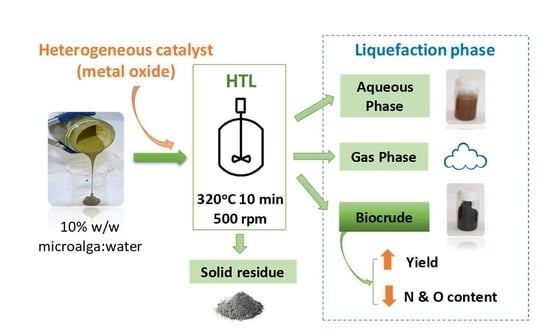
3.1. Effects of Catalysts on the Yields of Hydrothermal Liquefaction (HTL) Processes
The yields of the different fractions obtained from the catalytic HTL processes (biocrude, water-soluble products, gas phase and solid residue) are shown in
Table 2. The corresponding yields for the non-catalytic HTL (thermal) process are also included in the same table for comparison purposes.
The yield towards the fraction of greatest interest, i.e., biocrude, were mostly between 43 and 50 wt%, which were near or somewhat higher than the equivalent obtained under thermal non-catalysed conditions (42.60 ± 0.80 wt%). According to Shi et al. [
37], these catalysts prevent dehydration and promote the hydrolysis and cracking reactions. The use of CeO
2, MnO
2, La
2O
3 and Al
2O
3 as heterogeneous catalysts results in lower yields (43.80 ± 0.50 wt%, 44.11 ± 0.50 wt%, 42.66 ± 1.10 wt% and 44.22 ± 1.60 wt%, respectively) than the use of CaO (49.73 ± 0.90 wt%). If we attend at the electronegativity of these catalysts under the reaction conditions, the first four had a electronegativity between 8 and 11.5 while CaO had a value close to 5 [
38]. Consequently, the highest yield of biocrude was obtained with the most basic oxide catalyst (CaO), which promoted the hydrolysis and cracking reactions. In addition, the basicity of CaO could give rise to secondary reactions of saponification, decreasing the biocrude yield [
22]. However, the saponification reactions were not significant at the short reaction time used in this work (10 min) in comparison to the longer reaction times (60 min) in a previous study using palm biomass as raw material [
38].
The yields of the water-soluble products for basic metal oxides catalysts (CaO, CeO
2, MnO
2, La
2O
3) ranged from 37.43 ± 0.61 wt% to 44.12 ± 0.92 wt%, being higher than the corresponding value in the thermal HTL (31.98 ± 1.20 wt%). This implies that the metallic oxides also promote an increase of this fraction yield, which was also previously observed in the HTL of rice husk using these types of catalysts [
37]. In addition, the acid nature of these types of catalysts has an influence on the yield of the water-soluble products. Thus, the more acidic Al
2O
3 had a lower yield of water-soluble compounds than the more basic metal oxides.
The yields of the gas fraction obtained with CaO and CeO
2 were significantly lower (7.57 ± 0.50 wt% and 9.82 ± 0.01 wt%, respectively) than the thermal HTL (17.81 ± 2.27 wt%), whereas the yield of this fraction using La
2O
3 (12.50 ± 1.90 wt%), MnO
2 (18.27 ± 1.41 wt%) and Al
2O
3 (17.44 ± 3.21 wt%) was statistically the same as the one obtained without a catalyst. The decrease of the gas fraction yield could be related to the observed increase of the biocrude and water-soluble product yields because of the interaction between the different phases during HTL, according to the kinetic model proposed by Valdez [
39].
The liquefied fraction yields obtained with metallic oxides were generally higher than this fraction yield obtained in the HTL without catalyst (the liquefaction phase values of MnO
2, CeO
2 and Al
2O
3 were significantly higher than the value of the non-catalysed HTL), which is due to the detected increase in the yields of the liquid fractions (water-soluble products and biocrude) with these catalysts. These yields are in all cases higher than 94 wt%, even reaching 99.49 ± 0.70 wt% when MnO
2 was used. It is noteworthy, therefore, that the high yield of the liquefied fraction indicates a conversion of the microalga near 100%. These high yields are linked to the low amounts of solid residue obtained in the catalytic processes (0.50 wt% to just under 6 wt%) in comparison with the solid yield in the thermal reaction (7.60 ± 0.70 wt%). This increase in the liquefied fraction and a low yield of the solid residue was observed previously in the HTL of rice husk and microalga
Nannochloropsis with the same type of catalysts [
37,
40].
