Optimization of Synthesizing Upright ZnO Rod Arrays with Large Diameters through Response Surface Methodology
Abstract
:1. Introduction
2. Experimental Sections
2.1. The Pre-Treatment of the Substrates
2.2. Deposition of Seed Layers on the Substrates
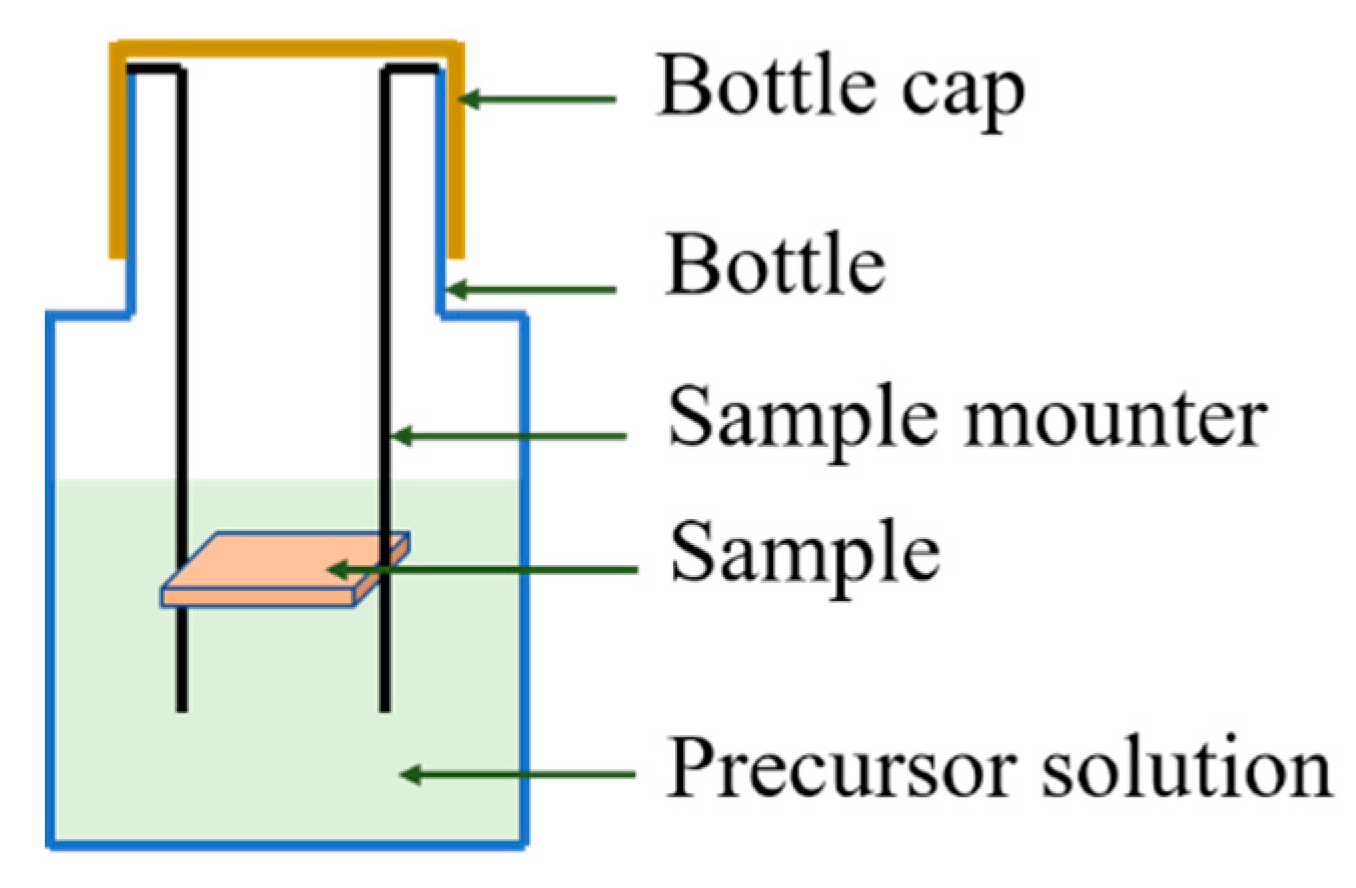
2.3. Pre-Growth of ZnO Nanorods
2.4. Preparation of Dense ZnO Films
2.5. Characterization of the Obtained ZnO Rods Arrays
2.6. Experimental Design
3. Results and Discussion
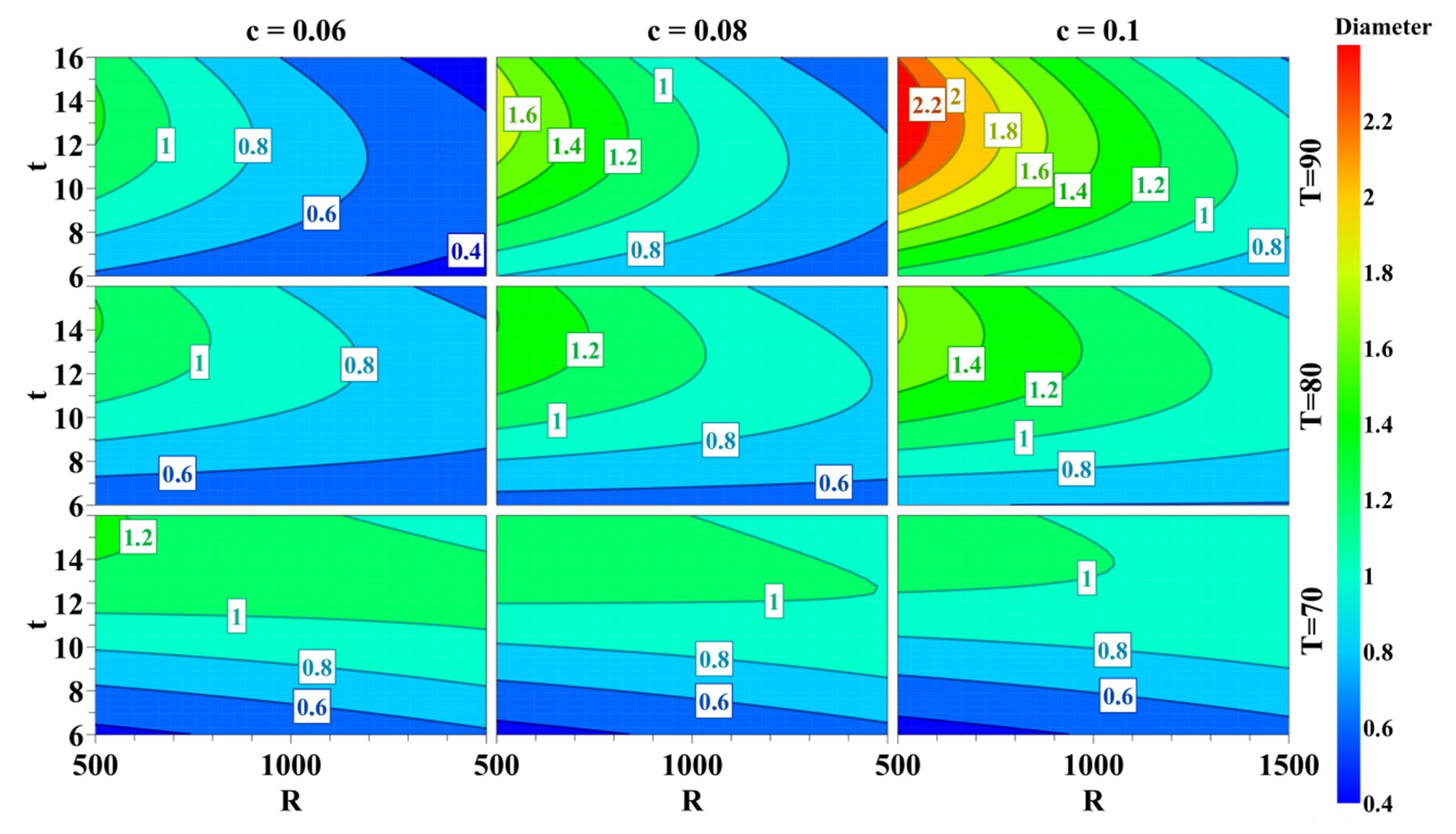
3.1. Optimization of the Experimental Parameters
3.2. Structure and Morphologies of the Obtained ZnO Rod Arrays
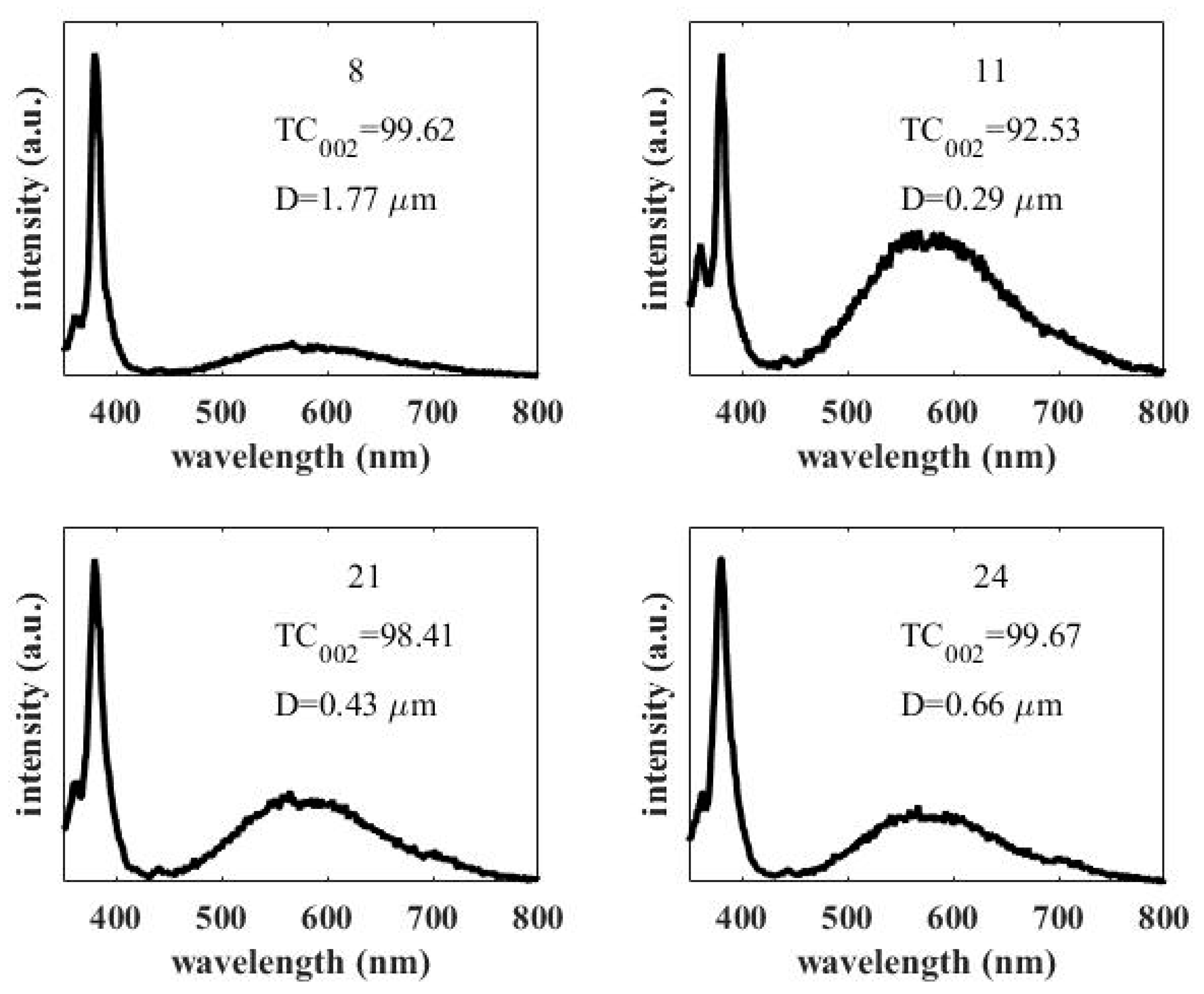
3.3. Photoluminescence Spectra of the Obtained ZnO Rod Arrays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiong, S.; Ma, J.; Volz, S.; Dumitrică, T. Thermally-Active Screw Dislocations in Si Nanowires and Nanotubes. Small 2014, 10, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiong, S.; Zhang, X.; Volz, S.; Hu, M. Thermal transport crossover from crystalline to partial-crystalline partial-liquid state. Nat. Commun. 2018, 9, 4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; Selli, D.; Neogi, S.; Donadio, D. Native surface oxide turns alloyed silicon membranes into nanophononic metamaterials with ultralow thermal conductivity. Phys. Rev. B 2017, 95, 180301. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Gao, R.; Lan, J.-L.; Wiranwetchayan, O.; Zhang, Q.; Li, C.; Cao, G. Growth of vertically aligned ZnO nanowalls for inverted polymer solar cells. Sol. Energy Mater. Sol. Cells 2013, 117, 34–40. [Google Scholar] [CrossRef]
