Abstract
Curcumin (CUR) has been used since ancient times to treat several ailments as it possesses many pharmacological activities. This study intended to explore the mechanism underlying the protective effects of CUR in remodeling oxidative stress and apoptotic signals in cyclophosphamide (CP)-induced pulmonary injury in albino rats. CUR was administered at a dose of 300 mg/kg/day for 7 days and on the seventh day a single dose of CP (200 mg/kg) was given. Histopathological and ultrastructural examinations of CP-intoxicated rats showed complete alveolar obstruction, thickened inter-alveolar septa, enlarged blood vessels, severe inflammatory edema with pyknotic nuclei, and disappearance of cytoplasmic organelles. Significant increases in caspase-3, malondialdehyde (MDA), and protein carbonyl (PCO) and significant decreases in superoxide dismutase (SOD) and glutathione peroxidase (GPx) were observed. In contrast, rats that received CUR showed clear and empty lumina with single row of pneumocytes, disappearance of edema, and no interstitial electron dense bodies in rats’ lung tissues. Additionally, CUR significantly reduced caspase-3, MDA, and PCO and increased SOD and GPx. In conclusion, these findings revealed the protective effects of CUR against CP-induced pulmonary injury in rats through suppressing oxidative damage and apoptosis.
1. Introduction
Cyclophosphamide (CP) is an antineoplastic alkylating agent extensively used for treating various malignancies, including lymphoma, leukemia, multiple myeloma, ovarian cancer, breast cancer, and sarcoma [1]. Injuries of normal tissues beside other side effects have been reported as a result of widespread usage of CP [2]. CP-induced lung injury is one of the most common forms of iatrogenic diseases encountered because of the extensive CP treatment [2,3]. CP decreases the activity of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) as well as levels of non-enzymatic antioxidants [4,5,6,7]. At the same time, CP increased the lipid peroxidation product malondialdehyde (MDA) [8] and tumor necrosis factor-α (TNF-α) [9] in the lungs of mice and rats, respectively.
Reactive oxygen species (ROS) are strong oxidants that are produced as an end product of large numbers of biological processes. ROS are responsible for many pathological conditions due to their ability to destroy many host factors through altering the dynamic redox balance between oxidants and antioxidants [10]. In addition, protein carbonyl (PCO), a type of oxidized protein formed as a result of reaction with ROS, plays an important role in oxidative stress processes. It can react with ROS or other molecules such as lipids and sugars to generate reactive carbonyl derivatives (RCD), which are highly reactive with protein. PCO content in the heart, lung, and liver was found to be increased significantly due to cellular proteins damage by ROS and its accumulation may impair the cell function [11]. It is highly interesting to use PCO as a marker of cell damage as it is highly stable, reliable, early developed, and has a long life span [12,13]. Although the exact mechanism of CP-induced lung injury is not entirely clarified, many studies have proposed CP and its metabolites-induced oxidative stress in lung cells as the general mechanism of lung injury. In this context, more than one pathway was found to be involved in the generation of toxic metabolites of CP. The metabolites 4-hydroxycyclophosphamide and acrolein have been suggested to induce direct toxic effect on lung cells. These two metabolites can directly damage the lung cells through minimizing microsomal enzyme activity [14] and disturbing the antioxidant activity in lung [15,16,17].
For decades, a wide range of natural agents have been used effectively to treat various illnesses as they are safe and possess strong antioxidant and anti-inflammatory effects [18]. Curcumin (CUR), an antioxidative and anti-inflammatory natural polyphenol compound, is one of the major compounds of Curcuma longa. CUR exhibited highly interesting beneficial effects on systemic oxidative stress and in the treatment of several induced diseases in animals and patients suffering from pulmonary complications induced by chronic chemotherapy [3,19]. So, it received great consideration among scientists and researchers. CUR exerts in vivo and in vitro antioxidant effects through different mechanisms and it has the ability to neutralize ROS and reactive nitrogen species (RNS) [20,21,22,23,24]. CUR inhibits ROS across several cellular and animal models of different disorders [20,21,22,23,24,25]. It decreases the expression rates of certain proteins and lipid oxidation markers, prevents the rise in oxidative derivative compounds, and increases the levels of SOD, reduced glutathione (GSH), GPx, and CAT [20,21,22,23,24]. In addition, CUR has the ability to modulate and control several signaling pathways resulting in accelerating apoptosis, antiproliferation, and cytotoxicity of different cancer cells [26]. Previous studies have demonstrated that CUR can inhibit lung injury in a mouse model of viral-induced acute respiratory distress syndrome [27], ventilator-induced lung injury (VILI) in rats [19], and Klebsiella pneumoniae-induced lung injury in mice [28]. However, no relevant study has explored the underlying mechanism behind the effect of CUR on CP-induced pulmonary injury in albino rats. We hypothesized that CUR could confer strong protective effects on pulmonary injury caused by CP in rats. Therefore, this study was designed to investigate the possible protective mechanism of CUR against CP-induced oxidative stress, apoptosis, and pulmonary injury in rats.
