Synthesis of the Hydrophobic Cationic Polyacrylamide (PADD) Initiated by Ultrasonic and its Flocculation and Treatment of Coal Mine Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
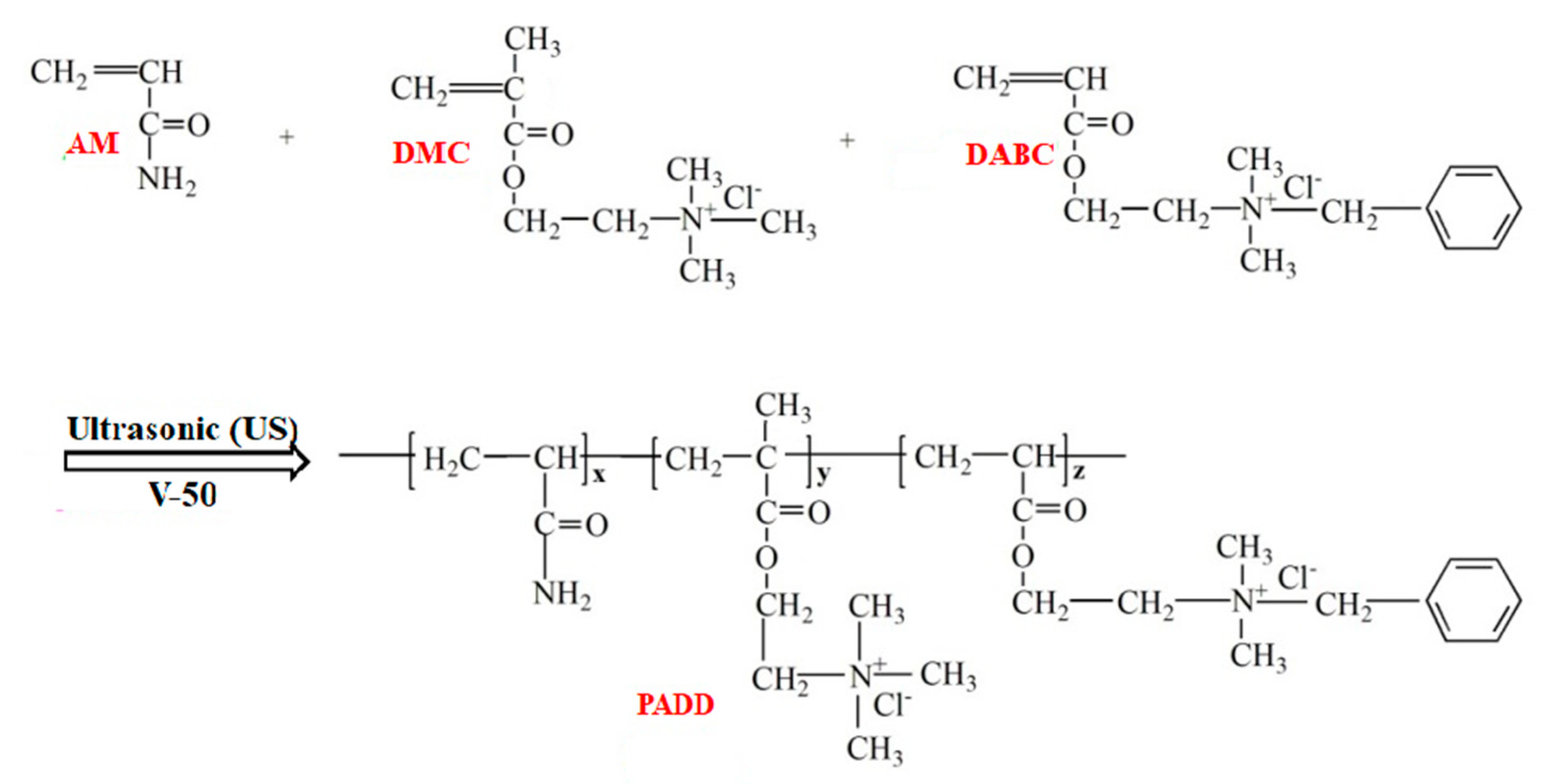
2.2. Preparation of Polymers
2.3. Characterization of Polymers
2.4. Analysis Method and Stirring Test
3. Results and Discussions
3.1. Effect of Single Factor Experimental Conditions on Polymerization
3.1.1. Effect of Ultrasonic Power on Polymerization
3.1.2. Effect of pH on Polymerization
3.1.3. Effect of Initiator Concentration on Polymerization
3.1.4. Effect of Mass Ratio of Cationic Monomers on Polymerization
3.2. FTIR Spectral Analysis
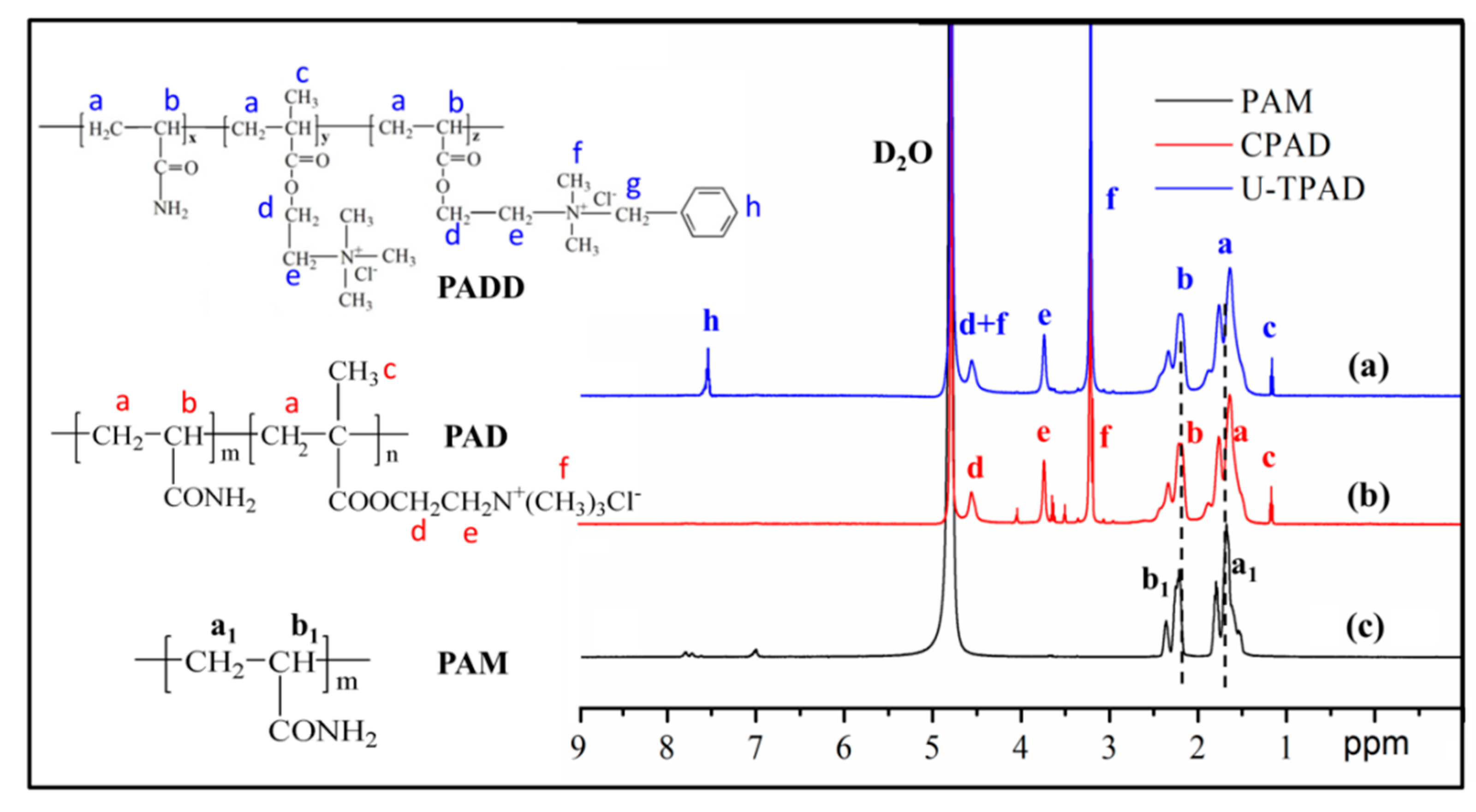
3.3. 1H NMR Spectrum Analysis
3.4. SEM of Polymer
4. Flocculation Performance
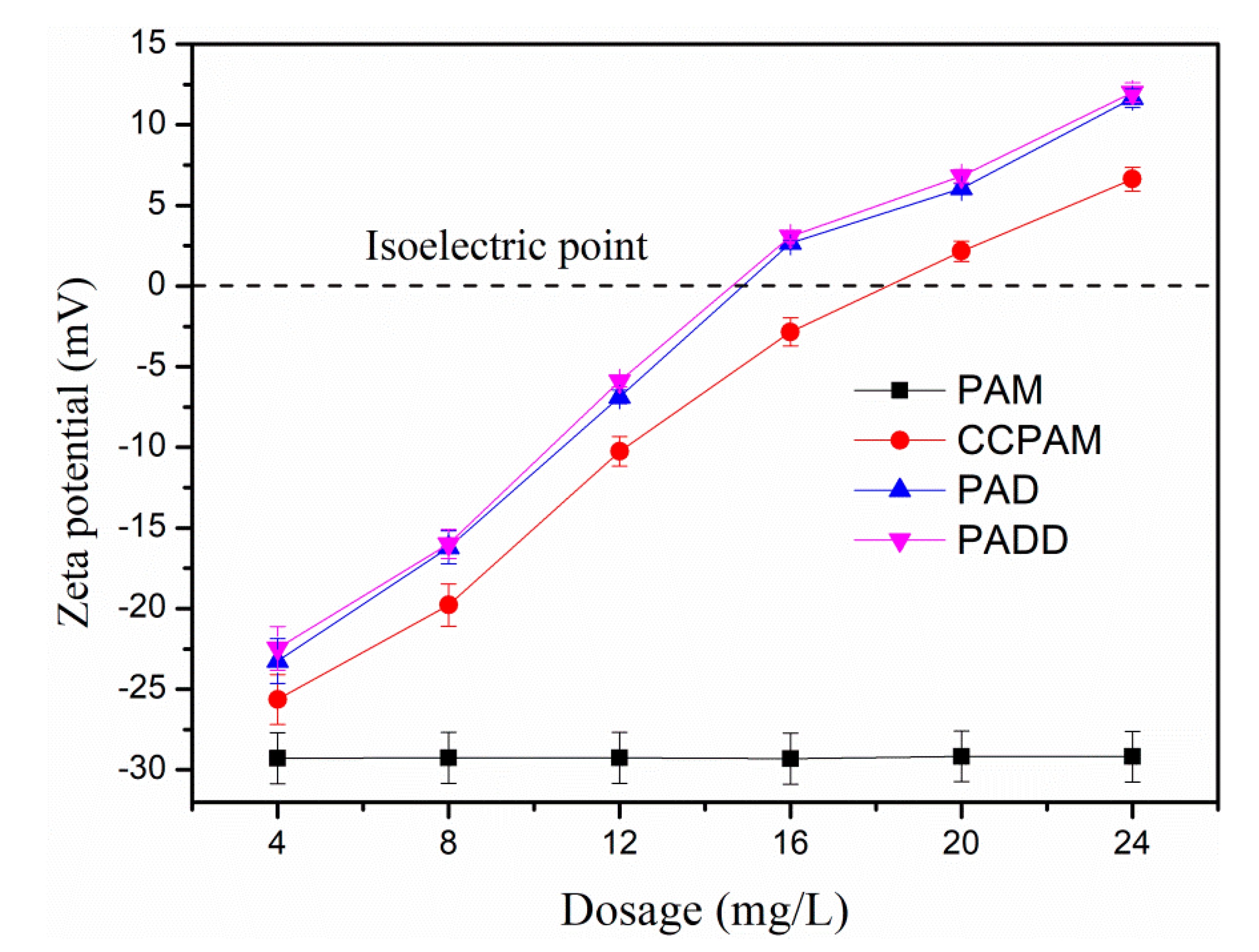
4.1. The Influence of Flocculant Dosage on Flocculation
4.2. The Effect of pH Value on Flocculation
4.3. Floc Characteristics and Flocculation Mechanism
4.3.1. Floc Dynamics Investigation
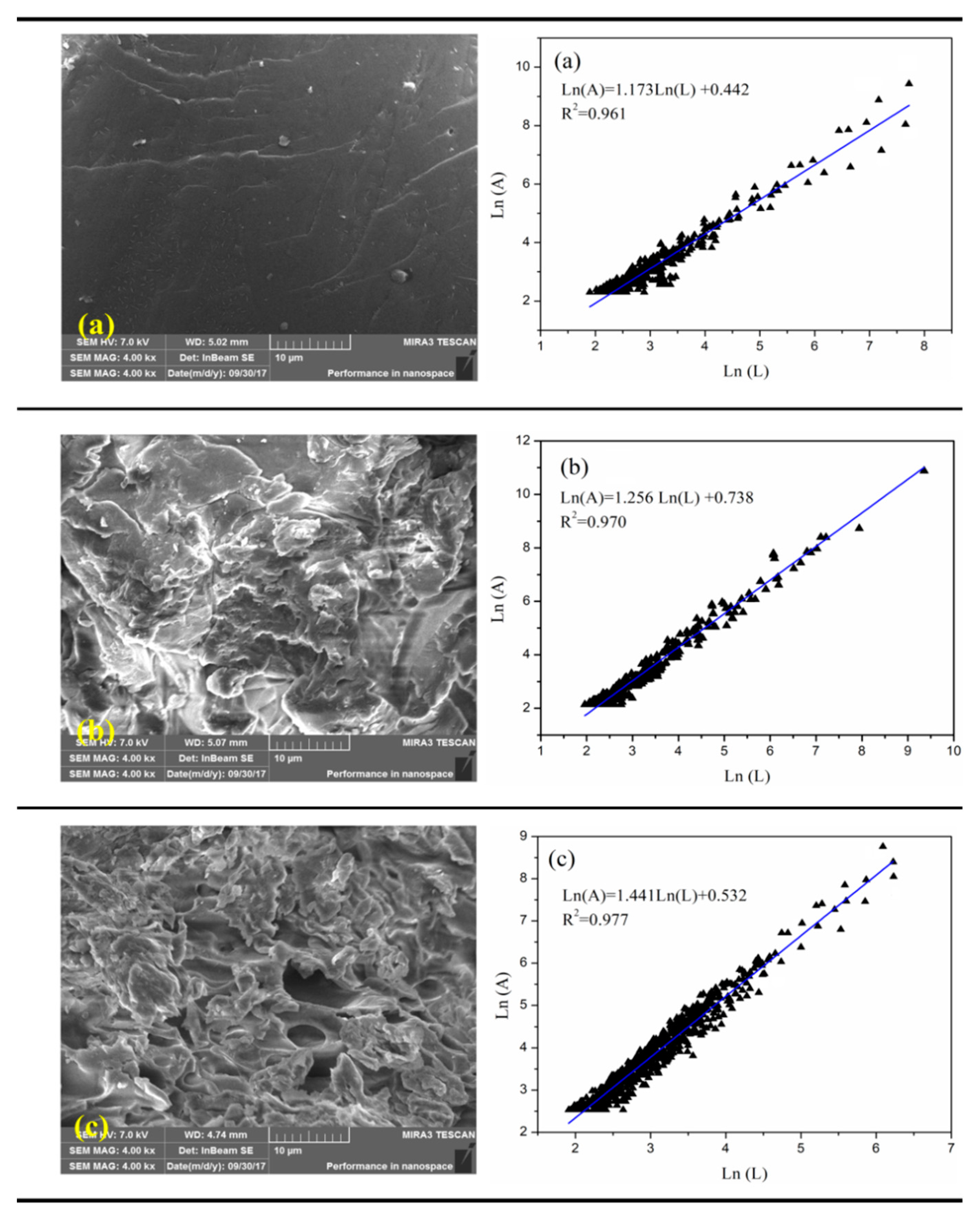
4.3.2. Fractal Dimension and Size of Flocs
4.3.3. Flocculation Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Zheng, H.L.; Wang, Y.L.; Sun, Y.J.; Xu, B.C.; Zhao, C.L. Fabricating an enhanced sterilization chitosan-based flocculants: Synthesis, characterization, evaluation of sterilization and flocculation. Chem. Eng. J. 2017, 319, 119–130. [Google Scholar] [CrossRef]
