
40], electrode’s exchange current density [
12,
41], NaCl concentration [
42,
43], salt type [
14], flow rate [
42,
43], temperature [
42,
43], electrical potentials [
36,
37], and electrolysis time [
37] on the physical and chemical properties of AcEW. In contrast, studies on the usage of AlEW are still not well defined in the literature.
Hsu et al. [
36] reported that a platinum–platinum (cathode–anode) pairing with higher electrical conductivity (
σ = 9.43 × 10
6 S/m) produced 3325 mg Cl
2/L, compared to a titanium–titanium pairing (
σ = 2.38 × 10
6 S/m), which only produced 3 mg Cl
2/L. Hsu et al. [
36] concluded that the electrical conductivity of an electrode is mainly responsible for the production of chlorine. However, studies conducted by Khalid et al. [
12] revealed that a titanium–silver pairing with higher electrical conductivity (
σ of silver = 6.3 × 10
7 S/m) produced a lower chlorine content compared with a titanium–stainless steel pairing (
σ of stainless steel= 1.45 × 10
6 S/m) (0.17 and 0.5 mg Cl
2/L, respectively). The quantity of chlorine generated is significantly low in the study conducted by Khalid et al. [
12] as the lab-scale electrolyzing unit has a low current efficiency. According to Natarajan [
41], higher exchange current density on electrodes will increase the hydrogen gas production. The exchange current density
io for H
+/H
2 reaction on platinum is about 10
−2 A/cm
2, while the
io for H
+/H
2 reaction on zinc is about 10
−11 A/cm
2. As such, reducing the hydrogen ions from the acidic electrolyte on the platinum electrode is easier, compared to the zinc electrode. The zinc electrode possesses a high hydrogen-overpotential (activation polarization). Lower
io leads to higher overpotential, while higher
io denotes lower overpotential. Thus, the reaction on zinc with higher
io, are tends towards reversibility. In our work, we are focusing on chlorine generation, and the work carried out by Natarajan [
41] can be used as a reference.
Salt concentration, temperature, and water flow rate do not affect the electrical potential and power consumption of an electrolysis generator [
42]. In the work by Hsu [
43], salt concentration, temperature, and water flow rate have no impact on the pH and dissolved oxygen (DO) of AcEW. An increase in flow rate, however, reduces the efficiency of electrolysis (production of chlorine) [
42,
43]. The increase in the ORP and electrical conductivity of AcEW can be explained by the increase in the salt concentration [
43]. The temperature has a minor effect on the total chlorine concentration [
43]. Increasing the electrical potential will increase the electrical current flow and eventually increase the chlorine production [
40]. Increasing the electrolysis time would then also increase chlorine production [
37]. However, as the total chlorine production approaches the maximum level, the electrolysis time has no significant effect on the total chlorine production.
Various electrolytic variables have been reported to affect the properties of EW. To obtain the desired EW properties, many electrolysis parameters and their interaction must be considered simultaneously. Response surface methodology (RSM) was employed to understand the functional relationship between EW properties and the electrolysis parameters. RSM has been commonly used for optimizing and improving several processes. For processes using multiple variables, RSM can be employed to determine the interactions among the tested variables at different ranges. The RSM models generated are used to describe the effects of different variables on the response [
37,
44,
45,
46,
47,
48]. In this research, the process optimization of electrolyzing parameters for electrolyzed water (alkaline and acidic) was investigated using RSM.
The aim of this research is to identify the optimum electrolysis parameters (the types of electrodes, electrical voltages, electrolysis times, and NaCl concentrations) based on the physico-chemical properties (free chlorine, total chlorine, pH, dissolved oxygen, oxidation-reduction potential (ORP), electrical conductivity, and pH) of electrolyzed water (acidic and alkaline). The optimum condition was used to prepare the best EW. The bactericidal activity of the EW was evaluated against Escherichia coli at different temperatures. The results from this paper can be used as a guideline to find the best operational parameters of an EW generator and to formulate a suitable green cleaning solution, as different types of fouling deposits require different properties of cleaning solutions.