2. Materials and Methods
2.1. Animals
Male albino rats weighing 200–250 g were obtained from the animal house of the Medical Research Center (MRC), Faculty of Veterinary Medicine, Zagazig University (Egypt). Rats were acclimatized to experimental conditions for one week and were fed a standard pellet ration (El-Nasr Chemical Company, Cairo, Egypt) and exposed to 12 h light/dark cycle. The study protocol and procedures have been implemented in compliance with the National Institutes of Health guidelines (NIH publication No. 85–23, revised 2011) and approved by the Local Animal Care Review Committee (ZU-IACUC/14/DEC/2018).
2.2. Experimental Design
Thirty–two rats were divided into four groups (n = 8) as follows:
- Group I (Control): Rats received saline containing 0.5% carboxymethylcellulose (CMC) orally for 7 days and a single intraperitoneal (i.p.) injection of saline at day 7.
- Group II (CUR): Rats received CUR (300 mg/kg/day, Sigma, St. Louis, MO, USA) [29] suspended in physiological saline containing 0.5% CMC orally for 7 days and a single i.p. injection of saline at day 7.
- Group III (CP): Rats received saline containing 0.5% CMC orally for 7 days and a single-dose of CP (200 mg/kg, Sigma, St. Louis, MO, USA) on the seventh day [30].
- Group IV (CUR + CP): Rats received CUR (300 mg/kg/day) [29] for 7 days and a single dose of CP (200 mg/kg) on the seventh day [30].
On the eighth day of the experiment, the animals were sacrificed under anesthesia and lung samples were collected immediately.
2.3. Histopathological Assessment
Lung tissue samples were fixed in 10% neutral buffered formalin solution for 48 h, dehydrated in gradual ascending ethyl alcohol (from 70% to absolute), cleared in xylene, and embedded in paraffin. The 5-µm-thick paraffin sections were sliced using a Leica RM 2155 microtome (Leica Biosystems, Buffalo Grove, IL, USA). The sections were prepared and routinely stained with hematoxylin and eosin (H&E) dyes for histopathology [31]. All chemicals used were supplied by Sigma (St. Louis, MO, USA). All section photos were photographed with a Leica® microscope combined with AmScope® microscope digital camera (Leica Biosystems, Buffalo Grove, IL, USA). Lesion scores were achieved following the lesion score system designed by Robert [32] and Gibson-Corley et al. [33]. The lesions scores were represented as follows: 0 = no alterations, +1 = mild, +2 = mild to moderate, and +3 = severe or diffuse alterations.
2.4. Ultrastructural Assessment
For ultrastructure examination, lung samples were trimmed into fine sections by a sharp blade, and immediately fixed in 2.5% glutaraldehyde (pH 7.2; Sigma, St. Louis, MO, USA) for 4 h and then transferred to 1.33% osmium tetroxide (Sigma, St. Louis, MO, USA) overnight at 4 °C. Following that, the specimens were prepared as previously described by Hayat [34]. Briefly, tissues were dehydrated, cleared, and embedded in epoxy resin, and semi-thin sections were obtained by ultra-microtome. The prepared sections were stained with toluidine blue (Sigma, St. Louis, MO, USA), evaluated by light microscope (Leica Biosystems, Buffalo Grove, IL, USA) to detect lesions, and uploaded on the grid, stained with lead citrate and uranium for examination by transmission electron microscopy (TEM, JEOL JEM-1230, JEOL Ltd, Tokyo, Japan).
2.5. Immunohistochemical Assessment of Caspase-3
Immunohistochemical staining was accomplished by using primary anti-caspase-3 (Abcam®, Cambridge, MA, USA) according to the previously described Avidin Biotin Complex (ABC) technique [35]. Briefly, 3-µm paraffin-embedded lung sections were deparaffinized in xylene followed by rehydration in descending graded ethanol and blocking in 5% bovine serum albumin (BSA; Sigma, St. Louis, MO, USA) in tris-buffered saline (TBS) for 2 h. After blocking, the sections were probed with rabbit polyclonal ant-caspase-3 overnight at 4 °C, washed thrice in TBS, and then incubated with goat anti-rabbit biotinylated secondary antibody (Abcam®, Cambridge, MA, USA) for 1 h at room temperature. Following washing in TBS, the sections were incubated for 10 min in 0.02% diaminobenzidine (DAB; Abcam®, Cambridge, MA, USA) containing 0.01% hydrogen peroxide and counterstained with hematoxylin (Sigma, St. Louis, MO, USA). The slides were visualized under light microscope and immunostaining densities were determined using ImagJ® software (version 1.32j, NIH, USA) as previously described [36].