- Jenkins, M.; Tiwari, S.; Darby, J. Bacterial, viral and turbidity removal by intermittent slow sand filtration for household use in developing countries: Experimental investigation and modeling. Water Res. 2011, 45, 6227–6239. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Fu, K.; Jiang, L.Y.; Ding, L.; Guan, Q.Q.; Zhang, S.H.; Zhang, H.W.; Shi, J.; Fu, X. Flocculation performance of cationic polyacrylamide with high cationic degree in humic acid synthetic water treatment and effect of kaolin particles. Sep. Purif. Technol. 2017, 181, 201–212. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.L.; Tang, X.M.; Zheng, X.Y.; Liu, S.; Sun, Q.; Wang, M.X. The investigation of the specific behavior of a cationic block structure and its excellent flocculation performance in high-turbidity water treatment. RSC Adv. 2018, 8, 15119–15133. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.Y.; Lo, S.L.; Chang, C.L.; Chen, F.L.; Wu, Y.D.; Ma, J.L. Treatment of highly turbid water using chitosan and aluminum salts. Sep. Purif. Technol. 2013, 104, 322–326. [Google Scholar] [CrossRef]
- Liao, L.N.; Zhang, P. Preparation and Characterization of Polyaluminum Titanium Silicate and its Performance in the Treatment of Low-Turbidity Water. Processes 2018, 6, 125. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, S.; Ghosh, A.; Bindal, R.; Tewari, P. Outside skin tubular ultrafiltration membranes for separation of proteins and polysaccharides and removal of turbidity from seawater. Desalin. Water Treat. 2011, 27, 231–236. [Google Scholar] [CrossRef]
- Nkurunziza, T.; Nduwayezu, J.; Banadda, E.; Nhapi, I. The effect of turbidity levels and Moringa oleifera concentration on the effectiveness of coagulation in water treatment. Water Sci. Technol. 2009, 59, 1551–1558. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.; Akram, M.; Uddin, A.; Khan, S.; Yeom, I. Effect of Dissolved Organic Matter on Agglomeration and Removal of CuO Nanoparticles by Coagulation. Processes 2019, 7, 455. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Y.; Shi, J.; Ding, H.C.; Zhu, G.C.; Fu, K.; Fu, X. Synthesis of cationic polyacrylamide by low-pressure UV initiation for turbidity water flocculation. Chem. Eng. J. 2017, 312, 20–29. [Google Scholar] [CrossRef]
- Koley, S.; Prasad, S.; Bagchi, S.; Mallick, N. Development of a harvesting technique for large-scale microalgal harvesting for biodiesel production. RSC Adv. 2017, 7, 7227–7237. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Y.; Fu, X.; Xia, W.; Fu, K.; Liao, Y. Flocculation of a High-Turbidity Kaolin Suspension Using Hydrophobic Modified Quaternary Ammonium Salt Polyacrylamide. Processes 2019, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, S.; Moghaddam, M.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Hankins, N.; Lu, N.; Hilal, N. Enhanced removal of heavy metal ions bound to humic acid by polyelectrolyte flocculation. Sep. Purif. Technol. 2006, 51, 48–56. [Google Scholar] [CrossRef]
- Zhang, P.; Liao, L.N.; Zhu, G.C. Performance of PATC-PDMDAAC Composite Coagulants in Low-Temperature and Low-Turbidity Water Treatment. Materials 2019, 12, 2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, T.; Wang, X.; Song, G.; Cui, G. Synthesis and flocculation performance of a chitosan-acrylamide-fulvic acid ternary copolymer. Carbohyd. Polym. 2017, 170, 182. [Google Scholar] [CrossRef]