- Lai, F.-I.; Yang, J.-F.; Hsu, Y.-C.; Kuo, S.-Y. Omnidirectional light harvesting enhancement of dye-sensitized solar cells decorated with two-dimensional ZnO nanoflowers. J. Alloy. Compd. 2020, 815, 152287. [Google Scholar] [CrossRef]
- Chandrasekhar, P.; Dubey, A.; Qiao, Q. High efficiency perovskite solar cells using nitrogen-doped graphene/ZnO nanorod composite as an electron transport layer. Sol. Energy 2020, 197, 78–83. [Google Scholar] [CrossRef]
- Wang, Z.L.; Zhu, G.; Yang, Y.; Wang, S.; Pan, C. Progress in nanogenerators for portable electronics. Mater. Today 2012, 15, 532–543. [Google Scholar] [CrossRef]
- Jin, C.; Hao, N.; Xu, Z.; Trase, I.; Nie, Y.; Dong, L.; Closson, A.; Chen, Z.; Zhang, J.X. Flexible piezoelectric nanogenerators using metal-doped ZnO-PVDF films. Sensors Actuators A Phys. 2020, 305, 111912. [Google Scholar] [CrossRef]
- Chen, M.-T.; Lu, M.-P.; Wu, Y.-J.; Song, J.; Lee, C.-Y.; Lu, M.-Y.; Chang, Y.-C.; Chou, L.-J.; Wang, Z.L.; Chen, L.J. Near UV LEDs Made with in Situ Doped p-n Homojunction ZnO Nanowire Arrays. Nano Lett. 2010, 10, 4387–4393. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, Y.; Yu, Y.; Han, X.; Wang, Y.; Shi, Z.; Dong, X.; Zhang, B.; Du, G.; Liu, Y. High-Performance Ultraviolet Light-Emitting Diodes Using n-ZnO/p-hBN/p-GaN Contact Heterojunctions. ACS Appl. Mater. Interfaces 2020, 12, 6788–6792. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.; Peleckis, G.; Wang, X.; Siegel, R.W.; Borca-Tasciuc, T.; Dou, S.; Ramanath, G. Al-Doped Zinc Oxide Nanocomposites with Enhanced Thermoelectric Properties. Nano Lett. 2011, 11, 4337–4342. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.; Singh, D.J.; Wu, P. Analysis of the thermoelectric properties ofn-type ZnO. Phys. Rev. B 2011, 83, 115110. [Google Scholar] [CrossRef]
- Xiong, S.; Sääskilahti, K.; Kosevich, Y.A.; Han, H.; Donadio, D.; Volz, S. Blocking Phonon Transport by Structural Resonances in Alloy-Based Nanophononic Metamaterials Leads to Ultralow Thermal Conductivity. Phys. Rev. Lett. 2016, 117, 025503. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Latour, B.; Ni, Y.; Volz, S.; Chalopin, Y. Efficient phonon blocking in SiC antiphase superlattice nanowires. Phys. Rev. B 2015, 91, 224307. [Google Scholar] [CrossRef]
- Sheng, X.; Li, Z.; Cheng, Y. Electronic and Thermoelectric Properties of V2O5, MgV2O5, and CaV2O5. Coatings 2020, 10, 453. [Google Scholar] [CrossRef]
- Menzel, A.; Subannajui, K.; Güder, F.; Moser, D.; Paul, O.; Zacharias, M. Multifunctional ZnO-Nanowire-Based Sensor. Adv. Funct. Mater. 2011, 21, 4342–4348. [Google Scholar] [CrossRef]
- Vinoth, E.; Gopalakrishnan, N. Fabrication of interdigitated electrode (IDE) based ZnO sensors for room temperature ammonia detection. J. Alloy. Compd. 2020, 824, 153900. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Jönsson, P.G.; Zhao, Z. Optimization of high-quality vertically aligned ZnO rod arrays by the response surface methodology. J. Alloy. Compd. 2015, 626, 180–188. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Jönsson, P.G.; Zhao, Z. Improvement and optimization of the growth quality of upright ZnO rod arrays by the response surface methodology. Appl. Surf. Sci. 2015, 351, 451–459. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Pramanik, A. Morphology control of ZnO with citrate: A time and concentration dependent mechanistic insight. CrystEngComm 2013, 15, 6349. [Google Scholar] [CrossRef]
- Nicholas, N.J.; Franks, G.; Ducker, W.A. Selective Adsorption to Particular Crystal Faces of ZnO. Langmuir 2012, 28, 7189–7196. [Google Scholar] [CrossRef] [PubMed]
- Lifson, M.L.; Levey, C.; Gibson, U.J. Diameter and location control of ZnO nanowires using electrodeposition and sodium citrate. Appl. Phys. A 2013, 113, 243–247. [Google Scholar] [CrossRef]
- Suwanboon, S.; Somraksa, W.; Amornpitoksuk, P.; Randorn, C. Effect of trisodium citrate on the formation and structural, optical and photocatalytic properties of Sr-doped ZnO. J. Alloy. Compd. 2020, 832, 154963. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; He, X.; Bensong, C.; Chen, B. Urchin-like ZnO-nanorod arrays templated growth of ordered hierarchical Ag/ZnO hybrid arrays for surface-enhanced Raman scattering. Nanotechnology 2020, 31, 165301. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, K.A.; Reidy, R.F. Handbook for Cleaning for Semiconductor Manufacturing: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 67. [Google Scholar]