2.6. Assessment of MDA, PCO, SOD, and GPx
The tissues samples were homogenized in cold Phosphate buffered saline (PBS; 10% w/v) using a tissue homogenizer. The homogenate was centrifuged at 10,000 g for 20 min at 4 °C, and supernatant was collected. The supernatant was used for the assessment of oxidative stress markers and antioxidant enzymes. Lipid peroxidation was determined using the thiobarbituric acid (TBA; Sigma, St. Louis, MO, USA) assay. This test was based on the reactivity of MDA with TBA to produce a red adduct [37]. SOD activity was assayed according to the method of Marklund and Marklund [38] based on the ability of SOD to inhibit pyrogallol autoxidation. GPx was assayed based on oxidation of GSH, and the reaction of the remaining GSH with 5,5-dithiobis-(2-nitrobenzoic acid; Sigma, St. Louis, MO, USA) produced a colored compound [39]. PCO was determined following the method of Levine et al. [40] and total protein content was estimated using Bradford reagent [41].
2.7. Statistical Analysis
Statistical significance was evaluated using Graphpad Prism 7 (La Jolla, CA, USA). The data were presented as mean ± standard error mean (SEM) and the means between the groups were assessed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The differences were considered significant at p value less than 0.05.
3. Results
3.1. CUR Prevents CP-Induced Lung Injury in Rats
3.1.1. Histopathological Findings
The examined sections from sacrificed rats at the end of experiment revealed normal histomorphology of pulmonary parenchyma, including normal airway bronchioles and blood vessels with apparently normal alveolar septa and pneumocytes in both control and CUR groups (Figure 1A,B). In contrast, the CP-treated group showed marked obstruction of alveoli and thickened interalveolar septal besides more enlarged blood vessels. This thickening was prominent due to severe inflammatory edema mixed with extravasated erythrocytes in the interalveolar septa along with inflammatory cells infiltration, mainly lymphocytes and few granulocytes. Other sections showed marked compensatory emphysema combined with thickened septa with prominent interstitial edema and inflammatory cells aggregations, which led to thickened septa (Figure 1C–G). On the other hand, CP-intoxicated rats treated with CUR (Figure 1H,I) revealed remodeling of the alveolar septa which were characterized by clear and empty lumen with single row of pneumocytes (Figure 1 and Table 1).
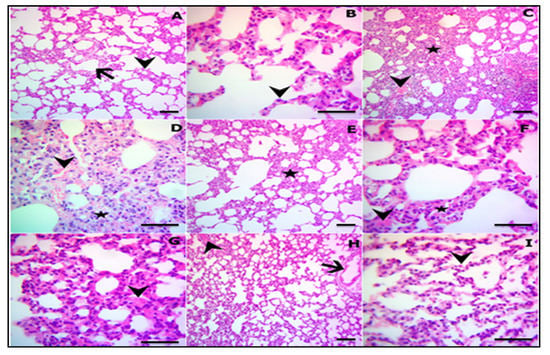
Figure 1.
Representative photomicrograph of the H&E-stained lung sections in control (A) and CUR groups (B) showing normal pulmonary parenchyma, air bronchioles, and blood vessels (arrow) with apparently normal alveolar septa (arrowhead) (A), and normal pneumocytes (arrowhead) (B). (C–G) CP-induced rats showing complete obstruction of the alveoli, thickened interalveolar septa (star), engorged blood vessels (arrowhead) (C), severe edema admixed with extravasated erythrocytes in the interalveolar septa (arrowhead), infiltration of inflammatory cells mainly lymphocytes and few granulocytes (star) (D), marked compensatory emphysema combined with thickened septa (star) (E), prominent interstitial edema (arrowheads), inflammatory cells aggregations, and thickened septa (F,G). (H,I) CP-induced rats pretreated with CUR showing restoration of the majority of lung structures, including airways (arrow) with still mlid congested blood vessels (arrowhead) (H), remodeled alveolar septa characterized by empty lumen and single row of pneumocytes (arrowhead) (I). (A,C,E,H X100; B,D,F,G,I X400).

Table 1.
Lesion severity grading of the 10 fields of lung sections.
3.1.2. Ultrastructure Findings
The ultrastructural findings revealed apparently normal alveolar capillary with erythrocytes, blood air barrier, pneumocytes type II with normal lamellar bodies, and surface microvilli in both control (Figure 2A) and CUR treated groups (Figure 2B). On the contrary, the CP-intoxicated group showed pyknotic nuclei with chromatolysis and disappearance of cytoplasmic organelles besides electron dense bodies in pneumocytes type II, and few collagen deposits in the proteinoid materials and nearly normal pneumocytes type I. Also, other sections showed marked thickened blood–air barrier due to proteinoid materials in the fused basal lamina, erythrocytes, disappearance of the cytoplasmic organelles which were replaced by electron dense bodies, and condensed chromatin in pneumocytes type II, which suffered loss of the surface microvilli. Moreover, marked necrotic pneumocytes type I and II with lysis of cytoplasm, few electron dense bodies, and numerous erythrocytes were seen in the CP group (Figure 2C–E). In addition, thickening of the air–blood barrier with collagen deposits, extravasated erythrocytes, interstitial electron dense bodies, and degenerated pneumocytes type I were also noticed in the CP group (Figure 2C–E). CP-intoxicated rats treated with CUR displayed apparently normal pneumocytes type I, mildly thickened blood–air barrier, restored pneumocytes type II components, including empty lamellar bodies and mitochondria, and prominent surface microvilli (Figure 2F). All the previous alterations and remodeling events were summarized and represented in Table 2.