- Sun, Y.J.; Sun, W.Q.; Shah, K.; Chiang, P.C.; Zheng, H.L. Characterization and flocculation evaluation of a novel carboxylated chitosan modified flocculant by UV initiated polymerization. Carbohyd. Polym. 2019, 208, 213–220. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhu, C.Y.; Sun, W.Q.; Xu, Y.H.; Xiao, X.F.; Zheng, H.L.; Wu, H.F.; Liu, C.Y. Plasma-initiated polymerization of chitosan-based CS-g-P(AM-DMDAAC) flocculant for the enhanced flocculation of low-algal-turbidity water. Carbohyd. Polym. 2017, 164, 222–232. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Sun, Y.; Sun, W.; Xu, Y.; Zheng, H. Synthesis and Characterization of Ampholytic Flocculant CPCTS-g-P (CTA-DMDAAC) and Its Flocculation Properties for Microcystis Aeruginosa Removal. Processes. 2018, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.L.; Sun, Y.J.; Guo, J.S.; Li, F.T.; Fan, W.; Liao, Y.; Guan, Q.Q. Characterization and Evaluation of Dewatering Properties of PADB, a Highly Efficient Cationic Flocculant. Ind. Eng. Chem. Res. 2014, 53, 2572–2582. [Google Scholar] [CrossRef]
- Kotlyarevskaya, O.; Navrotskii, V.; Orlyanskaya, M.; Navrotskii, A.; Novakov, I. Flocculation Properties of Polyelectrolytes Based on 2- (N, N -Dimethyl- N -Benzylammonio) ethyl Methacrylate Chloride. Russ. J. Appl. Chem. 2004, 77, 622–628. [Google Scholar] [CrossRef]
- Zheng, H.L.; Sun, Y.J.; Zhu, C.J.; Guo, J.S.; Zhao, C.; Liao, Y.; Guan, Q.Q. Uv-initiated polymerization of hydrophobically associating cationic flocculants: Synthesis, characterization, and dewatering properties. Chem. Eng. J. 2013, 234, 318–326. [Google Scholar] [CrossRef]
- Zhu, G.C.; Liu, J.F.; Yin, J.; Li, Z.W.; Ren, B.Z.; Sun, Y.J.; Wan, P.; Liu, Y.S. Functionalized polyacrylamide by xanthate for Cr (VI) removal from aqueous solution. Chem. Eng. J. 2016, 288, 390–398. [Google Scholar] [CrossRef]