2. Materials and Methods
2.1. Laboratory-Scale Batch Electrolysis Unit
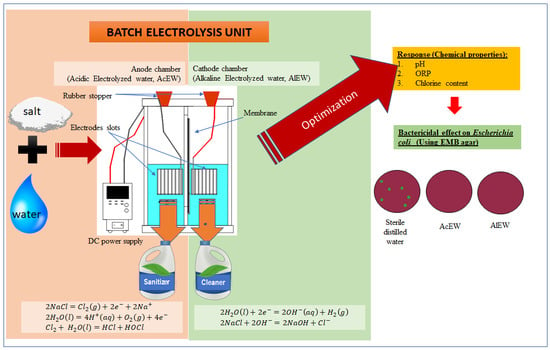
In this work, an electrolysis unit was used (
Figure 1). This lab benchtop batch electrolysis unit was designed and installed at the Process and Food Engineering Department, Engineering Faculty, Universiti Putra Malaysia (UPM). This unit consists of an acrylic glass electrolysis chamber (which can be filled with up to 3 L of electrolyte for each chamber) and direct current (DC) power supply (PSW 30–36, with output voltage range of 0–30 V and output current of 0–36 A, GW Instek, Taiwan). The electrolysis unit consists of a cathode chamber and an anode chamber, which are separated by a membrane (polyester ultrafiltration membrane) that allows ion exchange. The DC power supply allows for the manipulation of the current inlet. Electrode slots were designed to ensure the electrode plates faced each other, which maximizes the ion exchange between electrodes. The gap between the electrodes is 15 mm. This slot allows the electrodes to stay in an immobile position and maintain the same gap throughout the electrolysis process.
2.2. Electrode Preparation
In this work, titanium, zinc, copper, and stainless steel 316 were used. The electrode materials were chosen based on several criteria: high electrical conductivity, corrosion resistivity, erosion resistivity, ability to catalyze the electrode reactions, affordability, and availability in the Malaysian market. The electrode is 100 mm wide, 100 mm long, and 1 mm thick. The electrodes were soaked in acetone 1% (EAM, Malaysia) for 3 h. The electrodes were then rinsed with distilled water. Next, the electrodes were soaked in ethanol 1% (R&M Chemicals, United Kingdom) for 10 min. After that, the electrodes were rinsed three times with distilled water and air dried for 24 h. The electrodes were placed in a desiccator prior to the electrolysis experiments. Titanium, zinc, copper, and stainless steel were used alternately as an anode. Stainless steel was placed at the cathode. All of these materials were supplied by Jetro Engineering Works, Malaysia.
2.3. Electrolysis Experiments
Sodium chloride (R&M Chemicals, London, United Kingdom) was diluted with distilled water at different dilution concentrations (0.05, 0.53, and 1 wt.% NaCl). The diluted salt solution was then poured into the electrolysis chambers (1.7 L evenly for each chamber). The cathode and anode electrodes were assembled and inserted inside the electrolysis chambers. Stainless steel was used as the cathode, and copper was used as the anode. Different inlet voltages (5, 10, and 15 V) were applied to the electrodes. Stoppers were used to ensure that the wires connecting the electrodes and DC power supply stayed static during the electrolysis process. The slots were also designed to ensure that the anode and cathode electrodes stayed facing each other and did not move. The electrolysis process was conducted at different electrolyzing durations (5, 7.5, and 10 min). At the end of the electrolysis process, AcEW and AlEW were collected from the anode and cathode chambers, respectively. The final current inlet was recorded as well. A similar procedure was repeated using zinc, titanium, and stainless steel as the anode.
2.4. Analytical Measurement of Electrolyzed Water
A compact photometer (PF-3, Macherey-Nagel, Düren, Germany) was used to measure the free chlorine. Powder pillow free chlorine (Visocolor, Macherey-Nagel, Düren, Germany), which contains N,N-diethyl-1,4-phenylene diamine (DPD) was added to a 5 mL sample (electrolyzed water). The available free chlorine reacts with DPD to form a red-violet dye, which can be analyzed photometrically. The pH was measured through a portable pH meter (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The ORP was measured using a handheld ORP meter (Boeco, Hamburg, Germany). The DO was measured using a handheld DO meter (Hanna Instruments, Woonsocket, RI, USA). The electrical conductivity (unit in mS) was measured with a portable conductivity meter (Aqualytic, Germany). A rapid test using an H2O2 test stick (Macherey-Nagel, Germany) was used to test the H2O2 content in AcEW. In this work, the chemical properties of sodium hypochlorite, NaOCl (R&M Chemicals, United Kingdom), hydrogen peroxide, H2O2 (R&M Chemicals, United Kingdom), and household bleach (Kuat Harimau, Malaysia) were measured and compared with the optimized results of AcEW obtained from this research.