- Xiong, S.; Yang, K.; Kosevich, Y.A.; Chalopin, Y.; D’Agosta, R.; Cortona, P.; Volz, S. Classical to Quantum Transition of Heat Transfer between Two Silica Clusters. Phys. Rev. Lett. 2014, 112, 114301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, B.; Xiong, S.; Yang, Z.; Jing, Y.; Wang, Y. MoS 2 heterostructure with tunable phase stability: Strain induced interlayer covalent bond formation. Nanoscale 2017, 9, 8126–8132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Jönsson, P.G.; Zhao, Z. Controllable fabrication of large-area 2D colloidal crystal masks with large size defect-free domains based on statistical experimental design. Appl. Surf. Sci. 2014, 313, 144–151. [Google Scholar] [CrossRef]
- Liao, Z.-M.; Zhang, H.; Zhou, Y.; Xu, J.; Zhang, J.-M.; Yu, D. Surface effects on photoluminescence of single ZnO nanowires. Phys. Lett. A 2008, 372, 4505–4509. [Google Scholar] [CrossRef]





| No. | c/M | T/°C | t/h | R/ | TC002/ | Aspect Ratio/ | D/μm |
|---|---|---|---|---|---|---|---|
| 1 | 0.06 | 70 | 6 | 500 | 80.71 | 6.61 | 0.26 |
| 2 | 0.1 | 70 | 6 | 500 | 95.92 | 4.76 | 0.36 |
| 3 | 0.06 | 90 | 6 | 500 | 96.12 | 1.45 | 0.70 |
| 4 | 0.1 | 90 | 6 | 500 | 69.05 | 1.45 | 1.20 |
| 5 | 0.06 | 70 | 16 | 500 | 91.84 | 1.23 | 1.47 |
| 6 | 0.1 | 70 | 16 | 500 | 97.67 | 1.71 | 1.03 |
| 7 | 0.06 | 90 | 16 | 500 | 97.95 | 1.31 | 1.11 |