- Feng, L.; Liu, S.; Zheng, H.L.; Liang, J.J.; Sun, Y.J.; Zhang, S.X.; Chen, X. Using ultrasonic (US)-initiated template copolymerization for preparation of an enhanced cationic polyacrylamide (CPAM) and its application in sludge dewatering. Ultrason. Sonochem. 2018, 44, 53–63. [Google Scholar] [CrossRef]
- Feng, L.; Liu, J.Y.; Xu, C.; Lu, W.C.; Li, D.M.; Zhao, C.L.; Liu, B.Z.; Li, X.; Khan, S.; Zheng, H.L.; et al. Better understanding the polymerization kinetics of ultrasonic-Template method and new insight on sludge floc characteristics research. Sci. Total Environ. 2019, 689, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Feng, J.; Zheng, H.; Yang, H.; Xu, J. Preparation of pH-sensitive polymer by thermal initiation polymerization and its application in fluorescence immunoassay. Talanta 2005, 65, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, I.; Turro, N. Cage Effect Dynamics under Photolysis of Photoinitiators. Des. Monomers Polym. 2010, 13, 487–496. [Google Scholar]
- Ma, J.Y.; Shi, J.; Ding, L.; Zhang, H.W.; Zhou, S.; Wang, Q.J.; Fu, X.; Jiang, L.Y.; Fu, K. Removal of emulsified oil from water using hydrophobic modified cationic polyacrylamide flocculants synthesized from low-pressure UV initiation. Sep. Purif. Technol. 2018, 197, 407–417. [Google Scholar] [CrossRef]
- Song, S.X. Experimental studies on hydrophobic flocculation of coal fines in aqueous solutions and flotation of flocculated coal. Int. J. Oil Gas Coal Technol. 2008, 1, 180–193. [Google Scholar] [CrossRef]
- Zheng, H.L.; Feng, L.; Gao, B.Y.; Zhou, Y.; Zhang, S.X.; Xu, B.C. Effect of the cationic block structure on the characteristics of sludge flocs formed by charge neutralization and patching. Materials 2017, 10, 487. [Google Scholar]