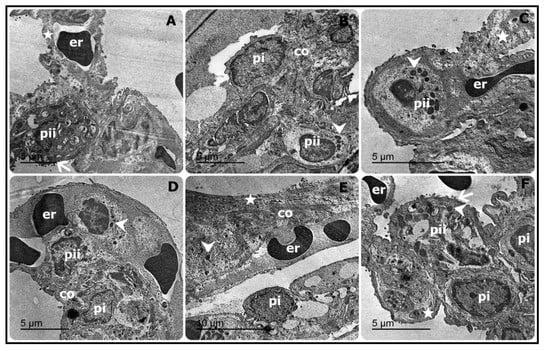
Figure 2.
Representative electron micrograph of the lung sections showing apparently normal alveolar capillary with erythrocytes (er), blood–air barrier (star), pneumocytes type II (pii) with normal lamellar bodies and surfaces microvilli (arrow) in control rats (A). Pyknotic nuclei with chromatolysis, disappearance of cytoplasmic organelles, electron dense bodies (arrowhead) in pneumocyte type II (pii), few collagen deposits (co) in the proteinoid materials along with nearly normal pneumocyte type I (pi) were observed in CP-induced rats (B). Marked thickening of blood–air barrier due to proteinoid materials in the fused basal lamina (star), erythrocytes (er), disappearance of the cytoplasmic organelles, which were replaced by electron dense bodies (arrowhead) along with condensed chromatin in pneumocyte type II (pii), which suffered loss of the surfaces microvilli (C), marked necrotic pneumocytes types I and II (pi and pii) with lysis of cytoplasm with still few electron dense bodies (arrowhead) besides numerous erythrocytes (er) (D) were also observed in CP-intoxicated rats. Moreover, CP-induced rats exhibited thickening of the air–blood barrier (star), collagen deposits (co), extravasated erythrocytes (er), interstitial electron dense bodies (arrowhead) and degenerated pneumocyte type I (pi) (E). In contrast, apparently normal pneumocytes type I (pi), mild thickened blood–air barrier (star), restored pneumocyte type II components (pii), including empty lamellar bodies, and mitochondria (arrowhead) as well as surface microvilli (arrow) were reported in CP-induced rats treated with CUR (F).

Table 2.
Ultrastructure criteria evaluations of pneumocytes type II and blood–air barriers among different experimental groups.
3.2. CUR Attenuates Apoptosis in Lung of CP-Induced Rats
Immunohistochemical staining of the apoptotic protien caspase-3 revealed normal expression in both control (Figure 3A) and CUR-supplemented rats (Figure 3B). In contrast, a marked increase in caspase-3 immunostaining, particularly in the thickened interstitial tissue, was seen in the CP-treated rat (Figure 3C), whereas mild to moderate expression was observed in numerous pneumocytes of CP-intoxicated rats treated with CUR (Figure 3D).
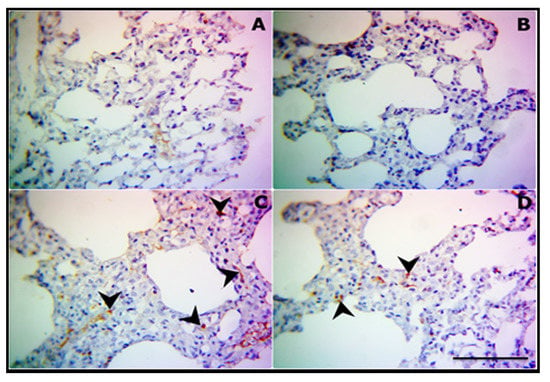
Figure 3.
CUR attenuates apoptosis in lung of CP-induced rats. Representative photomicrograph of caspase-3 expression in lung sections of control (A), CUR-supplemented (B), CP-intoxicated (C), and CP-intoxicated rats treated with CUR (D). (400×, scale bar = 50 µm).
The immuno-positve reaction areas percentage was determined in the stained sections from all groups and reperesented in Figure 4. The analysis revealed a significant increase (p ˂ 0.01) in caspase-3 in the lung of theCP group. CUR markedly decreased the expression levels of caspase-3 (p < 0.01) in the lung of the CP-intoxicated rats.
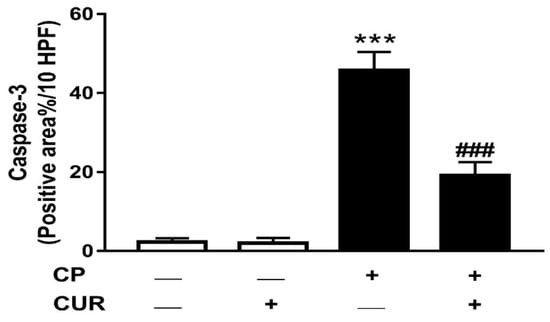
Figure 4.