| 8 | 0.1 | 90 | 16 | 500 | 99.62 | 0.96 | 1.77 |
| 9 | 0.06 | 70 | 6 | 1500 | 71.27 | 1.57 | 0.71 |
| 10 | 0.1 | 70 | 6 | 1500 | 95.09 | 2.51 | 0.52 |
| 11 | 0.06 | 90 | 6 | 1500 | 92.53 | 9.28 | 0.29 |
| 12 | 0.1 | 90 | 6 | 1500 | 97.90 | 2.55 | 0.71 |
| 13 | 0.06 | 70 | 16 | 1500 | 98.58 | 2.20 | 0.73 |
| 14 | 0.1 | 70 | 16 | 1500 | 91.27 | 2.30 | 0.75 |
| 15 | 0.06 | 90 | 16 | 1500 | 98.89 | 7.92 | 0.31 |
| 16 | 0.1 | 90 | 16 | 1500 | 97.04 | 2.42 | 0.71 |
| 17 | 0.06 | 80 | 11 | 1000 | 98.24 | 2.20 | 0.80 |
| 18 | 0.1 | 80 | 11 | 1000 | 94.53 | 2.18 | 0.96 |
| 19 | 0.08 | 70 | 11 | 1000 | 83.39 | 1.63 | 0.99 |
| 20 | 0.08 | 90 | 11 | 1000 | 98.35 | 1.95 | 0.74 |
| 21 | 0.08 | 80 | 6 | 1000 | 98.41 | 4.78 | 0.43 |
| 22 | 0.08 | 80 | 16 | 1000 | 98.43 | 1.62 | 1.08 |
| 23 | 0.08 | 80 | 11 | 500 | 94.76 | 2.30 | 1.20 |
| 24 | 0.08 | 80 | 11 | 1500 | 99.67 | 3.00 | 0.66 |
| 25 | 0.08 | 80 | 11 | 1000 | 93.11 | 1.65 | 1.18 |
| 26 | 0.08 | 80 | 11 | 1000 | 95.42 | 1.84 | 1.12 |
| 27 | 0.08 | 80 | 11 | 1000 | 93.10 | 1.78 | 1.27 |
| TC002 | DF a | SS b | MS c | F | p |
| Total | 27 | 236,668 | 8765.49 | ||
| Constant | 1 | 234,987 | 234,987 | ||
| Total corrected | 26 | 1681.06 | 64.656 | ||
| Regression | 6 | 764.311 | 127.385 | 2.779 | 0.039 |
| Residual | 20 | 916.751 | 45.838 | ||
| Lack of Fit (Model error) | 18 | 913.178 | 50.732 | 28.399 | 0.035 |
| Pure error (Replicate error) | 2 | 3.573 | 1.786 | ||
| Aspect Ratio | DF a | SS b | MS c | F | p |