- Feng, L.; Zheng, H.L.; Wang, Y.L.; Zhang, S.X.; Xu, B.C. Ultrasonic-template technology inducing and regulating cationic microblocks in CPAM: Characterization, mechanism and sludge flocculation performance. RSC Adv. 2017, 7, 23444–23456. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Zheng, H.; Qian, L.; Sun, Y.J.; Dai, L.; Xue, W.W. UV-Initiated Polymerization of Hydrophobically Associating Cationic Polyacrylamide Modified by a Surface-Active Monomer: A Comparative Study of Synthesis, Characterization, and Sludge Dewatering Performance. Ind. Eng. Chem. Res. 2014, 53, 11193–11203. [Google Scholar] [CrossRef]
- Wang, M.X.; Feng, L.; Fan, X.W.; Li, D.M.; Qu, W.Q.; Jiang, S.X.; Li, S.X. Fabrication of Bifunctional Chitosan-Based Flocculants: Characterization, Assessment of Flocculation, and Sterilization Performance. Materials 2018, 11, 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.C.; Zhu, Y.; Huang, W.Y.; Zhang, C.W.; Li, T.; Zhang, Y.M.; Li, A.F. Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium. Bioresour. Technol. 2012, 110, 496–502. [Google Scholar] [CrossRef]
- Suresha, P.R.; Manohar, V.B. Flocculation of kaolin from aqueous suspension using low dosages of acrylamide-based cationic flocculants. J. Appl. Polym. Sci. 2018, 136, 47286. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.L.; Gao, B.Y.; Zhao, C.L.; Zhang, S.X.; Chen, N. Enhancement of textile-dyeing sludge dewaterability using a novel cationic polyacrylamide: Role of cationic block structures. RSC Adv. 2017, 7, 11626–11635. [Google Scholar] [CrossRef] [Green Version]