2.5. Electrolysis Efficiency for Chlorine Production
Current efficiency is, according to Faraday’s law, the ratio of the actual mass of a substance liberated from an electrolyte by the current’s passage to the theoretical mass liberated [
12]. Equations (1) to (3) were used to calculate the theoretical total chlorine [
12]. The mass of chlorine produced from the electrolysis experiments was calculated using Equation (4). Equations (5) to (7) were used to calculate current density, current efficiency, and electrical efficiency, respectively [
36,
37,
40,
49].
2.6. Experimental Design, Statistical Analysis, and Optimization
In this work, an experimental design called Box–Behnken (BBD) for response surface method (RSM) was applied using the Design Expert software (Stat-Ease Inc., Minneapolis, MN, USA). Wang et al. [
46] and Cui et al. [
48] mentioned that BBD is sufficient to fit a quadratic model which contains squared terms and products of two factors. Therefore, BBD is adequate to test the statistical validity of the fitted model and the model’s lack of fit. For this three-factorial BBD, a total of 17 experiments were performed (12 experiments (each factor on three levels) plus five central experiments). In this particular study, the factors were voltages (5, 10, and 15 V), electrolyzing times (5, 7.5, and 10 min), and NaCl concentrations (0.05, 0.53, and 1 wt.%) as shown in
Table 1. The predicted responses were pH, ORP, and free chlorine electrolyzed.
Table 2 shows the desired target characteristics of EW.
The following quadratic equation in Equation (8) was applied to fit the experimental data [
47,
48]:
where
is the linear coefficient,
is the interaction coefficient, and
is the quadratic coefficient.
and
are the coded values of the electrolyzed water’s variables (voltages, electrolyzing times, and NaCl concentrations), while the predicted responses are pH, ORP, and chlorine content. At a confidence level of 95% (
p < 0.05), the correlations were considered statistically significant [
47]. The coefficient of determination (
R2) and the adjusted determination coefficient (
R2adj) were used to evaluate the adequacy and the reliability of the model [
44,
47].
2.7. Experimental Validation
The adequacy of the RSM models was validated. Two additional experiments were carried out by using the optimized condition of the best electrodes. Equation (9) was used to calculate the validity of this study.
2.8. Bacterial Culture Preparation
The optimized EW was used for microbiological testing.
Escherichia coli ATCC 10536 obtained from the Food Microbiology Research Laboratory 1, Food Science and Technology Faculty, UPM was used as the test bacteria. Under refrigeration (at or below 4 °C), stock cultures were kept on nutrient agar (Oxoid, United Kingdom). Prior to the microbiological testing, the inocula were prepared from overnight culture growth on nutrient agar at 37 °C. The turbidity of the bacterial suspension was adjusted to a 0.5 McFarland standard (comparable to a bacterial suspension of approximately 10
8 CFU/mL) [
50]. A loopful of the culture was diluted in phosphate-buffer saline 0.1% (Sigma-Aldrich, St. Louis, MO, USA). The final concentration of 10
8 CFU/mL was adjusted using a spectrophotometer according to the 0.5 McFarland standard.
2.9. EW Treatment and Microbiology Analysis
The antimicrobial activity of EW (AcEW and AlEW) against Escherichia coli ATCC 25922 was initially determined by mixing 2.0 mL of Escherichia coli (1.5 × 108 CFU/mL) with 18 mL of EW in sterile universal bottles. Survival of Escherichia coli was determined immediately after mixing for 30 s, by the spread-plate method with usage of Eosin Methylene Blue agar (Oxoid, England, United Kingdom) with serial dilution in sterile peptone water 0.1% (Oxoid, England, United Kingdom). Then, the plates were incubated for 24 h, and the colonies formed on the plates were subsequently counted. The result is expressed in log CFU/mL. Treatment using sterile distilled water was used as control. All treatments were conducted in triplicate.
2.10. Statistical Analysis
Using Minitab version 17.0, the selection of the best electrode was analyzed through two-way ANOVA. The types of electrodes and voltages were set as factors and the experimental chlorine, theoretical chlorine, current efficiency, and current were set as responses.
Meanwhile, the results of the microbiology tests were subjected to one-way ANOVA. The treatment was set as a factor and the surviving population of Escherichia coli was set as a response. The results are described as deviations of the means±standard error. ANOVA has been used to compare the means. Using the Tukey test, a significant difference between treatments was established at a significance level of p less than 0.05.