3.2. Element Content, Higher Heating Value (HHV) and Energy Recovery (ER) of Obtained Biocrude
Table 3 shows the elemental analysis, HHV and energy recovery (ER) of the biocrudes obtained with the metal oxide-catalysed HTL and the non-catalysed process.
Biocrudes obtained with the metal oxides CaO, CeO
2 and La
2O
3 had statistically similar N content (4.76 ± 0.02 wt%, 4.62 ± 0.15 wt% and 4.64 ± 0.01 wt%, respectively). Therefore, these catalysts are suitable to carry out denitrogenation reactions. However, the N content of the biocrude obtained with MnO
2 and Al
2O
3 were slightly higher (5.45 ± 0.11 wt% and 5.39 ± 0.21 wt%). In all cases, a significant decrease was observed in the N content with respect to the non-catalysed reaction (6.11 ± 0.02 wt%). This fact corroborates the power of metal oxides as catalysts of hydrolysis and cracking reactions breaking the macromolecules (proteins and lipids) to give compounds with N soluble in the aqueous phase fraction that decrease the content of this heteroatom in biocrude [
20,
38].
Regarding the O concentration in the biocrudes, the values obtained with the metal oxides ranged from 14.94 ± 0.58 wt% for MnO
2 to 21.68 ± 0.04 wt% for La
2O
3, being higher than those obtained by the thermal reaction (10.54 ± 0.50 wt%). This fact may be due to the hydrolysis of the main molecules (carbohydrates, lipids and proteins) into smaller ones that cannot undergo deoxygenation. However, the O content could be improved with these catalysts at a longer reaction time [
20]. In spite of that, in comparison with thermal reaction, no considerable growth was detected in the O concentration for CeO
2 and Al
2O
3 catalysts.
The HHV values obtained from the elemental analysis were between 30.12 ± 0.01 and 33.43 ± 0.91 MJ/kg (
Table 2), regardless of the type of metallic oxide used. These values are typical of biocrudes obtained from microalgae by HTL process (30–43 MJ/kg) [
20]. Conversely, the ER were between 52.88% ± 0.02% to 58.75% ± 1.62%. The HHV and ER results of the CeO
2 and Al
2O
3 were similar to those of the thermal reaction and the mentioned indexes for CaO and MnO
2 were slightly lower. These values were approximately similar to the ones reported previously for fast HTL (400 °C, 2 min) with Pd/C, Pt/C or dimethalic alumina catalysts [
15], but somewhat lower than those obtained in the HTL at 350 °C and 60 min with similar catalysts [
27]. Among the catalysts applied in this study, CeO
2 resulted in remarkable specifications for the obtained biocrude through HTL of microalgae,
N. gaditana. As can be clearly seen in
Table 2, the highest values of C and H content, HHV and ER, in addition to the lowest content of N, O and S occurred as a consequence of the HTL process using the CeO
2 catalyst.