- Manognya, B.; James, S.B.; Christopher, B.F.; Mohammad, S.I. Impact of Natural Cationic Polymers on Charge and Clarification of Microalgae Suspensions. Environ. Eng. Sci. 2015, 32, 212–221. [Google Scholar]
- Besra, L.; Sengupta, D.K.; Roy, S.K.; Ay, P. Influence of surfactants on flocculation and dewatering of kaolin suspensions by cationic polyacrylamide (PAM-C) flocculant. Sep. Purif. Technol. 2003, 30, 251–264. [Google Scholar] [CrossRef]
- Son, M.; Hsu, T.J. Flocculation model of cohesive sediment using variable fractal dimension. Environ. Fluid Mech. 2008, 8, 55–71. [Google Scholar] [CrossRef]
- Zhu, Z.; Peng, D.; Dou, J. Changes in the two-dimensional and perimeter-based fractal dimensions of kaolinite flocs during flocculation: A simple experimental study. Water Sci. Technol. 2018, 77, 861–870. [Google Scholar] [CrossRef]
- Chung, H.; Ju, S.; Lee, D. Hydrodynamic drag force exerted on activated sludge floc at intermediate Reynolds number. J. Colloid Interf Sci. 2003, 263, 498–505. [Google Scholar] [CrossRef]
- Wu, J.; He, C. Experimental and modeling investigation of sewage solids sedimentation based on particle size distribution and fractal dimension. Int. J. Environ. Sci. Technol. 2009, 7, 37–46. [Google Scholar]
- Huang, M.; Liu, Z.Z.; Li, A.M.; Hu, Y. Dual functionality of a graft starch flocculant: Flocculation and antibacterial performance. J. Environ. Manag. 2017, 196, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Gao, B.Y.; Huang, X.; Dong, H.Y.; Li, X.C.; Yue, Q.Y.; Qian, L. Compound bioflocculant and polyaluminum chloride in kaolin-humic acid coagulation: Factors influencing coagulation performance and floc characteristics. Bioresour. Technol. 