Effect of CUR on caspase-3 in lung of control and CP-induced rats. Values are represented as mean ± SEM, n = 8, *** p < 0.001 versus control and ### p < 0.001 versus CP.
3.3. CUR Suppresses Oxidative Stress in Lung of CP-Induced Rats
To evaluate the antioxidant activity of CUR, MDA, PCO, and antioxidant enzymes were determined in the lung of control and CP-intoxicated rats. The results showed nonsignificant differences in lung MDA (Figure 5A), PCO (Figure 5B), SOD (Figure 5C), and GPx (Figure 5D) between the control and CUR-supplemented rats. On the other hand, the levels of MDA and PCO showed statistically significant increase (p < 0.001) in CP-intoxicated rats when compared with the control group. SOD and GPx in lung of the CP group exhibited statistically significant decrease (p < 0.001). CP-intoxicated rats treated with CUR exhibited statistically significant increase (p < 0.001) in SOD and GPx activities and significant decrease in MDA and PCO levels.
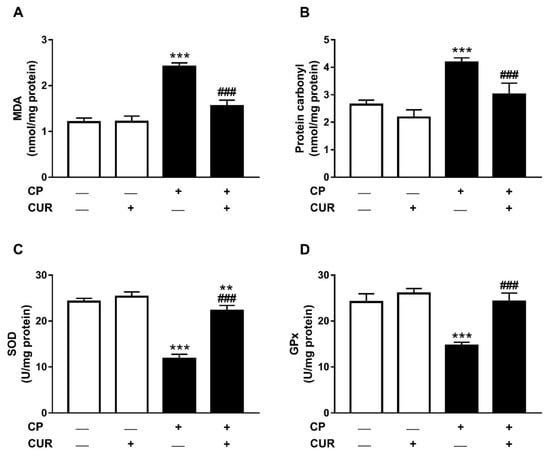
Figure 5.
Antioxidant effect of CUR in lung of CP-induced rats. CUR decreased MDA (A) and PCO (B), and increased SOD (C) and GPx (D) in lung of CP-induced rats. Values are expressed as mean ± SEM, n = 8, ** p < 0.01 and *** p < 0.001 versus control and ### p < 0.001 versus CP.
4. Discussion
CUR is a natural compound with well-acknowledged antioxidant and anti-inflammatory effects [42]. This study investigated the potential of CUR to prevent histological and ultrastructural changes, oxidative stress, and apoptosis provoked by CP in the lung of rats. CP is a well-known antineoplastic and immunosuppressive agent that could induce sever toxicity in various organs of experimental animals and humans [6,7,43,44]. In the present study, administration of CP resulted in adverse histopathological, ultrastructural, and biochemical changes in the lung of rats.
H&E staining of the lung tissues of the CP group showed the presence of sever obstruction of alveoli, thickened interalveolar septa, edema in the interalveolar septa, and compensatory emphysema combined with thickened septa. This was confirmed by the ultrastructural examination of lung tissues which showed the occurrence of pyknotic nuclei with chromatolysis, disappearance of cytoplasmic organelles besides electron dense bodies in pneumocytes type I and II, thickened blood–air barrier, and marked necrotic pneumocytes. CP has been shown to induce major cellular alterations, such as increased production of ROS and lipid peroxidation, possibly responsible for ultrastructural changes within the mitochondria that eventually trigger mitochondrial damage [45]. Accordingly, previous studies have demonstrated several alterations, including thickening in alveolar septa, alveolar cell injuries, erythrocytes in the alveolar lumen, and polymorphonuclear cells infiltration in the lung tissues of the experimental rats treated with CP [45,46]. Prior study stated that the injury of alveolar epithelial cells could provoke excessive production of cytokines and profibrotic mediators, which are associated with tissue alteration and deposition of connective tissues, leading to destruction of lung parenchyma and formation of fibrotic lesions [47].
According to previous studies, CUR has revealed many pharmacological activities, including anticancer, anti-inflammatory, antioxidant, and wound healing properties [20,21,22,23,24,48,49]. Therefore, we aimed to explore its protective effect against CP-induced lung injury in rats. CUR conferred protective effects in the CP-intoxicated group and didn’t induce any alterations when supplemented to normal rats. In CP-intoxicated rats, CUR restored the normal alveolar structure and vascular permeability, and suppressed polymorphonuclear cells infiltration. Furthermore, the reported deleterious effects of CP on the lung tissue of rats were found to be associated with increased MDA, PCO, and caspase-3 accompanied with diminished SOD and GPx. These alterations are attributed to CP and its active metabolites produced in the lung [14]. Therefore, oxidative stress and apoptosis are implicated in CP-induced lung injury. In this context, apoptosis and oxidative injury induced by CP have been reported in different tissues, including lung, liver, and kidney [6,7,43,44,45,46,50,51,52,53,54].