Because the biocrude properties are highly dependent on the H/C, N/C and O/C atomic ratios, these were calculated and compared with the corresponding ratios of the thermal biocrude and the initial microalgal biomass. In addition, the atomic ratios of the biocrudes were compared with a biodiesel obtained from rapeseed oil that complies European Biodiesel EN 14214 Standards, a biodiesel from
N. gaditana oil that meets the biodiesel specifications except for the content of polyunsaturated (≥4 double bonds) methylester and a reference fossil diesel complying European Standard EN-590. The atomic ratios were represented using a Van Krevelen diagram (
Figure 1a,b). The H/C, N/C and O/C ratio for the reference diesel were 1.84, 0.005 and 0.008, respectively. In the HTL process, deoxygenation and denitrogenation mechanisms are produced during the reaction [
12]. Therefore, O/C and N/C ratios decrease with respect to the initial biomass around 80% and 40%, respectively. The H/C ratios shown in
Figure 1 were similar for the five biocrudes regardless of the catalysts used, showing values around 1.50–1.65. However, the ratio of the thermal biocrude obtained was 1.25 because of its higher percentage of C in the composition. On the other hand, the N/C ratios were all between 0.05 and 0.06, slightly higher than that for the reference diesel oil (0.005) and somewhat lower than the thermal biocrude (0.084). Despite the fact that the N content was reduced with respect to the thermal reaction, a total recovery of C in the biocrude was not achieved and therefore, the N/C ratio obtained was somewhat higher than the limits reported in the literature [
20]. The values of O/C were between 0.10 and 0.20, which were higher values than the ones obtained previously for this heterogeneous catalysed process (0.004–0.053) [
15], which is associated to the fact that the O and C were not completely recovered in the biocrude phase. However, the O/C ratios obtained with oxygenated biofuels, such as biodiesels from rapeseed and
N. gaditana oils, were close to 0.10 and similar to the O/C ratios of the biocrudes obtained using CeO
2 (0.135) and Al
2O
3 (0.113). Longer reaction times are required to decrease the O concentrations of the biocrude obtained by HTL with heterogeneous catalysts. In spite of higher values of the H/C, N/C and O/C, we can also remark on the fact that, apart from higher NO
x emission due to the higher N content and lower heating value, using oxygenated biofuels such as biodiesel and bioethanol has significantly reduced the formation of air pollutants like CO, soot and unburned hydrocarbons and increased the combustion efficiency. In addition, developing engine technologies and fuel additives, besides optimizing biocrude production and upgrading systems, will provide a sustainable source of biofuel for commercial purposes [
41,
42,
43,
44].
The composition of the biocrudes obtained by GC–MS, grouped by families, is shown in
Table 4. A high content of acids was obtained in the biocrudes using CaO, CeO
2, La
2O
3 and Al
2O
3, achieving values of 28.08 wt%, 31.18 wt%, 24.99 wt% and 17.82 wt%, respectively. These catalysts promote hydrolysis reactions to a greater extent, increasing the amount of organic acids and alcohols. The latter achieved values of 9.46–18.48 wt%. Conversely, the content of organic acids in the biocrude obtained in the HTL with MnO
2 was remarkably higher (60.96 wt%). In this sense, this catalyst promotes the deamination reactions of aminoacids to organic acids, in addition to the hydrolysis of lipids to these organic acids. The high content of oxygenates (mainly acids, alcohols and amides) was around 65–70 wt% of the composition, which causes a low biocrude stability, a low HHV and high corrosive power [
45]. However, these values were lower than those of the thermal reaction were, where the oxygenated compounds were higher than 85 wt%. Attending to the N compounds (amines, amides and nitriles), they supposed between a 20.24 wt% and a 36.85 wt%. These compounds mainly come from the hydrolysis of the proteins [
12]. The content of amines was higher than that of the thermal reaction (9.74 wt%), while the amides were lower than the one obtained in the control thermal reaction (31.26 wt%). Finally, the amount of hydrocarbons in the biocrudes obtained with these metal oxides were higher (12.71–15.36 wt%) than the corresponding value obtained in the thermal control HTL (6.02 wt%), that is associated again with the decrease of heteroatoms in biocrude.
The boiling temperature of the biocrudes obtained by metal oxide catalysis was determined and compared with a diesel that complies with the EN-590 standard (
Table 5). The results obtained for the boiling point showed that the obtained biocrudes boiled completely at temperatures close to 529.20–551.80 °C and were similar to the one achieved in the thermal reaction (546.00 °C). However, these boiling points of the biocrude were higher than in the reference diesel (476.10 °C). Despite the decrease in the heteroatom content of the biocrudes obtained with the catalysts, the content of N and O remained high compared to the diesel that follows the regulations. This implies that the biocrude containing these electronegative heteroatoms is capable of forming more intense dipole–dipole interactions, which would lead to a higher apparent boiling point in the mixture. This fact was previously observed in the metal oxide-catalysed HTL of Malaysian oil palm biomass [
38] due to the presence of oxygenated compounds and aromatic compounds.