2014, 172, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Xue, F.; Jiang, L.Y.; Zhu, G.C.; Shi, J. Magnetic flocculants synthesized by Fe3O4 coated with cationic polyacrylamide for high turbid water flocculation. Environ Sci. Pollut. Res. 2018, 25, 25955–25966. [Google Scholar] [CrossRef] [PubMed]






| Flocculant | Molecular Weight (1 × 106 Da) | Cationic Degree (%) | Conversion Percent (%) |
|---|---|---|---|
| PADD | 400 | 40.0 | 99.8 |
| PAD | 400 | 40.0 | 99.6 |
| CCPAM | 400 | 20.0 | / |
| PAM | 300 | / | 99.8 |
| pH | Density (g·mL−1) | Zeta Potential (mV) | Turbidity (NTU) | Average Size Distribution (d50, μm) | Color |
|---|---|---|---|---|---|
| 7.47 | 1.169 | −29.30 | 313.9 | 76.2 | Gray and black |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Liu, J.; Wang, C.; Li, S.; Li, X.; Liang, Y.; Sarfaraz, K. Synthesis of the Hydrophobic Cationic Polyacrylamide (PADD) Initiated by Ultrasonic and its Flocculation and Treatment of Coal Mine Wastewater. Processes 2020, 8, 62. https://doi.org/10.3390/pr8010062
Qi X, Liu J, Wang C, Li S, Li X, Liang Y, Sarfaraz K. Synthesis of the Hydrophobic Cationic Polyacrylamide (PADD) Initiated by Ultrasonic and its Flocculation and Treatment of Coal Mine Wastewater. Processes. 2020; 8(1):62. https://doi.org/10.3390/pr8010062
Chicago/Turabian StyleQi, Xin, Junling Liu, Cheng Wang, Shiyao Li, Xiang Li, Yicong Liang, and Khan Sarfaraz. 2020. "Synthesis of the Hydrophobic Cationic Polyacrylamide (PADD) Initiated by Ultrasonic and its Flocculation and Treatment of Coal Mine Wastewater" Processes 8, no. 1: 62. https://doi.org/10.3390/pr8010062