| Total | 27 | 5.474 | 0.203 | ||
| Constant | 1 | 4.001 | 4.001 | ||
| Total corrected | 26 | 1.472 | 0.057 | ||
| Regression | 4 | 0.397 | 0.099 | 2.032 | 0.125 |
| Residual | 22 | 1.075 | 0.049 | ||
| Lack of Fit (Model error) | 20 | 1.074 | 0.054 | 106.606 | 0.009 |
| Pure error (replicate error) | 2 | 0.001 | 0.001 | ||
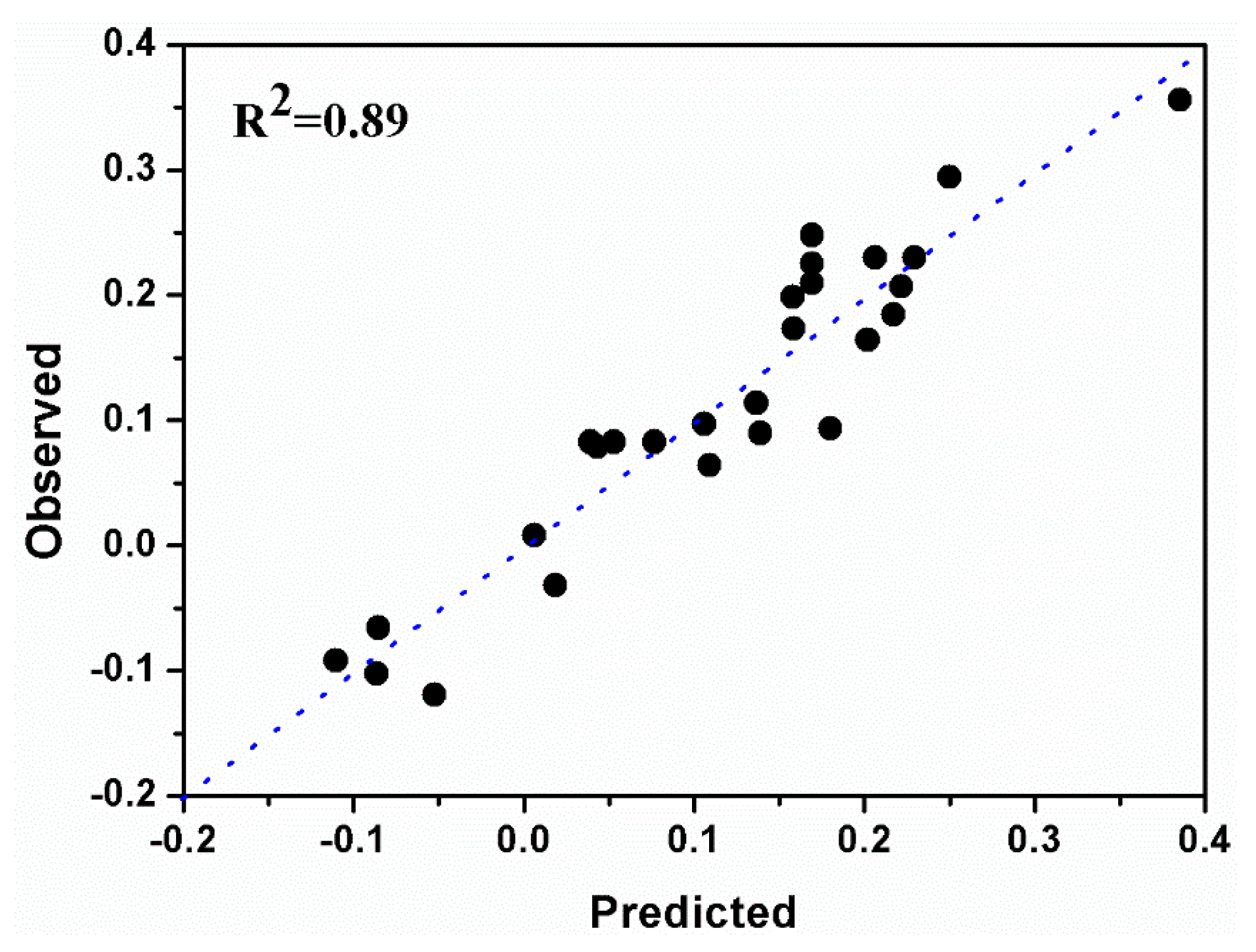
| Diameter | DF a | SS b | MS c | F | p |
| Total | 27 | 1.611 | 0.060 | ||
| Constant | 1 | 0.364 | 0.364 | ||
| Total corrected | 26 | 1.247 | 0.048 | ||
| Regression | 9 | 1.104 | 0.123 | 14.541 | 0.000 |
| Residual | 17 | 0.143 | 0.008 | ||
| Lack of Fit | 15 | 0.142 | 0.009 | 12.629 | 0.076 |
| (Model error) | |||||
| Pure error | 2 | 0.001 | 7.4 × 10−4 | ||
| (replicate error) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Cheng, Y.; Yao, Y.; Zhao, Z. Optimization of Synthesizing Upright ZnO Rod Arrays with Large Diameters through Response Surface Methodology. Processes 2020, 8, 655. https://doi.org/10.3390/pr8060655
Sheng X, Cheng Y, Yao Y, Zhao Z. Optimization of Synthesizing Upright ZnO Rod Arrays with Large Diameters through Response Surface Methodology. Processes. 2020; 8(6):655. https://doi.org/10.3390/pr8060655
Chicago/Turabian StyleSheng, Xiaofei, Yajuan Cheng, Yingming Yao, and Zhe Zhao. 2020. "Optimization of Synthesizing Upright ZnO Rod Arrays with Large Diameters through Response Surface Methodology" Processes 8, no. 6: 655. https://doi.org/10.3390/pr8060655