Oxidative stress is an imbalance between oxidants and antioxidants due to the excessive generation of ROS and the failure of cellular antioxidants to neutralize them [55]. ROS can damage different cellular components, including lipids and protein, and decline antioxidant enzymes. ROS provoke lipid peroxidation and this may affect the integrity of cell membrane, resulting in cell death [56]. Lipid peroxidation may boost the role of ROS via branched chain reactions and the levels of lipid peroxides can reflect the degree of cell damage. In addition to lipid peroxidation, ROS can oxidize cellular proteins and break DNA. Here, MDA and PCO were significantly increased in the lung of CP-induced rats, demonstrating lipid and protein damage, respectively. In the same context, CP has been recently reported to increase ROS generation and DNA damage in the liver of rats [51].
Treatment with CUR conferred protection against CP-induced lung injury possibly via its ability to suppress oxidative stress and apoptosis. CUR prevented structural alterations provoked by CP in the lung of rats as revealed by the histopathological and ultrastructure findings. In addition, antioxidant defenses were enhanced, whereas lipid and protein oxidation as well as apoptosis markers were all reduced in the lung of CP-intoxicated rats treated with CUR. These findings demonstrated the antioxidant and anti-apoptosis potential of CUR. Accordingly, previous work from Mahmoud’s lab revealed the antioxidant ant anti-apoptotic effects of CUR in gentamicin-, lead acetate-, and lipopolysaccharide/diclofenac-intoxicated rats [21,22,23,24]. In these studies, CUR prevented tissue injury, suppressed lipid peroxidation, DNA damage, inflammation, and pro-apoptotic proteins, and boosted antioxidants defenses [21,22,23,24]. CUR possesses the ability to suppress hydrogen peroxide-induced lipid peroxidation and increase thiols levels by inducing glutathione synthesis [57,58]. Besides its antioxidant activity, CUR attenuates inflammatory responses and the release of pro-inflammatory cytokines through interfering with the nuclear factor-kappaB (NF-κB) signaling [58]. Given the key role of oxidative stress and inflammation in eliciting apoptosis, CUR can inhibit cell death through its dual antioxidant and anti-inflammatory activity. The anti-apoptotic effect of CUR has been also connected to its ability to upregulate heme oxygenase 1 [24] and the Akt/GSK-3β signaling [21]. The role of CUR in the protection against lung injury induced by several insults has been previously reported. For instance, CUR inhibited inflammation and fibrosis in a mouse model of acute respiratory distress syndrome induced by plaque-forming units (pfu) reovirus 1/L [27]. In VILI in rats, CUR inhibited oxidative stress and suppressed inflammatory responses as reported by Wang et al. [19]. Moreover, CUR has shown potent anti-inflammatory and immune-modulatory effects and prevented lung injury in Klebsiella pneumoniae B5055-infected BALB/c mice [28]. Herein, we introduced new information that CUR prevents CP-induced lung injury through its ability to prevent histological and ultrastructural alterations and oxidative stress. Although CUR has shown potent antioxidant and anti-inflammatory activities, different studies have pointed to its low availability as the main factor that can limit its therapeutic applications [59,60]. Low aqueous solubility and intestinal absorption, systemic elimination, rapid metabolism, and degradation in alkaline pH are the main reasons for the low availability of CUR [59,60]. Therefore, different approaches, including the use of liposomes, adjuvants, and nanoparticles have been applied to increase the solubility of CUR [61]. The antioxidant and anti-inflammatory effects of CUR nanoparticles have been recently demonstrated in acute myocardial infarction in diabetic rats [62]. In comparison with the conventional form, nano CUR exhibited stronger antioxidative and anti-inflammatory effects [62]. Hence, the potential of nano CUR to protect against CP-induced oxidative stress, inflammation, and lung injury is worth being investigated.
5. Conclusions
This study introduced information on the protective effect of CUR against CP-induced lung injury in rats. CUR prevented histological alterations, suppressed inflammatory cells infiltration, protein damage, and lipid peroxidation, and enhanced antioxidant defenses in the lung of CP-induced rats. In addition, CUR diminished caspase-3 expression in the lung of CP-induced rats. Therefore, CUR could be employed as effective adjuvant therapy to prevent/attenuate lung injury in patients receiving CP. Further studies are needed to investigate the genetic and proteomic changes associated with the ameliorative effects of CUR on CP-induced lung injury.
Author Contributions
Conceptualization, S.A.M.S., A.M.M., and M.T.; methodology, S.A.M.S., A.M.M., S.A.A., M.A.A.-G., N.A.-G., H.Y.R., and M.T; validation, S.A.M.S. and A.M.M.; formal analysis, S.A.M.S., A.M.M., and M.T.; investigation, S.A.M.S., A.M.M., N.K.O., M.A.M.A., A.M.A.H., A.A.A., M.B.-J., and M.T.; resources, S.A.M.S., A.M.M., S.A.A., M.A.A.-G., N.A.-G., H.Y.R., and M.T.; data curation, S.A.M.S., M.T., and A.M.M.; writing, original draft preparation, S.A.M.S. and M.T.; writing, review and editing, A.M.M.; visualization, A.M.M. and M.T.; supervision, A.M.M. and M.T.; project administration, S.A.M.S., A.M.M., and M.T.; funding acquisition, M.B.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for supporting this research through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moignet, A.; Hasanali, Z.; Zambello, R.; Pavan, L.; Bareau, B.; Tournilhac, O.; Roussel, M.; Fest, T.; Awwad, A.; Baab, K. Cyclophosphamide as a first-line therapy in lgl leukemia. Leukemia 2014, 28, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Peng, C.H.; Ye, X. Interstitial pneumonia induced by cyclophosphamide: A case report and review of the literature. Respir. Med. Case Rep. 2019, 26, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Chandrakasan, G. Modulation of cyclophosphamide-induced early lung injury by curcumin, an anti-inflammatory antioxidant. Mol. Cell. Biochem. 1995, 142, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sulkowska, M.; Sulkowski, S.; Skrzydlewska, E.B.; Farbiszewski, R. Cyclophosphamide-induced generation of reactive oxygen species. Comparison with morphological changes in type ii alveolar epithelial cells and lung capillaries. Exp. Toxicol. Pathol. 1998, 50, 209–220. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Skrzydlewska, E.B.; Sulkowska, M.; Sulkowski, S.A. Effect of amifostine on lung oxidative stress after cyclophosphamide therapy. Bull. Vet. Inst. Pulawy 2002, 46, 87–94. [Google Scholar]
- Mahmoud, A.M.; Al Dera, H.S. 18β-glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: Potential role of pparγ and nrf2 upregulation. Genes Nutr. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Germoush, M.O.; Mahmoud, A.M. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J. Cancer Res. Clin. Oncol. 2014, 140, 1103–1109. [Google Scholar] [CrossRef]
- Ghosh, P.; Bhattacharjee, A.; Basu, A.; Singha Roy, S.; Bhattacharya, S. Attenuation of cyclophosphamide-induced pulmonary toxicity in Swiss albino mice by naphthalimide-based organoselenium compound 2-(5-selenocyanatopentyl)-benzo[de]isoquinoline 1,3-dione. Pharm. Biol. 2015, 53, 524–532. [Google Scholar] [CrossRef]
- Said, E.; Elkashef, W.F.; Abdelaziz, R.R. Tranilast ameliorates cyclophosphamide-induced lung injury and nephrotoxicity. Can. J. Physiol. Pharmacol. 2015, 94, 347–358. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Wadley, A.J.; Turner, J.E.; Aldred, S. Factors influencing post-exercise plasma protein carbonyl concentration. Free Radic. Res. 2016, 50, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yan, L.-J. Protein oxidative modifications: Beneficial roles in disease and health. J. Biochem. Pharmacol. Res. 2013, 1, 15. [Google Scholar] [PubMed]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Patel, J. Metabolism and pulmonary toxicity of cyclophosphamide. Pharmacol. Ther. 1990, 47, 137–146. [Google Scholar] [CrossRef]
- Kachel, D.; Martin, W. Cyclophosphamide-induced lung toxicity: Mechanism of endothelial cell injury. J. Pharmacol. Exp. Ther. 1994, 268, 42–46. [Google Scholar] [PubMed]
- Patel, J.; Block, E.; Hood, C. Biochemical indices of cyclophosphamide-induced lung toxicity. Toxicol. Appl. Pharmacol. 1984, 76, 128–138. [Google Scholar] [CrossRef]
- Patel, J.M.; Block, E.R. Cyclophosphamide-induced depression of the antioxidant defense mechanisms of the lung. Exp. Lung Res. 1985, 8, 153–165. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Wang, X.; An, X.; Wang, X.; Bao, C.; Li, J.; Yang, D.; Bai, C. Curcumin ameliorated ventilator-induced lung injury in rats. Biomed. Pharmacother. 2018, 98, 754–761. [Google Scholar] [CrossRef]
- Yonar, M.E.; Yonar, S.M.; İspir, Ü.; Ural, M.Ş. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (oncorhynchus mykiss) against aeromonas salmonicida subsp. Achromogenes. Fish Shellfish. Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating akt/gsk-3beta signaling pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ahmed, O.M.; Galaly, S.R. Thymoquinone and curcumin attenuate gentamicin-induced renal oxidative stress, inflammation and apoptosis in rats. EXCLI J. 2014, 13, 98–110. [Google Scholar] [PubMed]
- Galaly, S.R.; Ahmed, O.M.; Mahmoud, A.M. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J. Physiol. Pharmacol. 2014, 65, 823–832. [Google Scholar]
- Al-Dossari, M.H.; Fadda, L.M.; Attia, H.A.; Hasan, I.H.; Mahmoud, A.M. Curcumin and selenium prevent lipopolysaccharide/diclofenac-induced liver injury by suppressing inflammation and oxidative stress. Biol. Trace Elem. Res. 2019, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Abdou, R.H. Protective effects of diallyl sulfide and curcumin separately against thallium-induced toxicity in rats. Cell J. 2015, 17, 379–388. [Google Scholar] [PubMed]
- Guo, Y.; Shu, L.; Zhang, C.; Su, Z.-Y.; Kong, A.-N.T. Curcumin inhibits anchorage-independent growth of ht29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene dlec1. Biochem. Pharmacol. 2015, 94, 69–78. [Google Scholar] [CrossRef]
- Avasarala, S.; Zhang, F.; Liu, G.; Wang, R.; London, S.D.; London, L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS ONE 2013, 8, e57285. [Google Scholar] [CrossRef]
- Bansal, S.; Chhibber, S. Curcumin alone and in combination with augmentin protects against pulmonary inflammation and acute lung injury generated during klebsiella pneumoniae b5055-induced lung infection in balb/c mice. J. Med. Microbiol. 2010, 59, 429–437. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Sharma, S.S. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004, 74, 969–985. [Google Scholar] [CrossRef]
- Olama, N.K.; Taha, M.; Rady, H.Y. The potential protective role of coenzyme q10 on the cyclophosphamide-induced lung toxicity in adult male albino rats: A histological and ultrastructural study. Int. J. 2018, 4, 225. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Oxford, UK, 2018. [Google Scholar]
- Klopfleisch, R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology-a systematic review. BMC Vet. Res. 2013, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.E. Fixation for Electron Microscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hsu, S.; Raine, L.; Fanger, H. Use of biotin-avidin-peroxidase conplex (abc) in immunoperoxidase techniques: A comparison between abc and unlabeled antibody techniques. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Matkovics, B.; Szabo, L.; Varga, I.S. Determination of enzyme activities in lipid peroxidation and glutathione pathways (in hungarian). Lab. Diagn. 1998, 15, 248–249. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. [49] determination of carbonyl content in oxidatively modified proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 464–478. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Noorafshan, A.; Ashkani-Esfahani, S. A review of therapeutic effects of curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar]
- Alqahtani, S.; Mahmoud, A.M. Gamma-glutamylcysteine ethyl ester protects against cyclophosphamide-induced liver injury and hematologic alterations via upregulation of ppargamma and attenuation of oxidative stress, inflammation, and apoptosis. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of nrf2 and ppargamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmcother. 2017, 86, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ashry, N.A.; Gameil, N.M.; Suddek, G.M. Modulation of cyclophosphamide-induced early lung injury by allicin. Pharm. Biol. 2013, 51, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Şengül, E.; Gelen, V.; Gedikli, S.; Özkanlar, S.; Gür, C.; Çelebi, F.; Çınar, A. The protective effect of quercetin on cyclophosphamide-induced lung toxicity in rats. Biomed. Pharmcother. 2017, 92, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; King, T.E.; Pardo, A. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 2001, 134, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Verderio, P.; Bonetti, P.; Colombo, M.; Pandolfi, L.; Prosperi, D. Intracellular drug release from curcumin-loaded plga nanoparticles induces g2/m block in breast cancer cells. Biomacromolecules 2013, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ceber, M.; Topcu, B.; Ozen, O.A. The effects of topical treatment with curcumin on burn wound healing in rats. J. Mol. Histol. 2013, 44, 83–90. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Ramadan, S.A. Amelioration of cyclophosphamide-induced hepatotoxicity by the brown seaweed turbenaria ornata. Int. J. Clin. Toxicol. 2013, 1, 9–17. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.M.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef]
- Mahmoud, A.M. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of pparγ and abrogation of oxidative stress and inflammation. Can. J. Physiol. Pharmacol. 2014, 92, 717–724. [Google Scholar] [CrossRef]
- ALHaithloul, H.A.S.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates nrf2/are/ho-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmcother. 2019, 111, 676–685. [Google Scholar] [CrossRef]
- Kamel, E.M.; Mahmoud, A.M.; Ahmed, S.A.; Lamsabhi, A.M. A phytochemical and computational study on flavonoids isolated from trifolium resupinatum l. And their novel hepatoprotective activity. Food Funct. 2016, 7, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.C. Oxidative stress. J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, F.; Siassi, F.; Minaie, S.; Djalali, M.; Rahimi, A.; Chamari, M. Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women? Arya Atheroscler. 2010, 2, 189–192. [Google Scholar]
- Borra, S.K.; Mahendra, J.; Gurumurthy, P.; Jayamathi; Iqbal, S.S.; Mahendra, L. Effect of curcumin against oxidation of biomolecules by hydroxyl radicals. J. Clin. Diagn. Res. 2014, 8, 1–5. [Google Scholar]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurological symptoms in eae model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef]
- Boarescu, P.-M.; Boarescu, I.; Bocșan, I.C.; Gheban, D.; Bolboacă, A.E.; Nicula, C.; Pop, R.M.; Râjnoveanu, R.-M.; Bolboacă, S.D. Antioxidant and anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants 2019, 8, 504. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).